Abstract
Topical delivery of nitric oxide (NO) through a wound dressing has the potential to reduce wound infections and improve healing of acute and chronic wounds. This study characterized the antibacterial efficacy of an ointment containing NO-loaded, zinc-exchanged zeolite A that releases NO upon contact with water. The release rate of NO from the ointment was measured using a chemiluminescence detection system. Minimum bactericidal concentration assays were performed using five common wound pathogens, including Gram-negative bacteria (Escherichia coli and Acinetobacter baumannii), Gram-positive bacteria (Staphylococcus epidermidis and meticillin-resistant Staphylococcus aureus) and a fungus (Candida albicans). The time dependence of antimicrobial activity was characterized by performing log-reduction assays at four time points after 1–8 h ointment exposure. The cytotoxicity of the ointment after 24 h was assessed using cultured 3T3 fibroblast cells. Minimum microbicidal concentrations (MMCs) for bacterial organisms (5×107 c.f.u.) ranged from 50 to 100 mg ointment (ml media)−1; the MMC for C. albicans (5×104 c.f.u.) was 50 mg ointment (ml media)−1. Five to eight log reductions in bacterial viability and three log reductions in fungal viability were observed after 8 h exposure to NO–zeolite ointment compared with untreated organisms. Fibroblasts remained viable after 24 h exposure to the same concentration of NO–zeolite ointment as was used in antimicrobial tests. In parallel studies, full-thickness cutaneous wounds on Zucker obese rats healed faster than wounds treated with a control ointment. These data indicate that ointment containing NO-loaded zeolites could potentially be used as a broad-spectrum antimicrobial wound-healing dressing.
Introduction
Nitric oxide (NO) is naturally produced by the immune system to combat a broad spectrum of pathogens (Jones et al., 2010). Impaired wound healing has been associated with decreased levels of endogenous NO production (Rizk et al., 2004), which suggests that exogenous NO has the potential to accelerate healing in such wounds. However, systemic delivery of NO is complicated by the short half-life of NO in vivo (Thomas et al., 2001) and potential unwanted effects on non-wound physiology (Kröncke & Suschek, 2008). Controlled, localized delivery of NO through a topical wound dressing has the potential to reduce wound infections and improve healing of both acute and chronic wounds.
Several technologies have been proposed as controlled delivery vehicles for NO, including compressed gas cylinders that deliver NO directly to a wound (Ghaffari et al., 2006, 2007; Miller et al., 2004), nitrite creams that release a burst of NO in acidic environments (Ormerod et al., 1999; Weller & Finnen, 2006), glassy matrix nanoparticles containing nitrites that undergo sugar-mediated thermal reduction to release NO in neutral environments (Friedman et al., 2008), molecules such as diazeniumdiolates (Keefer, 2005; Shin et al., 2007) and nitrosothiols (Li & Lee, 2010) that give off NO spontaneously and nanoporous materials such as zeolites and metal organic frameworks that adsorb NO gas and release it upon contact with moisture (Morris & Wheatley, 2008). Here, we report on a topical ointment that contains NO-loaded zeolites.
Zeolites are negatively charged nanoporous aluminosilicate structures that can be balanced using a variety of transition metal cations; these metal-exchanged zeolites are capable of storing more than 1 mmol NO (g zeolite)–1 under anhydrous conditions (Wheatley et al., 2006). Anhydrous zeolites have a high affinity for water, which causes NO to be displaced and released from the zeolite structure upon contact with moisture (Wheatley et al., 2006). Previously published research has demonstrated that topically applied NO-loaded, manganese-exchanged zeolites in an aqueous cream base are capable of delivering NO to the dermis without the inflammatory effects that can be caused by acidified nitrite creams (Mowbray et al., 2008). NO-loaded, cobalt-exchanged zeolites in electrospun polylactic acid polymer fibres are capable of releasing NO over a period of several hours (Liu & Balkus, 2009). The antibacterial efficacy of solid discs made from NO-loaded, zinc-exchanged zeolites mixed with a polytetrafluoroethylene polymer that release NO over periods of less than 1 h has been demonstrated (Fox et al., 2010). The present study characterized the microbicidal efficacy of a topical ointment containing NO-loaded, zinc-exchanged zeolites that releases NO over several hours.
Methods
Ointment formulation and storage.
