Abstract
Previous studies of blood pressure and mortality in haemodialysis have yielded mixed results, perhaps due to confounding by comorbid conditions. We hypothesized that after improved accounting for confounding factors, higher systolic blood pressure (SBP) would be associated with higher all-cause mortality. We conducted a secondary analysis of data from the haemodialysis study, a randomized trial in prevalent haemodialysis patients. We used three proportional hazard models to determine the relative hazard at different levels of SBP: (1) Model-BL used baseline SBP; (2) Model-TV used SBP as a time-varying variable; and (3) Model-TV-Lag added a 3-month lag to Model-TV to de-emphasize changes in SBP associated with acute illness. In all the models, pre-dialysis SBP o120 mm Hg was associated with a higher risk of mortality compared with the referent group (140–159 mm Hg); higher pre-dialysis SBP was not associated with higher risk of mortality. In conclusion, we observed a robust association between lower pre-dialysis SBP and higher risk for all-cause and cardiovascular mortality in a well-characterized cohort of prevalent haemodialysis patients. Randomized clinical trials are needed to define optimal blood pressure targets in the haemodialysis population.
Keywords: blood pressure, dialysis, mortality, cardiovascular, survival
Introduction
The prevalence of hypertension in patients with end-stage renal disease on dialysis ranges from 50–90% as defined by blood pressure (BP) 4140/90 mm Hg,1 and cardiovascular (CV) disease is the most common cause of death.2 In the general population, large observational studies have shown direct, linear relationship among BP and the risks of CV disease and death.3–5 In contrast, studies in dialysis populations have yielded mixed results, with some reports of a higher mortality risk associated with higher systolic BP (SBP),6,7 others of a higher mortality risk with lower SBP8–10 or a ‘U’-shaped association, with higher mortality risk at both extremes of SBP.11 Disparate results may be attributed in part to differences in dialysis modality and adequacy, misclassification of hypertension or confounding by comorbid conditions. Previous studies included incident and prevalent patients or patients on peritoneal dialysis and haemodialysis. Cohort studies completed in the late 1980s and early 1990s often had a mean dialysis dose below current standards, whereas others lacked information on CV disease and other important comorbid conditions. In addition, many previous studies determined cause-specific mortality from the Centers for Medicare and Medicaid Services Death Notification form, which is particularly inaccurate in classification of CV death.12
We hypothesized that after improved accounting for confounding factors, higher SBP would be associated with a higher risk of all-cause and CV mortality in patients on haemodialysis. To test this hypothesis, we carried out a secondary analysis of data from the Haemodialysis (HEMO) Study,13 a randomized clinical trial that tested the effects of higher versus conventional dialysis dose and higher versus lower membrane flux on mortality and morbidity. The HEMO study subjects were relatively uniform, as only prevalent patients on haemodialysis with minimal residual kidney function were included. The majority of study subjects achieved an adequate dialysis dose, and classification of cause-specific mortality was adjudicated by a committee after a review of the medical records. In addition, the HEMO study data included multiple assessments of serum albumin concentration and comorbid conditions. These features of the HEMO study allowed us to address some of the limitations of previous studies.
Materials and methods
Study population
Details of the HEMO study have been published previously.13,14 Briefly, the HEMO study was a randomized clinical trial of prevalent haemodialysis patients between 18 and 80 years of age from 15 US centres. Subjects were enrolled between March 1995 and October 2000 and randomly assigned in a 2 2 factorial design to standard-dose or high-dose equilibrated Kt/Vurea and low-flux or high-flux dialyser membranes. Subjects were followed until death or December 2001 and censored at the time of kidney transplant. Subjects were excluded if they had serum albumin concentration ≤2.6 g per 100 ml, residual urea clearance of ≥1.5 ml min –1 per 35 l of urea distribution volume, or if they were unable to achieve an equilibrated Kt/Vurea of >1.30 within 4.5 h during two of three baseline kinetic modelling sessions.
SBP
SBP was measured before (pre-dialysis) and after (post-dialysis) the dialysis session in a seated position using a sphygmomanometer as per the dialysis unit routine. Pre-dialysis SBP (mm Hg) was categorized as follows: SBP: <120, 120–139, 140–159, 160–179 and ≥180 (values of SBP <40 were coded as missing); we considered SBP 140–159 mm Hg as the referent group. We also examined post-dialysis SBP and categorized in the same way. Baseline SBP values were defined as the mean of the first two measurements recorded from two separate dialysis sessions during the pre-randomization period.
