Summary
Photorespiration raises cellular CO2 levels about 3-fold in leaves of C3–C4 intermediate Flaveria species. This was shown by using 14C-based fluxomics to determine the Rubisco in vivo carboxylation-to-oxygenation ratios.
Key words: 14CO2 labelling, C3–C4 intermediate plants, carbon-concentrating mechanism, Flaveria, glycine decarboxylation, photorespiration, photosynthesis.
Abstract
Formation of a photorespiration-based CO2-concentrating mechanism in C3–C4 intermediate plants is seen as a prerequisite for the evolution of C4 photosynthesis, but it is not known how efficient this mechanism is. Here, using in vivo Rubisco carboxylation-to-oxygenation ratios as a proxy to assess relative intraplastidial CO2 levels is suggested. Such ratios were determined for the C3–C4 intermediate species Flaveria pubescens compared with the closely related C3 plant F. cronquistii and the C4 plant F. trinervia. To this end, a model was developed to describe the major carbon fluxes and metabolite pools involved in photosynthetic–photorespiratory carbon metabolism and used quantitatively to evaluate the labelling kinetics during short-term 14CO2 incorporation. Our data suggest that the photorespiratory CO2 pump elevates the intraplastidial CO2 concentration about 3-fold in leaves of the C3–C4 intermediate species F. pubescens relative to the C3 species F. cronquistii.
Introduction
Land plants form three major classes characterized by specific modes of photosynthetic CO2 assimilation. In C3 plants, CO2 enters metabolism directly via ribulose 1,5-bisphosphate (RubP) carboxylase/oxygenase (Rubisco). In the mesophyll of C4 plant leaves and in CAM (crassulacean acid metabolism) plants, CO2 is initially fixed by phosphoenolpyruvate carboxylase. The resulting four-carbon (C4) compounds are decarboxylated in the Rubisco-containing bundle-sheath of C4 plants (Hatch and Slack, 1970) or become stored in the vacuoles of CAM plants for daytime decarboxylation and refixation of the released CO2 by Rubisco (Lüttge, 2004). Both modifications to the C3 mode of CO2 assimilation are adaptations to specific environmental conditions such as low CO2 or water availability. While C4 plants represent only about 3% of all land plant species, they dominate nearly all grasslands in the tropics, subtropics, and warm temperate zones (Sage, 2004). They also include highly productive crops, such as corn and sugar cane, and there is much interest to introduce yield-relevant features of C4 photosynthesis into C3 crops.
Given the ecological and agricultural significance of C4 plants, it is important to understand how they evolved and what were the crucial steps in this process. A number of studies have shown that the evolution of C4 photosynthesis was not a unique event but occurred at least 66 times during the past 35 million years (Sage, 2004; Sage et al., 2012). Among these plant lineages, the small genus Flaveria (Yellowtops) has received particular attention because it includes species with CO2 assimilation modes ranging from C3 via a broad range of C3–C4 intermediate species to C4 (Powell, 1978; Apel and Maass, 1981; Ku et al., 1983; Bauwe, 1984). Notably, extant Flaveria C3–C4 intermediate species represent true evolutionary intermediates between C3 and C4 photosynthesis (Kopriva et al., 1996; McKown et al., 2005). Major physiological features of such plants are low apparent photorespiration (Apel and Maass, 1981; Holaday et al., 1982, 1984) in combination with an enhanced refixation of photorespiratory CO2 (Holbrook et al., 1985; Bauwe et al., 1987) and high glycine accumulation (Holaday and Chollet, 1983, 1984).
Mechanistically, corresponding to the distribution of the photorespiratory enzyme glycine decarboxylase (GDC) in leaves of C4 plants (Ohnishi and Kanai, 1983), these specific characteristics are closely related to a confinement of GDC activity to the leaf bundle sheath (Hylton et al., 1988; Moore et al., 1988). Based on these and other data, it was hypothesized that C3–C4 intermediate species reduce apparent photorespiration by an efficient refixation of photorespired CO2 in the bundle sheath (Monson et al., 1984; Edwards and Ku, 1987; Rawsthorne, 1992). This initial focus on the importance of CO2 refixation was later extended by the hypothesis that the confinement of glycine decarboxylase could result in a concentration of CO2 in the bundle sheath of C3–C4 intermediate plants (von Caemmerer, 1989; Monson and Rawsthorne, 2000). Today, such a mechanism, in which photorespiratory glycine serves as a vehicle to move ‘CO2’ from the mesophyll to the GDC-containing bundle sheath, is seen as a crucial step during the evolution of C4 photosynthesis (Bauwe, 2011; Sage et al., 2012). In other words, the multiple evolution of C4 photosynthesis might have been triggered by and possibly even required the preceding presence of a much simpler CO2 concentration system than the C4 cycle, based on relatively small alterations to the high-flux photorespiratory glycine metabolism.
This hypothesis is now widely accepted and the genetic alterations necessary to restrict photorespiratory GDC activity to the bundle sheath are being unravelled (Wiludda et al., 2012; Schulze et al., 2013). On the other hand, it is not known how efficient this photorespiratory CO2 pump could be. Here, 14CO2 incorporation studies designed to obtain an estimate of the in vivo rates of the two Rubisco-catalysed reactions in the C3–C4 species Flaveria pubescens relative to the control C3 species Flaveria cronquistii are reported. The ratio of these reactions, carboxylation versus oxygenation of RuBP, is co-determined by kinetic parameters of Rubisco and by the CO2/O2 concentration ratio (Laing et al., 1974; Peisker, 1974; Farquhar et al., 1980). Hence, a higher in vivo carboxylation/oxygenation ratio in F. pubescens relative to a control C3 species would not only indicate an elevated CO2/O2 concentration ratio but also allow quantifying the efficiency of the photorespiratory CO2 pump.