Zinc-exchanged zeolite A was loaded with NO gas according to the procedure described by Wheatley et al. (2006). NO-loaded zeolite powder (1.6 g) was combined with emulsifying ointment BP (3.2 g), resulting in a 33 % (w/w) NO–zeolite ointment. NO–zeolite ointment samples were vacuum sealed under inert gas in 3 ml nylon/poly bags to prevent the release of NO until the start of experiments. Stability under these storage conditions was observed for 4 months (data not shown). All samples used in these experiments were stored for less than 1 week.
Control zeolite ointment was prepared by mixing sodium zeolite A without zinc exchange or NO loading into emulsifying ointment BP to a final concentration of 33 % (w/w).
Measurement of NO release.
The release profile of NO from NO–zeolite ointment was measured using a Sievers Nitric Oxide Analyser (NOA 280i; GE Analytical Instruments). Nitrogen gas was flowed through a sintered glass frit into a glass bubbler (Ace Glass) containing 15 ml 1 × PBS. Ointment samples weighing 25 mg were evenly spread over a 1×1 cm square of laboratory tissue (Kimwipe; Kimberly-Clark), forming a layer with less than 0.5 mm thickness. This ointment geometry is representative of a thin layer of ointment spread over skin or a wound. The ointment and tissue were placed directly into the PBS. Nitrogen gas flowed through the PBS and carried NO to the analyser as it was released from the ointment. The system was calibrated using standard volumes of PBS that had been equilibrated with 2000 p.p.m. NO gas in nitrogen gas (Airgas USA); the concentration of NO in each volume of PBS was calculated from the solubility of NO in PBS (1.75×10−6 mol ml−1 atm–1) (Zacharia & Deen, 2005).
Concentration dependence of antimicrobial activity.
Experiments were conducted to determine the minimum microbicidal concentration (MMC) of NO–zeolite ointment for micro-organisms incubated in growth media over a 24 h period. Escherichia coli (American Type Culture Collection [ATCC] 25922), Acinetobacter baumannii (ATCC 19606), Staphylococcus epidermidis (ATCC 12228) and meticillin-resistant Staphylococcus aureus (MRSA, BAA1680) were cultured in tryptic soy broth to a concentration of 109 c.f.u. ml−1. The concentration was adjusted to 107 c.f.u. ml−1 and 5 ml aliquots were placed in 50 ml conical tubes. Samples of NO–zeolite ointment and control zeolite ointment ranging in mass from 125 to 1000 mg [25–200 mg ointment (ml media)−1] were added to each tube. All tubes were sealed with Parafilm, vortexed for 30 s and incubated for 24 h under gentle agitation at 37 °C. Samples of 100 µl from each tube were plated onto Trypticase soy agar and incubated overnight. Plates were inspected for colonies. The minimum amount of NO–zeolite ointment that yielded a plate with no colonies was considered the MMC. Because only 100 µl of each original sample was plated, a plate with no colonies indicated that the concentration of microbes was less than 10 c.f.u. ml−1.
This procedure was repeated for Candida albicans (a gift from Dr Thomas Edlind, Drexel University College of Medicine, Philadelphia, PA, USA), except that yeast peptone glucose broth and agar were used instead of tryptic soy broth and Trypticase soy agar, and the initial concentration of organisms was 104 c.f.u. ml−1 instead of 107 c.f.u. ml−1. All experiments were conducted in triplicate.
Time dependence of antimicrobial activity.
Experiments were conducted to quantify the number of micro-organisms inactivated by NO–zeolite ointment across a range of exposure durations. E. coli, A. baumannii, S. epidermidis and MRSA were cultured in tryptic soy broth to a concentration of 109 c.f.u. ml−1. The concentration was adjusted to 107 c.f.u. ml−1 and 5 ml aliquots were placed in three 50 ml conical tubes. Tubes were assigned to one of three treatment groups: (1) NO–zeolite ointment (0.5 g), (2) control zeolite ointment (0.5 g) or (3) no treatment (control). All tubes were sealed with Parafilm, vortexed for 30 s and incubated under gentle agitation at 37 °C. After 0, 2, 4, 6 and 8 h, 100 µl samples were removed from each tube and serially diluted five times in 100-fold increments. Samples of 100 µl of each dilution and undiluted media were plated onto Trypticase soy agar, and c.f.u.s were counted after overnight incubation. Because only 100 µl of each undiluted sample was plated, the minimum detectable concentration of organisms was 10 c.f.u. ml−1.
This procedure was repeated for C. albicans, except that yeast peptone glucose broth and agar were used instead of tryptic soy broth and Trypticase soy agar, the initial concentration of organisms was 104 c.f.u. ml−1 instead of 107 c.f.u. ml−1, and samples were plated at 0, 1, 2, 3 and 4 h. All experiments were conducted in triplicate.