Covariates
In multivariable models, we adjusted for age (10-year increments), sex, race (Black versus non-Black), vintage (years on dialysis), diabetes, mean baseline serum albumin concentration (averaged from two values obtained during the pre-randomization period) (g per 100 ml), residual kidney function (urea clearance in ml min –1 per 35 l), intervention group (high-dose or standard-dose dialysis and high-flux or low-flux dialyser membrane) and modified Index of Coexistent Diseases (ICED) score. Trained study coordinators assessed the ICED score at baseline and annually thereafter, which aggregates the presence and severity of congestive heart failure, ischaemic heart disease, peripheral vascular disease, cerebral vascular disease, 15 other medical conditions and 11 physical impairments. ICED scores range from 0–3, with higher scores indicating increasing severity of comorbid conditions.15 The HEMO study used a modified ICED score excluding diabetes, and accounted for this comorbid condition as a separate covariate.
We chose these covariates because they were pre-specified in the HEMO study and/or because significant differences existed among the categories of baseline pre-dialysis SBP. In Model-TV and Model-TV-Lag, we included serum albumin concentration and modified ICED score as time-varying covariates. Although we expected differences in antihypertensive medication use depending on baseline category of SBP, we did not include an adjustment for baseline antihypertensive medication use in our main analyses given the problem of indication bias inherent to observational studies. However, to check the robustness of our results, we conducted additional analyses that included an adjustment for baseline antihypertensive medication use.
Outcomes
The primary outcome of interest was time from randomization to all-cause mortality. Secondary outcomes included time to CV mortality and time to infection-related mortality, the two major categories of cause-specific mortality in patients on dialysis. The cause of death was adjudicated by an outcomes committee.
Statistical methods
We compared baseline clinical characteristics using general linear models or the Cochran–Armitage trend test for trend, as appropriate. We evaluated the association of SBP parameters with all-cause and cause-specific mortality using Cox regression in three sets of models. First, we analysed mean baseline SBP (Model-BL) as a time-constant variable. Second, we analysed SBP as a time-varying covariate (Model-TV), which incorporates all values of SBP. Third, we added a 3-month lag to Model-TV (Model-TV-Lag) to deemphasize changes in SBP associated with acute illness preceding death. Thus, Model-TV calculates the hazard ratio using the closest SBP recorded preceding the event, whereas Model-TV-Lag uses the SBP recorded approximately 3 months before the event. Analyses were stratified by clinical centre.
We tested the proportionality assumption with log-negative-log and Schoenfeld residual plots. As we divided SBP into five categories, we first assessed the overall association of SBP with the outcome before evaluating each SBP category separately. To do this, we conducted a Wald test and reported the result as a global P-value. We tested for effect modification by including multiplicative interaction terms with SBP for age, sex, race (Black versus non-Black), diabetes and baseline antihypertensive medication use. In these models, we expressed SBP as linear and quadratic terms given the curvilinear association with mortality in models without interaction terms. We considered two-tailed P-values <0.05 as statistically significant. All analyses were conducted with SAS Enterprise Guide 4.2 (Cary, NC, USA).
Results
A total of 1846 subjects participated in the HEMO study. Subjects with higher baseline pre-dialysis SBP were more likely to be female, Black, on antihypertensive medications, to have diabetes and of shorter dialysis vintage (Table 1). Median follow-up time was 2.5 years (interquartile range 1.3–4.3 years) after accounting for deaths and censoring at the time of kidney transplant. CV deaths accounted for 47% (N ¼ 408 out of 871) of total deaths.
Table 1.