Materials and methods
Plant growth and 14C labelling
Flaveria cronquistii A.M. Powell (C3), Flaveria pubescens Rydberg (C3–C4), and Flaveria trinervia (Spreng.) C. Mohr (C4) were grown in soil in a controlled environment chamber at 28/22 °C (day/night) and 250–300 µmol photons m–2 s–1 at a photoperiod of 16h. Fully expanded leaves excised from 40–60-d-old plants were fixed by thin wires in a frame positioned in a purpose-built fast-acting 14CO2 labelling device (Pärnik et al., 1987). Leaves were pre-illuminated at 30 Pa 12CO2 and 210 kPa O2 for 10–15min at about 1200 µmol photons m–2 s–1 and 25 °C to ensure maximum stomata opening and achievement of the steady-state rate of photosynthesis. Plants were then exposed to 14CO2 (2000 MBq mmol–1) for 0.6, 1.2, 2.4, 5, 15, 60, 120, and 360 s at the same concentrations of CO2 and O2, temperature and light as applied during pre-illumination. At the given time points, within 0.1 s, the leaf samples were automatically transferred into boiling 80% ethanol. 14CO2 incorporation was linear over the whole experiment. All experiments were performed in triplicate (three individual plants in three consecutive days, resulting in three leaf samples per time-point for each species).
Metabolite analysis
All leaf samples were individually extracted as described before (Värk et al., 1968) with slight modifications. After 2min in boiling 80% ethanol, the samples were extracted for 15min at 86 °C with 5ml of 80% ethanol (twice) and 20% ethanol (once). All four ethanolic fractions were combined. The remaining samples were then further extracted for 15min at 86 °C with 5ml 96% ethanol acidified with 3 drops of 3 N HCl. The two extracts were separately (to avoid the hydrolysis of disaccharides) dried at 37 °C, individually re-dissolved in 5ml H2O each and cleared by centrifugation. The supernatants were combined, dried as above, and the metabolites re-dissolved in 1ml H2O. This final extract was used to determine total extractable radioactivity, radioactivity in amino acids (AAA 339 analyzer, Mikrotechna, Czech Republic), and other metabolites by using two-dimensional paper chromatography. Residual radioactivity in the fully extracted, dried, and triturated leaf samples was determined by using a non-aqueous scintillation cocktail. These analytical methods including the protocol used for starch analysis were described in more detail elsewhere (Keerberg et al., 2011).
Photosynthetic–photorespiratory gas exchange
Rates of net and true photosynthesis, photorespiratory CO2 evolution from the leaf, intracellular decarboxylation of early photosynthates, and rates of reassimilation of photorespiratory CO2 were determined during steady-state photosynthesis by using standard gas-exchange measurement techniques in combination with a radiogasometric method described before (Pärnik and Keerberg, 1995, 2007). In short, this method is based on the analysis of time curves of 14CO2 evolution from labelled photosynthates in leaves previously exposed to 14CO2. Photorespiration (210 kPa O2) and day respiration (15 kPa O2) were distinguished by measurement under different O2 concentrations. Re-fixation ratios (D) of photorespiratory CO2 were calculated from 14CO2 evolution at the very high concentration of 3 kPa 12CO2, where re-fixation of 14CO2 evolved inside the cell is close to zero, relative to 14CO2 evolution at air levels of 12CO2.
Modelling and data analysis
From the radioactivity values for individual metabolites in combination with the specific radioactivity of the 14CO2 fed to leaves, the amounts of carbon incorporated at the selected time points were calculated and plotted against the duration of feeding with 14CO2. The amounts of carbon fixed in individual compounds were expressed in absolute (µmol C m–2) and relative (per cent of total carbon fixed) units. These experimental labelling curves contain the information about rates of all relevant carbon fluxes and corresponding metabolite pool sizes.
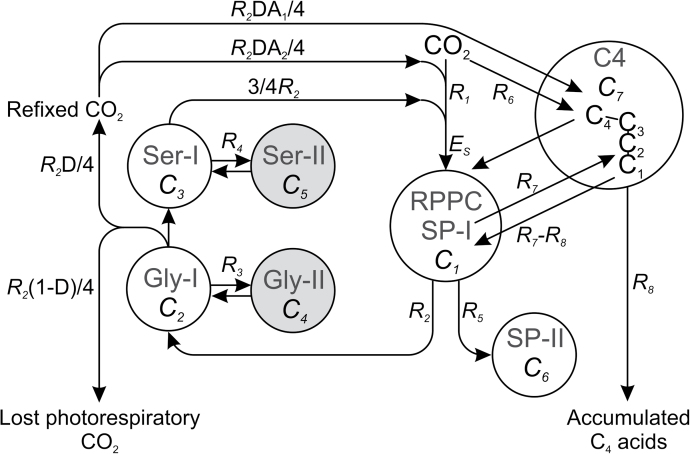
To extract this information on Flaveria photosynthetic–photorespiratory metabolism, the model shown in Fig. 1 was used. The model allows CO2 incorporation into the reductive pentose phosphate cycle (RPPC) either directly with rate R 1 or via the C4 cycle with rate R 6. Total carbon flux through the photorespiratory cycle is denoted R 2. R5 is the export rate of phosphorylated sugars into other pathways, for example, sucrose biosynthesis. R 7 denotes the rate of carbon efflux from the RPPC to the C3 skeleton of C4 acids, while R 8 describes the rate of accumulation of C4-acids. In order to simplify calculations, metabolites were grouped into four pools: (i) pool ‘SP’ with sugar phosphates plus 3-phosphoglycerate, (ii) pool ‘Gly’ with metabolites of the two-carbon branch of the photorespiratory cycle, (iii) pool ‘Ser’ with metabolites of the three-carbon branch of the photorespiratory cycle, and (iv) pool ‘C 4’ with malate and aspartate. Each of these four pools comprises two metabolic sub-pools with different labelling kinetics, for example, photorespiratory pools with rapid turnover in peroxisomes and mitochondria (Gly-I and Ser-I with pools C 2 and C 3, respectively) or less mobility in the cytosol and chloroplasts (Gly-II and Ser-II with pools C 4 and C 5, respectively). At steady-state photosynthesis, these pools are in diffusional equilibrium with exchange rates R 3 and R 4, respectively. At the glycine-into-serine conversion step, one molecule of CO2 is released per serine molecule formed, corresponding to a glycine decarboxylation rate of R 2/4. The resulting CO2 is re-fixed in the RPPC or the C4 cycle or escapes from the leaf. The extent of re-fixation is described by the re-fixation coefficient D, which was experimentally determined as described above.