Cytotoxicity testing.
3T3 fibroblast cells (ATCC) were seeded on 12-well Corning CellBIND culture plates at a density of 5000 cells cm−2. After 72 h incubation at 37 °C and 5 % CO2, 200 mg ointment was spread onto the polyester membranes of Corning Transwell inserts with 0.4 µm pores. Three inserts were treated with NO–zeolite ointment, three were treated with control zeolite ointment and three were left untreated. The inserts were placed into the 12-well plates, which each contained 1.5 ml growth medium (Eagle’s minimal essential medium with 10 % horse serum; ATCC), and 0.5 ml growth medium was added to each Transwell insert. After 24 h incubation, the Transwell inserts were removed and the viability of the cells in each well was quantified using the AlamarBlue cell viability assay (Life Technologies). AlamarBlue is a trademark for resazurin (7-hydroxy-3H-phenoxazin-3-one 10-oxide), a blue and almost non-fluorescent dye that is converted by cellular respiration (thus, the viable cells) into resorufin (reduced form), a red and highly fluorescent product that can be measured fluorometrically. Briefly, 150 µl AlamarBlue reagent was added to each well and incubated for 2.5 h. A total of 100 µl medium from each well was transferred to a 96-well plate and fluorescence was measured using a Tecan Infinite 200 plate reader with excitation and emission wavelengths of 570 and 610 nm, respectively. This procedure was conducted five times.
Wound closure testing.
The effect of NO on the wound closure rate was tested by applying NO–zeolite ointment to full-thickness cutaneous wounds in obese rats. This protocol was approved by the Institutional Animal Care & Use Committee at the Drexel University College of Medicine. Ten male obese Zucker rats were purchased from Charles River Laboratories. One full-thickness cutaneous wound was made on the left dorsal region of each rat using a scalpel and circular template (surface area 4.6 cm2). All wounds were covered with a sterile transparent wound dressing (Tegaderm; 3M) for 24 h. Treatments were applied three times per week starting the day after wound surgery. Five rats received NO–zeolite ointment and five received control zeolite ointment; the amount of ointment applied was enough to cover the wound with a layer approximately 0.5–1.0 mm thick. All ointments were covered with a sterile Tegaderm dressing and the animals were wrapped with elastic bandaging (Vetrap; 3M) to prevent them from removing their dressings. Animals were killed 20 days after the surgery.
During the course of the study, all animals were supplied with food and water ad libitum and were housed in individual cages. During each dressing change, wounds were digitally photographed using a Fujifilm FinePix s700 digital camera with cross-polarizing filters to reduce surface reflection. A ruler was held in the imaging plane of each photograph to allow calculation of an absolute value for the wound area. The boundaries of each wound were manually traced using a computer mouse and Microsoft Paint software, and wound areas were calculated from the traced photographs using an image analysis code developed with matlab (MathWorks) software. This image analysis code counted the number of pixels within the traced wound boundary, and then calculated the wound area by approximating the size of each pixel from the image of the ruler in the imaging plane of each wound.
Results
The purpose of this study was to understand the in vitro responses of various bacterial and fungal pathogens against NO–zeolite, and to evaluate antimicrobial efficacy, NO release over time and relative cytotoxicity.
NO release
The mean release profile of NO from three emulsifying ointment samples is shown in Fig. 1. The NO release rate peaked at 4.4±1.2 nmol min–1 (mg zeolite)–1 (mean±sd) after 1 min exposure to water and fell to 0.06±0.05 nmol min–1 (mg zeolite)–1 after 3 h. Cumulative NO released after 3 h was 104±14 nmol (mg zeolite)–1.
Fig. 1.

NO release profiles from samples of emulsifying ointment containing 33 % (w/w) NO-loaded zeolites. (a) NO released per minute. (b) Cumulative NO release. Shaded areas represent the sd. n = 3. All samples were stored for 1–7 days before measurement.
Concentration dependence of antimicrobial activity
MMCs [mg ointment (ml medium)−1] for each organism after 24 h incubation with NO–zeolite ointment were as follows: E. coli, 100; A. baumannii, 100; S. epidermidis, 50; MRSA, 100; and C. albicans, 50. None of the control zeolite samples was microbicidal. Based on the cumulative NO release curve of NO–zeolite ointment (Fig. 1b), and assuming that the release of NO after 3 h was negligible, MMC values of 100 and 50 mg ointment (ml medium)−1 corresponded to cumulative concentrations of 3.5 and 1.7 µmol NO (ml medium)−1, respectively.