Baseline characteristics of the HEMO study cohort
| Baseline characteristic | Entire cohort (N = 1846) | By category of baseline pre-dialysis SBP (mmHg) |
|||||
|---|---|---|---|---|---|---|---|
| <120 (N = 116) | 120-139 (N = 427) | 140-159 (N = 658) | 160-179 (N = 444) | ≥180 (N = 199) | P-trenda | ||
| Age, years, mean (s.d.) | 57.6 (14.0) | 58.6 (14.8) | 56.5 (15.1) | 57.7 (14.1) | 57.7 (13.6) | 59.1 (11.8) | 0.19 |
| Female sex (%) | 56.2 | 44.8 | 54.8 | 56.4 | 56.5 | 64.8 | < 0.01 |
| Black race (%) | 62.7 | 51.3 | 62.3 | 64.9 | 62.4 | 63.8 | 0.08 |
| On antihypertensive medication (%) | 79.1 | 55.9 | 67.5 | 82.8 | 86.3 | 89.5 | < 0.01 |
| Current smoker (%) | 17.3 | 15.5 | 19.2 | 16.4 | 16.7 | 19.1 | 0.49 |
| Cause of ESRD (%) | |||||||
| Diabetic nephropathy | 37.2 | 20.7 | 25.1 | 37.7 | 43.7 | 56.3 | |
| Hypertension | 31.7 | 33.6 | 37.7 | 32.1 | 27.9 | 25.6 | < 0.01 |
| Glomerular disease | 13.8 | 17.2 | 26.5 | 14.0 | 13.7 | 6.5 | |
| Other or unknown | 17.3 | 28.5 | 21.6 | 16.3 | 14.6 | 11.6 | |
| Diabetes (%) | 44.6 | 27.6 | 33.5 | 45.9 | 48.7 | 64.3 | < 0.01 |
| Modified ICED score (%) | |||||||
| 0–1 | 35.6 | 35.3 | 39.1 | 36.6 | 34.2 | 27.6 | |
| 2 | 31.3 | 29.3 | 28.3 | 32.4 | 34.2 | 28.6 | 0.03b |
| 3 | 33.2 | 35.3 | 32.6 | 31.0 | 31.5 | 43.7 | |
| BMI, mean (s.d.) | 25.5 (5.3) | 24.8 (5.4) | 25.3 (5.0) | 25.6 (5.3) | 25.4 (5.0) | 26.1 (5.9) | 0.08 |
| Vintage, years, median (IQR) | 2.2 (0.9–4.7) | 2.6 (1.4–6.4) | 2.3 (0.9–5.0) | 2.1 (0.9–4.6) | 2.2 (0.9–4.2) | 2.0 (1.0–4.0) | < 0.01 |
| Mean baseline serum albumin, g per 100ml, mean (s.d.) | 3.6 (0.4) | 3.6 (0.3) | 3.6 (0.4) | 3.6 (0.4) | 3.6 (0.4) | 3.6 (0.4) | 0.66 |
| Baseline serum total cholesterol, mg per 100ml, mean (s.d.) | 172.6 (40.7) | 164.8 (35.7) | 168.5 (40.3) | 176.3 (42.1) | 172.9 (40.3) | 173.1 (39.3) | 0.05 |
| Residual kidney function >0 (%) | 32.9 | 23.3 | 29.0 | 37.2 | 32.2 | 34.2 | 0.03 |
| Mean baseline spKt/V, mean (s.d.) | 1.6 (0.2) | 1.6 (0.2) | 1.6 (0.2) | 1.6 (0.2) | 1.6 (0.2) | 1.6 (0.2) | 0.24 |
Abbreviations: BMI, body mass index; ESRD, end-stage renal disease; HEMO study, haemodialysis study; ICED, index of coexistent diseases, modified to exclude diabetes; IQR, interquartile range; SBP, systolic blood pressure; spKt/V, single-pool Kt/Vure^_
P-value for trend across the categories of baseline SBP calculated using one-sided Cochran-Armitage trend test or linear regression as appropriate.
P-value is from χ2-test.
Pre-dialysis SBP and all-cause mortality
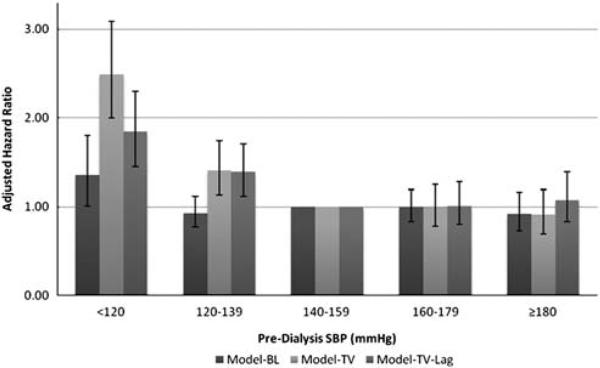
Globally, baseline pre-dialysis SBP was not significantly associated with all-cause mortality (P = 0.15) in an adjusted analysis. Pre-dialysis SBP evaluated as a time-varying covariate (Model-TV) was globally associated with all-cause mortality (P<0.0001). Specifically, pre-dialysis SBP <120 mm Hg and 120–139 mm Hg were associated with a significantly higher risk of all-cause mortality compared with the referent group of 140–159 mm Hg (Model-TV, Figure 1). After the addition of a 3-month lag, the relationship between lower pre-dialysis SBP and higher risk of all-cause mortality persisted (Table 2 and Figure 1). Higher pre-dialysis SBP was not associated with higher risk of all-cause mortality in any of the three models (Figure 1).
Figure 1.