Fig. 1.
Model of major photosynthetic-photorespiratory carbon fluxes in Flaveria including the reductive pentose phosphate cycle (RPPC) with the attached photorespiratory pathway and the C4 photosynthetic pathway. R 1, rate of CO2 fixation in RPPC; R 2, rate of carbon flux through the glycolate cycle; R 3, rate of carbon exchange between different pools of glycine; R 4, rate of carbon exchange between different pools of serine; R 5, rate of transport of sugar phosphates out of the RPPC; R 6, rate of CO2 fixation by the C4 pathway; R 7, rate of carbon flux from RPPC into ‘C3 skeletons’ of C4 acids, R 8, rate of accumulation of C4 acids; C 1, total pool of sugar phosphates in the RPPC; C 2, active pool of the glycine branch of the photorespiratory pathway; C 3, active pool of the serine branch of the photorespiratory pathway; C 4 and C 5, corresponding non-photorespiratory metabolite pools; C 6, extra-cyclic pool of sugar phosphates; C 7, total pool of C4 acids. D (reassimilation coefficient) describes the fraction of refixed relative to total photorespiratory CO2. A 1 and A 2 are the partition coefficients describing the relative contributions of the RPPC and the C4 pathway to refixation of photorespiratory CO2. Note that Gly-I and Ser-I also include all other metabolites from the respective branches of the photorespiratory pathway. Gly-II and Ser-II represent less mobile (cytosolic, plastidial, vacuolar) pools of these metabolites.
Formally, the metabolic model is described by the four analytical functions shown as equations 1–4, one for each major metabolite pool (similar to Keerberg et al., 2011). To determine individual pool sizes C i and carbon fluxes R i, the experimental values of the radioactivity of sugar phosphates, metabolites of the glycine and serine branches of the photorespiratory pathway, and of C4-acids were simultaneously fitted to these functions by multi-component non-linear regression analysis. These functions also consider the time-dependent dilution of the applied tracer CO2 by unlabelled photorespiratory CO2, which is important particularly at the start of tracer feeding under steady-state photosynthesis. A more detailed explanation of these functions is provided in the Supplementary data at JXB online.
Results and discussion
The analysis of in vivo Rubisco carboxylation and oxygenation rates is not trivial. Potentially, such data can be extracted from gas exchange experiments (Pärnik and Keerberg, 1995), but this approach is biased by limited knowledge of the internal diffusion pathways for CO2 and O2. Bias becomes even stronger at a varying intercellular distribution of photosynthetic tasks, such as the operation of CO2-concentrating mechanisms. Assuming that there is no large variation in the plastidial O2 concentrations (Tolbert et al., 1995), it should be possible approximately to assess the efficiency of the photorespiratory CO2 pump in C3–C4 intermediate plants by the quantification of carbon fluxes through the individual routes of the photosynthetic–photorespiratory biochemical network. Speed and complexity of the biochemical processes involved require fast and, consequently, sensitive labelling techniques using 14CO2 as a tracer in combination with model-based data analysis.
For our study, three Flaveria species were used, F. cronquistii (C3), F. pubescens (C3–C4 intermediate), and F. trinervia (C4). These species have previously been examined for their photosynthetic types (Apel and Maass, 1981; Ku et al., 1983; Rumpho et al., 1984), kinetic properties of Rubisco (Bauwe, 1984; Wessinger et al., 1989; Kubien et al., 2008), and phylogenetic position within the genus (Powell, 1978; Kopriva et al., 1996; McKown et al., 2005). These studies include the observation (Bassüner et al., 1984; Monson et al., 1986) that C3–C4 intermediate Flaveria species fix a small fraction of CO2 via the C4 pathway (R 6 in the model shown in Fig. 1) while most of the CO2 enters metabolism directly via the RPPC (R 1 in Fig. 1). It was not our intention to perform a comprehensive re-analysis of photosynthetic–photorespiratory carbon metabolism of these species. Instead, we wanted to focus on the quantification of key fluxes including control data confirming adequate fidelity of our approach.