Association of pre-dialysis SBP with adjusted risk of all-cause mortality, using three analytical models. All models adjusted for age, sex, Black race, serum albumin concentration, vintage, modified ICED score, diabetes, residual kidney function and randomized intervention group. Error bars indicate 95% confidence intervals. SBP, systolic blood pressure; Model-BL, baseline SBP as a time-constant variable; Model-TV, SBP as a time-varying variable; Model-TV-Lag, Model-TV + 3-month lag.
Table 2.
Multivariable-adjusted Model-TV-Lag analysis of all-cause mortality
| Predictor variable | Cox regression coefficient | Relative risk of all-cause mortality | P-value | |
|---|---|---|---|---|
| Pre-dialysis SBP (mmHg) | HR | 95% CI | ||
| <120 | 0.60±0.12 | 1.84 | 1.45–2.30 | <0.0001 |
| 120–139 | 0.33 ±0.11 | 1.39 | 1.12–1.70 | 0.003 |
| 140–159 | Ref. | 1.00 | Ref. | - |
| 160–179 | 0.01 ±0.12 | 1.01 | 0.80–1.28 | 0.93 |
| ≥180 | 0.07±0.28 | 1.07 | 0.83–1.39 | 0.59 |
| Age (per 10-year increment) | 0.31± 0.04 | 1.36 | 1.27–1.46 | < 0.0001 |
| Female sex | –0.11 ±0.08 | 0.89 | 0.76–1.05 | 0.16 |
| Black race | –0.35 ±0.09 | 0.70 | 0.59–0.85 | 0.0002 |
| Diabetes | 0.17± 0.08 | 1.19 | 1.01–1.40 | 0.04 |
| Dialysis vintage (per 1-year increment) | 0.02± 0.01 | 1.02 | 1.00–1.40 | 0.15 |
| Serum albumin (per 0.1 g per 100 ml increment) | –0.13± 0.01 | 0.88 | 0.86–0.89 | < 0.0001 |
| Modified ICED (per 1-unit increment) | 0.51± 0.06 | 1.66 | 1.48–1.86 | < 0.0001 |
| Residual kidney function (per 1mlmin-1 per 351) | –0.10± 0.09 | 0.90 | 0.76–1.08 | 0.27 |
| High-dose dialysis intervention group | –0.01± 0.08 | 0.99 | 0.85–1.15 | 0.88 |
| High-flux membrane intervention group | –0.11± 0.08 | 0.89 | 0.76–1.04 | 0.14 |
Abbreviations: CI, confidence interval; HR, hazard ratio; ICED, Index of Coexistent Diseases score, modified to exclude diabetes; Model-TV-Lag, SBP as time-varying variable+3-month lag; ref., referent group; SBP, systolic blood pressure. Plus-minus values are ± s.e.
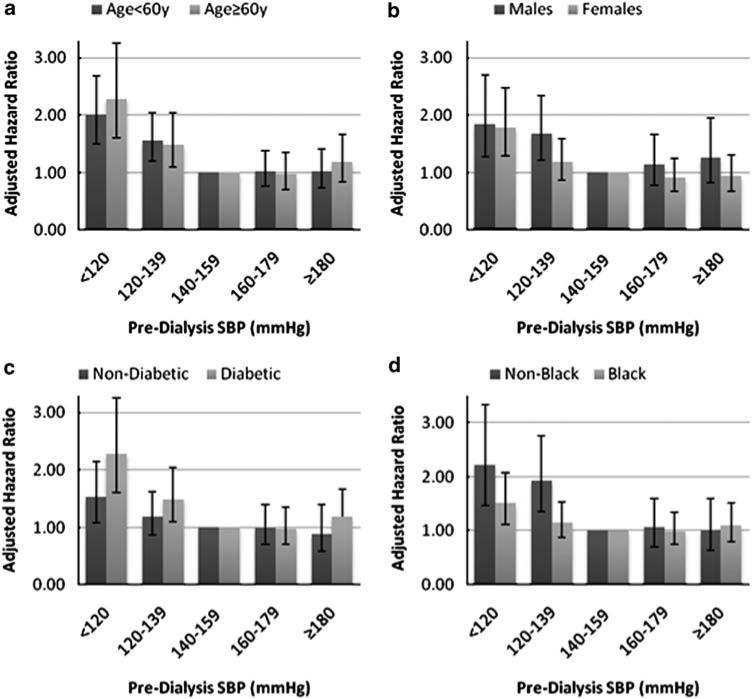
Other important independent predictors of all-cause mortality included age, diabetes, modified ICED score, serum albumin concentration and race (Table 2). However, none of these covariates was as strongly related to all-cause mortality as pre-dialysis SBP <120 mm Hg. Female sex, dialysis vintage and intervention group were not significantly associated with all-cause mortality. We conducted additional analyses that included an adjustment for baseline antihypertensive medication use in Model-TV and Model-TV-Lag, which did not materially change the results (data not shown). We found no evidence of effect modification by age, gender, diabetes or race (Black versus non-Black) on the relationship between pre-dialysis SBP and all-cause mortality (Figures 2a–d). In addition, there was no evidence of effect modification by antihypertensive medication use on the relationship between pre-dialysis SBP and all-cause mortality (P = 0.26).
Figure 2.
(a–d). Association of pre-dialysis SBP and adjusted all-cause mortality risk stratified by subgroup using Model-TV-Lag. All models adjusted for age, sex, Black race, serum albumin concentration, vintage, modified ICED score, diabetes, residual kidney function and randomized intervention group, unless stratified by that variable. Error bars indicate 95% confidence intervals. SBP, systolic blood pressure; Model-TV-Lag, SBP as time-varying variable + 3-month lag. P-values for interaction of SBP2 with (a) age = 0.31 (b) sex = 0.99 (c) diabetes = 0.40 (d) Black race = 0.34.
Pre-dialysis SBP and cause-specific mortality
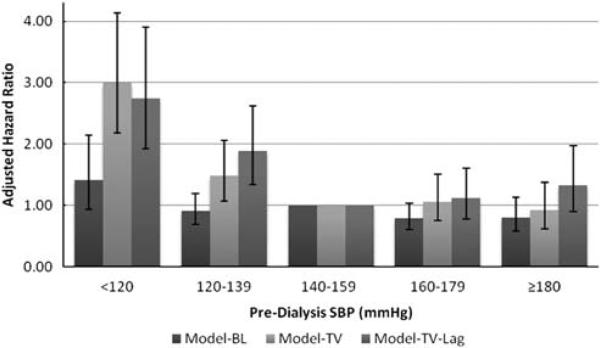
In the analyses of pre-dialysis SBP and CV mortality, we observed a similar pattern of results as with all-cause mortality (Figure 3). With the addition of the 3-month lag, the association between pre-dialysis SBP and CV mortality appeared curvilinear (Model-TV-Lag, Figure 3), with a 34% (95% confidence interval (CI) –10 to 97%) higher risk of CV mortality among subjects with pre-dialysis SBP ≥180 mm Hg; however, this association did not achieve statistical significance.
Figure 3.
Association of pre-dialysis SBP with adjusted risk of cardiovascular mortality, using three analytical models. All models adjusted for age, sex, Black race, serum albumin concentration, vintage, modified ICED score, diabetes, residual kidney function and randomized intervention group. Error bars indicate 95% confidence intervals. SBP, systolic blood pressure; Model-BL, baseline SBP as a time-constant variable; Model-TV, SBP as a time-varying variable; Model-TV-Lag, Model-TV + 3-month lag.
In the analyses of pre-dialysis SBP and infection-related mortality, using Model-TV, pre-dialysis SBP <120 mm Hg was associated with higher infection-related mortality compared with the referent group of 140–159 mm Hg (hazard ratio 2.64, 95% CI 1.66–4.21). However, this relationship was attenuated and not statistically significant in Model-TV-Lag (hazard ratio 1.31, 95% CI 0.79–2.15). As with all-cause mortality, models that included an adjustment for baseline antihypertensive medication use did not materially change the results when analysing cause-specific mortality (data not shown).
Post-dialysis SBP and mortality
Globally, we observed no significant association between baseline post-dialysis SBP and all-cause mortality (P = 0.24). In Model-TV, post-dialysis SBP <120 mm Hg was associated with 36% (95% CI 11–68%) higher risk of all-cause mortality, but in Model-TV-Lag a significant relationship between post-dialysis SBP and all-cause mortality was no longer observed (P = 0.95). Similarly, using Model-TV-Lag, we found no significant association between post-dialysis SBP and CV mortality (P = 0.43) or infection-related mortality (P = 0.38). As with our analyses of pre-dialysis SBP, higher = post-dialysis SBP was not associated with a significantly higher risk of all-cause or cause-specific mortality (data not shown).
Discussion
In this analysis, we determined the relationship among various levels of pre- and post-dialysis SBP with all-cause and cause-specific mortality in patients on haemodialysis using three analytical models. Our results are consistent with previous studies, which have also shown the highest risk of mortality with lower, rather than higher SBP.10,16–18 We observed the highest risk of all-cause and CV mortality among subjects with pre-dialysis SBP <120 mm Hg compared with the referent group of 140–159 mm Hg, whereas significant associations among higher pre-dialysis SBP and these clinical outcomes were not observed. Post-dialysis SBP was not consistently associated with clinical outcomes. The results from our analysis extend findings described in other cohorts by (1) including long-itudinal determinations of pre- and post-dialysis SBP as a time-varying covariate; (2) adjusting for demographic factors, comorbid conditions and selected laboratory results not only at baseline, but also as time-varying covariates; and (3) incorporating adjudicated outcomes for cause-specific mortality.