Building upon previous studies (Pärnik et al., 1987; Keerberg et al., 2011), the model schematically shown in Fig. 1 was developed which embraces, in a generalized form, all the relevant information that is necessary to determine Rubisco carboxylation/oxygenation ratios in vivo. It considers time- and flux-dependent changes in the tracer’s specific radioactivity at all nodes of the network and allows the separation of high- and low-turnover pools of key metabolites of photosynthetic CO2 and photorespiratory O2 fixation. In order to simplify the model and make it as robust as possible, the metabolically related metabolites of the four major pathways were combined into four pools, each of which is described by a labelling function P(t,C i,R i) shown as equations 1–4.
| (1) |
| (2) |
| (3) |
| (4) |
Essentially, these four functions describe the time dependence of the radioactivity P incorporated under steady-state conditions into each of the four major model components sugar phosphates plus 3-phosphoglycerate [equation (1); SP-I plus SP-II], the glycine branch [equation (2); Gly-I plus Gly-II] and the serine branch [equation (3); Ser-I plus Ser-II] of the photorespiratory pathway, and the C4 pathway [equation (4); C 4]. S S and S C are time-dependent functions that describe changes in the specific radioactivity of CO2 fixed in the RPPC and the C4 pathways, respectively. Functions P(SP), P(Gly), P(Ser), and P(C 4) were simultaneously fitted to experimental data points collected over a time scale from 0.6 to 360 s during steady-state photosynthesis. Quantitative values for carbon fluxes Ri between the sub-pools directly involved in photosynthetic CO2 fixation and photorespiration, for example, from SP-I (pool size C 1) via Gly-I (pool size C 2) to Ser-I (pool size C 3), were calculated by multi-component non-linear regression analysis.
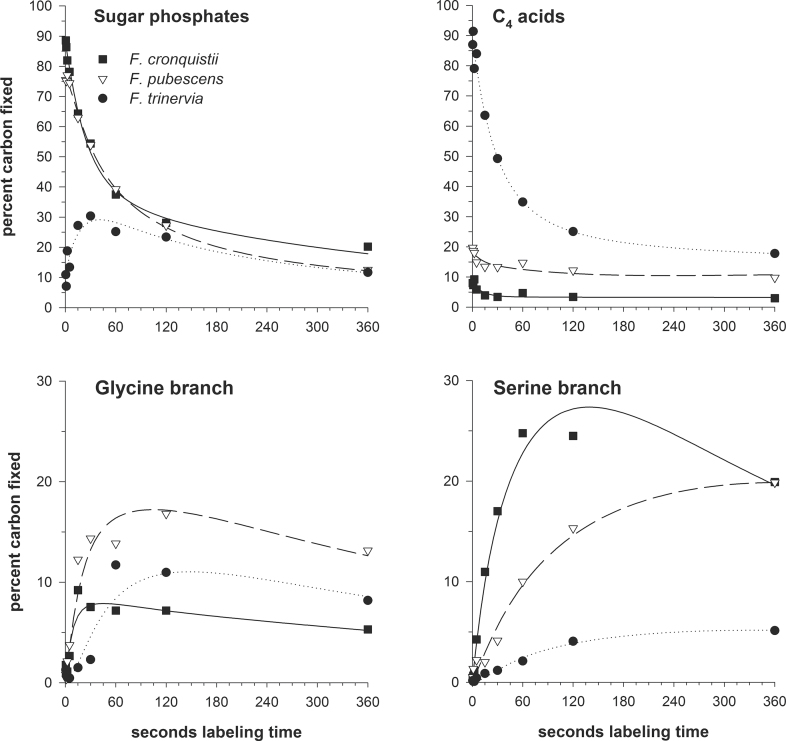
Figure 2 demonstrates that the model approximations for all four major metabolite pools represented by the model fit very well to the experimental data points. This includes initial CO2 fixation by the C4 pathway in F. trinervia in combination with final refixation of CO2 released from C4 acids by the RPPC as well as the ‘glycine anomaly’ of the C3–C4 intermediate plant F. pubescens. As mentioned in the Introduction, the specific alterations to glycine metabolism of C3–C4 intermediate plants are due to a specific distribution of photorespiratory GDC activity (Rawsthorne, 1992), which represents the enzymatic backbone of the photorespiratory CO2 pump.
Fig. 2.
Time-courses of CO2 incorporation into sugar phosphates, C4 acids, and intermediates of the two branches of the photorespiratory pathway. Shown are time-courses relative to true photosynthesis, which was set to 100% for easier comparison. Symbols represent mean values from three data points (biological replicates). Solid (F. cronquistii), dashed (F. pubescens), and dotted lines (F. trinervia) are best fits to the labelling functions (Equations 1–4) and were calculated by multi-component non-linear regression analysis.