We used three alternative proportional hazards models in our analysis. Pre-dialysis SBP analysed as a time-varying predictor yielded strong associations among categories of SBP <140 mm Hg and higher risk of all-cause and CV mortality. Associations between lower SBP and higher mortality have been reported in other high-risk groups not on dialysis, including the elderly19 and persons with congestive heart failure.20 It may be that although hypertension predisposes patients with and without kidney disease to CV morbidity, lower SBP acts as a potent marker for other, ultimately fatal comorbid conditions. Results from our analysis support this explanation. An infection leading to death would be expected to result in lower SBP, and we demonstrated a strong association between lower pre-dialysis SBP and infection-related mortality in Model-TV. After adding a 3-month lag (Model-TVLag), the association between lower pre-dialysis SBP and infection-related mortality was no longer statistically significant, supporting the hypothesis that lower SBP within 3 months of death may result from (rather than cause) the acute illness that ultimately leads to death. However, the 3-month lag did not fully attenuate the relationship between lower SBP and all-cause and CV mortality, perhaps due to residual confounding.
Why did we not demonstrate a significant association between hypertension and mortality, despite improved accounting for confounders over time? Most studies that have shown associations between higher SBP and higher mortality risk in end-stage renal disease were conducted in healthier cohorts with fewer comorbid conditions. For example, a Japanese study of a relatively young cohort (mean age 52.3 years) with a low mortality rate (60.3 per 1000 patient-years) showed a 4% higher risk of all-cause mortality per 10 mm Hg increment in SBP.6 Similarly, a study of haemodialysis patients7 with a low prevalence of diabetes (12.3%) showed an association between pre-dialysis SBP ≥160 mm Hg and a twofold higher risk of death after ≥2 years of follow-up. In contrast, Li et al.10, describing a US-based cohort with a high prevalence of diabetes was able to demonstrate a higher risk of mortality only with extremely elevated levels of SBP (≥200 mm Hg), and the relationship was much weaker than the association of SBP <120 mm Hg with mortality.
A mortality rate of 10% per year has been suggested as a ‘threshold’, above which an independent effect of higher SBP on mortality is difficult to demonstrate;21 in the HEMO study the annual crude mortality rate was ~14%.13 The HEMO study cohort of prevalent haemodialysis patients, with a median dialysis vintage of 2.2 years, may have had a high burden of unmeasured comorbid disease or severity of illness, thereby masking the impact of hypertension on mortality. In addition, as only 10–15% of the cohort had a pre-dialysis SBP ≥180 mm Hg at any given time, we may have lacked the statistical power to detect a small effect of higher SBP on mortality.
We did not observe a consistent association between post-dialysis SBP and mortality, in contrast to Zager et al.11 who showed a U-shaped association between post-dialysis SBP and mortality. In that report, the patients were less likely to have diabetes and were of shorter dialysis vintage; moreover, there was no adjustment for comorbid conditions beyond the cause of end-stage renal disease. Similarly, Port et al.18 demonstrated a higher risk of mortality at both extremes of post-dialysis SBP; however, their cohort also had a relatively low prevalence of diabetes and the analysis only considered baseline, rather than time-varying, SBP. As noted above, the analytic method seems to materially influence the results; we could have reached different conclusions, had time-varying exposures not been considered.
Our study has several limitations. First, BP measurements in the HEMO study were conducted as per the usual dialysis centre routine and not standardized. Although current clinical practice guidelines22 target routine pre- and post-dialysis BP measured in the dialysis unit, a recent prospective study by Agarwal23 showed that although higher quartiles of home-based and ambulatory SBP strongly predicted mortality, routine in-centre pre- and post-dialysis SBP measurements did not. Routine in-centre SBP measurements can variably overestimate SBP,24–26 and misclassification of hypertension due to inaccurate SBP measurements could explain the lack of association between higher SBP and mortality in our analysis. Second, we did not include analyses of diastolic BP or pulse pressure in this report. We chose to focus on SBP for two reasons: clinically, SBP is of primary consideration when making decisions about dry weight or medication adjustments; practically, by focusing on SBP, which was correlated with diastolic BP and pulse pressure, we avoided the statistical pitfalls associated with multiple comparisons. Third, information on CV function such as echocardiography was not collected as part of the HEMO study, which might have helped elucidate the underlying mechanisms of the observed association between lower SBP and higher mortality. Finally, although appearing similar to the US prevalent haemodialysis population in most measured characteristics, the generalizability of the HEMO study data might be restricted by its nature as a randomized clinical trial.
In summary, these analyses confirm and extend previous studies linking lower pre-dialysis SBP with higher risks of all-cause and CV mortality. This association may be explained by considering lower SBP as a marker of underlying comorbid illnesses not captured by the ICED score. Although two recent meta-analyses27,28 showed a reduced risk of CV morbidity and mortality with antihypertensive medication treatment in dialysis patients, the possibility remains that lowering pre-dialysis SBP to <140/90 mm Hg as recommended by current clinical practice guidelines22 may cause harm. A recent study showed that dialysis centres with a higher proportion of haemodialysis patients reaching an SBP of <140 mm Hg had higher rates of symptomatic intradialytic hypotension.29 Intradialytic hypotension can reduce cardiac perfusion and is associated with the intradialytic myocardial stunning phenomenon.30 Results from our analysis cannot answer questions about cause and effect, and should not be used to argue against treating hypertension in haemodialysis. However, our results, together with several other studies, underscore the need for a randomized clinical trial to define safe and efficacious BP targets in the haemodialysis population.
What is known about topic
In the general population, higher systolic blood pressure is consistently associated with a higher risk of death, but previous studies in patients on dialysis have yielded mixed results.
Although some studies in dialysis have shown a direct relationship between systolic blood pressure and death, others have shown an inverse or even a U-shaped relationship; these differences may have stemmed, in part, from heterogeneous study cohorts or residual confounding.
What this study adds
After improved accounting for confounding factors, we demonstrated that in a relatively uniform cohort of prevalent patients on haemodialysis, lower pre-dialysis systolic blood pressure is associated with higher risk of all-cause and cardiovascular mortality.
Our analysis underscores the need for a randomized clinical trial to define safe and efficacious blood pressure targets in patients on haemodialysis.
Acknowledgements
Dr Chang is supported by a Ruth L Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant (5 T32 DK7357).
Footnotes
An abstract based on these data was presented as a free communication at the 2009 American Society of Nephrology Annual Meeting in San Diego, CA, USA.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Horl MP, Horl WH. Hemodialysis-associated hypertension: pathophysiology and therapy. Am J Kidney Dis. 2002;39(2):227–244. doi: 10.1053/ajkd.2002.30542. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Renal Data System . USRDS 2007 Annual Data Report. Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health National Institute of Diabetes and Digestive and Kidney Disease; Bethesda, MD: 2007. [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 4.Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345(18):1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 5.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. The Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 6.Tozawa M, Iseki K, Iseki C, Takishita S. Pulse pressure and risk of total mortality and cardiovascular events in patients on chronic hemodialysis. Kidney Int. 2002;61(2):717–726. doi: 10.1046/j.1523-1755.2002.00173.x. [DOI] [PubMed] [Google Scholar]
- 7.Mazzuchi N, Carbonell E, Fernandez-Cean J. Importance of blood pressure control in hemodialysis patient survival. Kidney Int. 2000;58(5):2147–2154. doi: 10.1111/j.1523-1755.2000.00388.x. [DOI] [PubMed] [Google Scholar]
- 8.Klassen PS, Lowrie EG, Reddan DN, DeLong ER, Coladonato JA, Szczech LA, et al. Association between pulse pressure and mortality in patients undergoing maintenance hemodialysis. JAMA. 2002;287(12):1548–1555. doi: 10.1001/jama.287.12.1548. [DOI] [PubMed] [Google Scholar]
- 9.Stidley CA, Hunt WC, Tentori F, Schmidt D, Rohrscheib M, Paine S, et al. Changing relationship of blood pressure with mortality over time among hemodialysis patients. J Am Soc Nephrol. 2006;17(2):513–520. doi: 10.1681/ASN.2004110921. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Lacson E, Jr, Lowrie EG, Ofsthun NJ, Kuhlmann MK, Lazarus JM, et al. The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am J Kidney Dis. 2006;48(4):606–615. doi: 10.1053/j.ajkd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, et al. U’ curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int. 1998;54(2):561–569. doi: 10.1046/j.1523-1755.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- 12.Rocco MV, Yan G, Gassman J, Lewis JB, Ornt D, Weiss B, et al. Comparison of causes of death using HEMO Study and HCFA end-stage renal disease death notification classification systems. The National Institutes of Health-funded Hemodialysis. Health care financing administration. Am J Kidney Dis. 2002;39(1):146–153. doi: 10.1053/ajkd.2002.29905. [DOI] [PubMed] [Google Scholar]