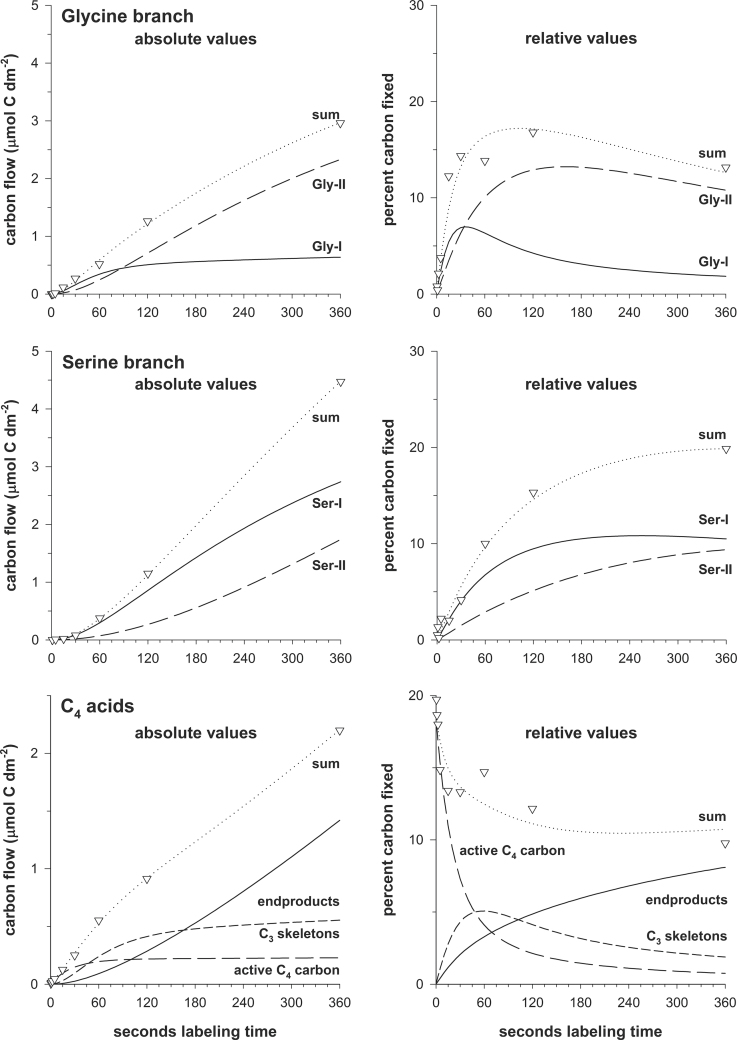
Another apparent feature is the overlap of primary and secondary labelling kinetics, which is best seen with the C4 acids but also within the glycine and serine branches of the photorespiratory pathway (Keerberg et al., 2011). In the case of the C4 acids, the complex labelling kinetics results from direct CO2 fixation (R 6 in Fig. 1), secondary labelling of carbons 1–3 by the synthesis of phosphoenolpyruvate from RPPC intermediates (via phosphoglycerate mutase and enolase; R 7), and export as a metabolically less mobile pool (probably to the vacuole; R 8). Also, two metabolic pools with different labelling kinetics exist in both branches of the photorespiratory pathway. This is because one fraction each (Gly-II and Ser-II with pools C 4 and C 5, respectively) is present in cellular compartments that do not directly contribute to photorespiratory reactions. These fractions show a lower turnover than the photorespiratory most active pools (Gly-I and Ser-I with pools C 2 and C 3, respectively). At steady-state photosynthesis, the pools equilibrate pairwise with exchange rates R 3 and R 4. To consider such effects, and specifically calculate fluxes between metabolite pools directly involved in CO2 fixation and photorespiration, the model allows overlapping pools with different labelling kinetics to be separated by component analysis. Figure 3 provides examples of how the sequestration of metabolites into different pools was quantified and how the separation of primary and secondary labelling was achieved in the case of F. pubescens. The example data display carbon incorporation into high- (Gly-I and Ser-I) and low-turnover (Gly-II and Ser-II) pools within the glycine and serine branches of the photorespiratory pathway. They also demonstrate the quantitative separation of the ‘active’ C 4 carbon pool of C4 acids from label appearing in carbon atoms 1–3 and in C4 acids exported to the vacuole. Collectively, these data show that the chosen model is an adequate tool for the calculation of fluxes through the major routes of photosynthetic CO2 fixation from quantitative 14CO2 labelling data.
Fig. 3.
Examples for the model-based separation of fast- and slow-turnover pools in the ‘Gly’ and ‘Ser’ branches of the photorespiratory pathway and for primary versus secondary labelling and accumulation of C4 acids. All data are for F. pubescens.
The relevant fluxes are summarized in Table 1 and complemented by results from radiogasometric measurements performed in parallel with the same set of plants. These independent data show rates of true photosynthesis, total decarboxylation, and photorespiratory CO2 evolution. They allowed calculating the extent to which photorespiratory CO2 is re-fixed.
Table 1.
Carbon fluxes in photosynthetic–photorespiratory carbon metabolism of Flaveria speciesValues marked with an asterisk represent means ±SE from three measurements on different plants by using a radiogasometric method (Pärnik and Keerberg, 2007). All other values were calculated as means ±SE by multi-component non-linear regression analysis from the time-course of 14C-incorporation (simultaneous fit to equations 1–4; labelling data from three independent experiments).
| Carbon fluxes | F. cronquistii µmol m–2 s–1 | % P T | F. pubescens µmol m–2 s–1 | % P T | F. trinervia µmol m–2 s–1 | % P T | |
|---|---|---|---|---|---|---|---|
| P T* | True photosynthesis | 3.76±0.10 | 7.93±0.70 | 10.37±0.28 | |||
| R 1 | CO2 incorporation directly into RPPC | 3.82±0.49 | 101.6 | 6.23±0.07 | 78.6 | 0.45±0.25 | 4.3 |
| R 6 | CO2 incorporation directly into C4 acids | 0.32±0.01 | 8.5 | 1.29±0.32 | 16.3 | 9.42±0.10 | 90.8 |
| R 7 | Secondary labelling of C4 acids C1–C2–C3 | 0.43±0.07 | 11.4 | 1.71±0.03 | 21.6 | 1.76±0.21 | 17.0 |
| R 8 | C4 acid immobilization as end-products | 0.10±0.01 | 2.7 | 0.66±0.06 | 8.3 | 0.94±0.15 | 9.1 |
| R 1+R 6 | Total CO2 incorporation | 4.14±0.49 | 110.1 | 7.52±0.33 | 94.8 | 9.87±0.27 | 95.2 |
| *of which sucrose formation amounts to | 0.95±0.02 | 25.3 | 2.11±0.09 | 26.6 | 5.98±0.63 | 57.7 | |
| *of which starch formation amounts to | 0.86±0.02 | 22.9 | 1.58±0.13 | 19.9 | 1.80±0.12 | 17.4 | |
| *of which insoluble material amounts to | 0.55±0.02 | 14.6 | 0.75±0.02 | 9.5 | 1.62±0.30 | 15.6 | |
| R 2 | C flow through photorespiratory pathway | 6.64±0.25 | 176.6 | 3.66±0.20 | 46.2 | 2.56±0.21 | 24.7 |
| R 2/4 | Decarboxylation of glycine | 1.66±0.06 | 44.1 | 0.92±0.05 | 11.6 | 0.64±0.05 | 6.2 |
| DEC* | Photorespiratory and C4 decarboxylation | 2.18±0.08 | 58.0 | 1.84±0.05 | 23.2 | 6.70±0.21 | 64.6 |
| R P* | Photorespiratory CO2 evolution | 1.35±0.05 | 35.9 | 0.16±0.02 | 2.0 | 0.03±0.02 | 0.3 |
| D* | Reassimilation in % of DEC | 38.1 | 91.3 | 99.5 | |||
| R 2/2 | Oxygenation | 3.3 | 1.8 | 1.3 | |||
| R 1+R 6+D*R 2/4 | Carboxylation | 4.8 | 8.3 | 10.5 | |||
| Mean relative CO2 at Rubisco sites | 1.0 | 3.2 | 5.7 |
CO2 can become incorporated into the RPPC either directly with rate R 1 or indirectly via the C4 pathway with rate R 6. The sums R 1+R 6 then represent total CO2 incorporation from external sources and show an increasing contribution by the C4 cycle, very low in F. cronquistii, low in F. pubescens, and, as expected, very high in F. trinervia. These total influx rates correspond reasonably well to directly measured rates for true photosynthesis P T, which provides a strong argument for the soundness of all other flux calculations. Higher values for P T (C3<C3–C4<C4) go together with increased rates of sucrose formation (R 5; directly measured in Table 1) and C4 acid accumulation as end-products (R 8). Moreover, the photosynthetically active pools of C4 acids (C 7; not listed in Table 1) increased from 13±1 (C3) via 57±19 (C3–C4) to 161±39 µmol C m–2 (C4). It is important to note that the increase of C4 cycle activity from F. cronquistii to F. pubescens (5.8% to 8.3% of P T, calculated as R6–R8) is only very small in comparison with the activity of the C4 cycle in F. trinervia (81.7% of P T). This suggests that CO2 accumulation occurs mainly by glycine-shuttling and less by C4 cycle activity in the bundle sheath of F. pubescens.
Carbon flux through the glycolate cycle, R 2, is stoichiometrically related to the rate of RuBP oxygenation, R 2/2. As a result of the operation of CO2-concentrating mechanisms in F. pubescens and in F. trinervia, photorespiration-related fluxes become distinctly lower from C3 towards C4 metabolism. To determine the true rates of RuBP carboxylation, in addition to the sum of R 1 and R 6, it was necessary to consider the refixation of CO2 generated from internal sources. In C3 and C3–C4 plants, photorespiration is the dominating internal source of CO2 during photosynthesis. R 2 is stoichiometrically related to photorespiratory glycine decarboxylation as R 2/4, because one molecule of CO2 is released per one molecule of serine formed from two glycine molecules. The extent to which refixation occurs must be separately determined. This was done by radiogasometric measurements (Pärnik and Keerberg, 1995, 2007), which allowed direct quantification of the sum DEC of photorespiratory glycine decarboxylation plus C4 acid decarboxylation plus minor CO2 releasing processes. It is reasonable to assume that all fractions of internally generated CO2 are re-assimilated with the same efficiency. In combination with the rate R P of CO2 losses from the leaf (simplifying referred to as photorespiratory CO2 evolution), this assumption allows assessing the partitioning D between re-fixation and loss of CO2 from the leaf. The calculated total rates with which Rubisco fixes CO2 arriving by diffusion from the stomata (R 1), from decarboxylation in the C4 cycle (R 6), and from photorespiration (D*R 2/4) were related to RuBP oxygenation rates (R 2/2). The comparison shows that the resulting in vivo carboxylation-to-oxygenation ratio of Rubisco is more than three times higher in F. pubescens relative to F. cronquistii under the same experimental conditions.
Rubisco from C4 Flaveria species has a somewhat lower affinity to CO2, but it is also known that Rubisco from C3 and C3–C4 Flaveria species show more or less identical kinetics (Bauwe, 1984;Wessinger et al., 1989; Kubien et al., 2008). Since the oxygen compensation point of C3 plants is only slightly above air levels (Tolbert et al., 1995), plastidial oxygen concentrations are probably close to air oxygen concentrations in F. cronquistii and F. pubescens but presumably also in the C4 species F. trinervia. Therefore, in a comparison of these species, measurement of in vivo carboxylation-to-oxygenation ratios allows the calculation of the relative CO2 concentration in chloroplasts. Considering the reported K m values of Rubisco for CO2, which are even somewhat higher than steady-state internal CO2 levels, our data suggest that the photorespiratory CO2 pump elevates the mean intraplastidial CO2 concentration during steady-state photosynthesis about 3-fold in leaves of the C3–C4 intermediate species F. pubescens relative to the C3 species F. cronquistii. This is considered to be a sound estimate because small contributions from C4 photosynthesis are balanced by the operation of a significant fraction of Rubisco at non-elevated CO2 levels in the mesophyll of F. pubescens.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary data. An explanation of the labelling functions of the model shown in Fig. 1 used for the quantitative analysis of the labelling kinetics.