- 13.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 14.Greene T, Beck GJ, Gassman JJ, Gotch FA, Kusek JW, Levey AS, et al. Design and statistical issues of the hemodialysis (HEMO) study. Control Clin Trials. 2000;21(5):502–525. doi: 10.1016/s0197-2456(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 15.Miskulin DC, Athienites NV, Yan G, Martin AA, Ornt DB, Kusek JW, et al. Comorbidity assessment using the Index of Coexistent Diseases in a multicenter clinical trial. Kidney Int. 2001;60(4):1498–1510. doi: 10.1046/j.1523-1755.2001.00954.x. [DOI] [PubMed] [Google Scholar]
- 16.Foley RN, Herzog CA, Collins AJ. Blood pressure and long-term mortality in United States hemodialysis patients: USRDS Waves 3 and 4 Study. Kidney Int. 2002;62(5):1784–1790. doi: 10.1046/j.1523-1755.2002.00636.x. [DOI] [PubMed] [Google Scholar]
- 17.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. Impact of hypertension on cardiomyopathy, morbidity and mortality in end-stage renal disease. Kidney Int. 1996;49(5):1379–1385. doi: 10.1038/ki.1996.194. [DOI] [PubMed] [Google Scholar]
- 18.Port FK, Hulbert-Shearon TE, Wolfe RA, Bloembergen WE, Golper TA, Agodoa LY, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33(3):507–517. doi: 10.1016/s0272-6386(99)70188-5. [DOI] [PubMed] [Google Scholar]
- 19.Staessen J, Bulpitt C, Clement D, De Leeuw P, Fagard R, Fletcher A, et al. Relation between mortality and treated blood pressure in elderly patients with hypertension: report of the European Working Party on High Blood Pressure in the Elderly. BMJ. 1989;298(6687):1552–1556. doi: 10.1136/bmj.298.6687.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raphael CE, Whinnett ZI, Davies JE, Fontana M, Ferenczi EA, Manisty CH, et al. Quantifying the paradoxical effect of higher systolic blood pressure on mortality in chronic heart failure. Heart. 2009;95(1):56–62. doi: 10.1136/hrt.2007.134973. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal R. Hypertension and survival in chronic hemodialysis patients—past lessons and future opportunities. Kidney Int. 2005;67(1):1–13. doi: 10.1111/j.1523-1755.2005.00050.x. [DOI] [PubMed] [Google Scholar]
- 22.K/DOQI Workgroup K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 Suppl 3):S1–153. [PubMed] [Google Scholar]
- 23.Agarwal R. Blood pressure and mortality among hemodialysis patients. Hypertension. 2010;55(3):762–768. doi: 10.1161/HYPERTENSIONAHA.109.144899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fagugli RM, Ricciardi D, Rossi D, Ricciardi D, Gaetano Ad, Taglioni C. Blood pressure assessment in haemodialysis patients: comparison between pre-dialysis blood pressure and ambulatory blood pressure measurement. Nephrology. 2009;14(3):283–290. doi: 10.1111/j.1440-1797.2009.01090.x. [DOI] [PubMed] [Google Scholar]
- 25.Rahman M, Griffin V, Kumar A, Manzoor F, Wright JT, Jr, Smith MC. A comparison of standardized versus ‘usual’ blood pressure measurements in hemodialysis patients. Am J Kidney Dis. 2002;39(6):1226–1230. doi: 10.1053/ajkd.2002.33395. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal R, Peixoto AJ, Santos SFF, Zoccali C. Pre- and postdialysis blood pressures are imprecise estimates of interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol. 2006;1(3):389–398. doi: 10.2215/CJN.01891105. [DOI] [PubMed] [Google Scholar]
- 27.Heerspink HJL, Ninomiya T, Zoungas S, de Zeeuw D, Grobbee DE, Jardine MJ, et al. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet. 2009;373(9668):1009–1015. doi: 10.1016/S0140-6736(09)60212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agarwal R, Sinha AD. Cardiovascular protection with antihypertensive drugs in dialysis patients: systematic review and meta-analysis. Hypertension. 2009;53(5):860–866. doi: 10.1161/HYPERTENSIONAHA.108.128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davenport A, Cox C, Thuraisingham R. Achieving blood pressure targets during dialysis improves control but increases intradialytic hypotension. Kidney Int. 2008;73(6):759–764. doi: 10.1038/sj.ki.5002745. [DOI] [PubMed] [Google Scholar]
- 30.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4(5):914–920. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]