Acknowledgements
We wish to acknowledge help during the experiments by Hille Keerberg. This work was supported by the Akademie der Wissenschaften der DDR, the Estonian Science Foundation (grants 4173 and 5989), the Estonian Ministry of Education and Research (IUT-8-3), the EU’s 7th Framework Programme (KBBE-2011-289582), the European Regional Fund (Center of Excellence in Environmental Adaptation), and by the Deutsche Forschungsgemeinschaft (FOR 1186).
Glossary
Abbreviations:
- CAM
crassulacean acid metabolism
- GDC
glycine decarboxylase
- RPPC
reductive pentose phosphate cycle (Calvin–Benson cycle)
- RubP
ribulose 1,5-bisphosphate.
References
- Apel P, Maass I. 1981. Photosynthesis in species of Flaveria. CO2 compensation concentration, O2 influence on photosynthetic gas exchange and δ13C values in species of Flaveria (Asteraceae). Biochemie und Physiologie der Pflanzen 176, 396–399 [Google Scholar]
- Bassüner B, Keerberg O, Bauwe H, Pärnik T, Keerberg H. 1984. Photosynthetic CO2 metabolism in C3–C4 intermediate and C4 species of Flaveria (Asteraceae). Biochemie und Physiologie der Pflanzen 179, 631–634 [Google Scholar]
- Bauwe H. 1984. Photosynthetic enzyme activities and immunofluorescence studies on the localization of ribulose-1,5-bisphosphate carboxylase/oxygenase in leaves of C3, C4, and C3–C4 intermediate species of Flaveria (Asteraceae). Biochemie und Physiologie der Pflanzen 179, 253–268 [Google Scholar]
- Bauwe H. 2011. Photorespiration: the bridge to C4 photosynthesis. In: Raghavendra AS, Sage R, eds. C4 photosynthesis and related CO2 concentrating mechanisms, Vol. 32 New York: Springer Science+Business Media BV, 81–108 [Google Scholar]
- Bauwe H, Keerberg O, Bassüner R, Pärnik T, Bassüner B. 1987. Reassimilation of carbon dioxide by Flaveria (Asteraceae) species representing different types of photosynthesis. Planta 172, 214–218 [DOI] [PubMed] [Google Scholar]
- Edwards GE, Ku MSB. 1987. Biochemistry of C3–C4 intermediates. In: Hatch MD, Boardman NK, eds. The biochemistry of plants, Vol. 10 London: Academic Press, 275–325 [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90 [DOI] [PubMed] [Google Scholar]
- Hatch MD, Slack CR. 1970. Photosynthetic CO2 fixation pathways. Annual Review of Plant Physiology 21, 141–162 [Google Scholar]
- Holaday AS, Chollet R. 1983. Photosynthetic/photorespiratory carbon metabolism in the C3–C4 intermediate species, Moricandia arvensis and Panicum milioides . Plant Physiology 73, 740–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaday AS, Chollet R. 1984. Photosynthetic/photorespiratory characteristics of C3–C4 intermediate species. Photosynthesis Research 5, 307–323 [DOI] [PubMed] [Google Scholar]
- Holaday AS, Harrison AT, Chollet R. 1982. Photosynthetic/photorespiratory CO2 exchange characteristics of the C3–C4 intermediate species, Moricandia arvensis . Plant Science Letters 27, 181–189 [Google Scholar]
- Holaday AS, Lee KW, Chollet R. 1984. C3–C4 intermediate species in the genus Flaveria: leaf anatomy, ultrastructure, and the effect of O2 on the CO2 compensation concentration. Planta 160, 25–32 [DOI] [PubMed] [Google Scholar]
- Holbrook GP, Jordan DB, Chollet R. 1985. Reduced apparent photorespiration by the C3–C4 intermediate species, Moricandia arvensis and Panicum milioides . Plant Physiology 77, 578–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylton CM, Rawsthorne S, Smith AM, Jones DA, Woolhouse HW. 1988. Glycine decarboxylase is confined to the bundle-sheath cells of leaves of C3–C4 intermediate species. Planta 175, 452–459 [DOI] [PubMed] [Google Scholar]
- Keerberg O, Ivanova H, Keerberg H, Pärnik T, Talts P, Gardeström P. 2011. Quantitative analysis of photosynthetic carbon metabolism in protoplasts and intact leaves of barley. Determination of carbon fluxes and pool sizes of metabolites in different cellular compartments. BioSystems 103, 291–301 [DOI] [PubMed] [Google Scholar]
- Kopriva S, Chu CC, Bauwe H. 1996. Molecular phylogeny of Flaveria as deduced from the analysis of nucleotide sequences encoding H-protein of the glycine cleavage system. Plant, Cell and Environment 19, 1028–1036 [Google Scholar]
- Ku MSB, Monson RK, Littlejohn RO, Nakamoto H, Fisher DB, Edwards GE. 1983. Photosynthetic characteristics of C3–C4 intermediate Flaveria species. I. Leaf anatomy, photosynthetic responses to O2 and CO2, and activities of key enzymes in the C3 and C4 pathways. Plant Physiology 71, 944–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubien DS, Whitney SM, Moore PV, Jesson LK. 2008. The biochemistry of Rubisco in Flaveria . Journal of Experimental Botany 59, 1767–1777 [DOI] [PubMed] [Google Scholar]
- Laing WA, Ogren WL, Hageman RH. 1974. Regulation of soybean net photosynthetic CO2 fixation by the interaction of CO2, O2, and ribulose 1,5-diphosphate carboxylase. Plant Physiology 54, 678–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttge U. 2004. Ecophysiology of Crassulacean Acid Metabolism (CAM). Annals of Botany 93, 629–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKown AD, Moncalvo JM, Dengler NG. 2005. Phylogeny of Flaveria (Asteraceae) and inference of C4 photosynthesis evolution. American Journal of Botany 92, 1911–1928 [DOI] [PubMed] [Google Scholar]
- Monson RK, Edwards GE, Ku MSB. 1984. C3–C4 intermediate photosynthesis in plants. BioScience 34, 563–574 [Google Scholar]
- Monson RK, Moore BD, Ku MSB, Edwards GE. 1986. Co-function of C3- and C4-photosynthetic pathways in C3, C4 and C3–C4 intermediate Flaveria species. Planta 168, 493–502 [DOI] [PubMed] [Google Scholar]
- Monson RK, Rawsthorne S. 2000. C3–C4 intermediate photosynthesis. In: Leegood RC, Sharkey TD, von Caemmerer S, eds. Photosynthesis, physiology and metabolism, Vol. 9 Dordrecht: Kluwer Academic Publishers, 553–550 [Google Scholar]
- Moore BD, Monson RK, Ku MSB, Edwards GE. 1988. Activities of principal photosynthetic and photorespiratory enzymes in leaf mesophyll and bundle sheath protoplasts from the C3–C4 intermediate Flaveria ramosissima . Plant and Cell Physiology 29, 999–1006 [Google Scholar]
- Ohnishi J, Kanai R. 1983. Differentiation of photorespiratory activity between mesophyll and bundle sheath cells of C4 plants. I. Glycine oxidation by mitochondria. Plant and Cell Physiology 24, 1411–1420 [Google Scholar]
- Pärnik T, Keerberg O. 1995. Decarboxylation of primary and end-products of photosynthesis at different oxygen concentrations. Journal of Experimental Botany 46, 1439–1447 [Google Scholar]
- Pärnik T, Keerberg O. 2007. Advanced radiogasometric method for the determination of the rates of photorespiratory and respiratory decarboxylations of primary and stored photosynthates under steady-state photosynthesis. Physiologia Plantarum 129, 34–44 [Google Scholar]
- Pärnik T, Keerberg OF, Yurisma EY. 1987. Fast-acting exposure chamber for studying photosynthesis with C-14 CO2 . Soviet Plant Physiology 34, 676–683 [Google Scholar]
- Peisker M. 1974. A model describing the influence of oxygen on photosynthetic carboxylation. Photosynthetica 8, 47–50 [Google Scholar]
- Powell AM. 1978. Systematics of Flaveria (Flaveriinae-Asteraceae). Annals of the Missouri Botanical Garden 65, 590–636 [Google Scholar]
- Rawsthorne S. 1992. C3–C4 intermediate photosynthesis: linking physiology to gene expression. The Plant Journal 2, 267–274 [Google Scholar]
- Rumpho ME, Ku MSB, Cheng SH, Edwards GE. 1984. Photosynthetic characteristics of C3–C4 intermediate Flaveria species. III. Reduction of photorespiration by a limited C4 pathway of photosynthesis. Plant Physiology 75, 993–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF. 2004. The evolution of C4 photosynthesis. New Phytologist 161, 341–370 [DOI] [PubMed] [Google Scholar]
- Sage RF, Sage TL, Kocacinar F. 2012. Photorespiration and the evolution of C4 photosynthesis. Annual Review of Plant Biology 63, 19–47 [DOI] [PubMed] [Google Scholar]
- Schulze S, Mallmann J, Burscheidt J, Koczor M, Streubel M, Bauwe H, Gowik U, Westhoff P. 2013. Evolution of C4 photosynthesis in the genus Flaveria: establishment of a photorespiratory CO2 pump. The Plant Cell 25, 2522–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert NE, Benker C, Beck E. 1995. The oxygen and carbon dioxide compensation points of C3 plants - possible role in regulating atmospheric oxygen. Proceedings of the National Academy of Sciences, USA 92, 11230–11233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Värk E, Keerber H, Keerberg O, Pärnik T. 1968. On the extraction of the products of photosynthesis by ethanol of different concentrations (in Russian). Proceedings of the Estonian Academy of Sciences (Biology) 17, 367–373 [Google Scholar]
- von Caemmerer S. 1989. A model of photosynthetic CO2 assimilation and carbon-isotope discrimination in leaves of certain C3–C4 intermediates. Planta 178, 463–474 [DOI] [PubMed] [Google Scholar]
- Wessinger ME, Edwards GE, Ku MSB. 1989. Quantity and kinetic properties of ribulose 1,5-bisphosphate carboxylase in C3, C4, and C3–C4 intermediate species of Flaveria (Asteraceae). Plant and Cell Physiology 30, 665–671 [Google Scholar]
- Wiludda C, Schulze S, Gowik U, Engelmann S, Koczor M, Streubel M, Bauwe H, Westhoff P. 2012. Regulation of the photorespiratory GLDPA gene in C4 Flaveria: an intricate interplay of transcriptional and posttranscriptional processes. The Plant Cell 24, 137–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.