Time dependence of antimicrobial activity
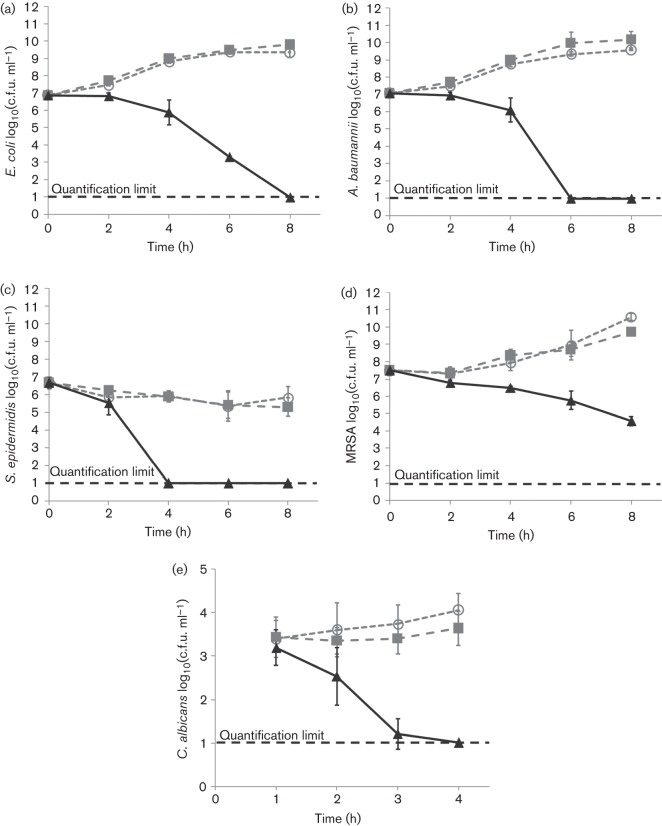
The results of the time-dependency experiments are shown in Fig. 2. Log reductions in the concentrations of each organism exposed to NO–zeolite ointment for 4 or 8 h are summarized in Table 1. The growth of organisms exposed to control ointment was similar to that of untreated organisms, indicating that the zeolite ointment itself does not inactivate microbes.
Fig. 2.
Results of time-dependency experiments for (a) E. coli, (b) A. baumannii, (c) S. epidermidis, (d) MRSA and (e) C. albicans. ▴, Organisms treated with NO–zeolite ointment [100 mg (ml medium)–1];  , organisms treated with control zeolite ointment [100 mg (ml medium)–1]; ○, untreated organisms. Each point represents the mean of three trials. Error bars represent sds.
, organisms treated with control zeolite ointment [100 mg (ml medium)–1]; ○, untreated organisms. Each point represents the mean of three trials. Error bars represent sds.
Table 1. Log reductions in viable microbe colonies after exposure to NO–zeolite ointment for 8 h (E. coli, A. baumannii, S. epidermidis and MRSA) or 4 h (C. albicans).
Log reduction (NO–zeolite versus initial) = log(initial microbe concentration) – log(final microbe concentration after NO–zeolite exposure). Log reduction (NO–zeolite versus untreated) = log(final untreated microbe concentration) – log(final microbe concentration after NO–zeolite exposure).
| Organism | Log reduction (NO–zeolite versus initial) | Log reduction (NO–zeolite versus untreated) |
| E. coli | 5.9 | 8.4 |
| A. baumannii | 6.1 | 8.6 |
| S. epidermidis | 5.7 | 5.1 |
| MRSA | 2.9 | 6.0 |
| C. albicans | 3.0 | 3.1 |
Cytotoxicity testing
Percentage viability of cells treated with NO–zeolite ointment and control zeolite ointment was calculated by dividing the mean fluorescence intensity of medium in treated wells by the mean fluorescence intensity of medium in untreated wells. The percentage viability values (mean±sd) for cells treated with NO–zeolite and control zeolite ointment were 34±8 and 91±21 %, respectively. Assuming that NO was able to diffuse freely across the Transwell membrane, the concentration of NO–zeolite ointment in growth medium was equal to the ointment concentration used in time-dependent antibacterial tests [100 mg ointment (ml medium)–1, corresponding to 3.5 µmol NO (ml medium)−1].
In vivo obese rat studies
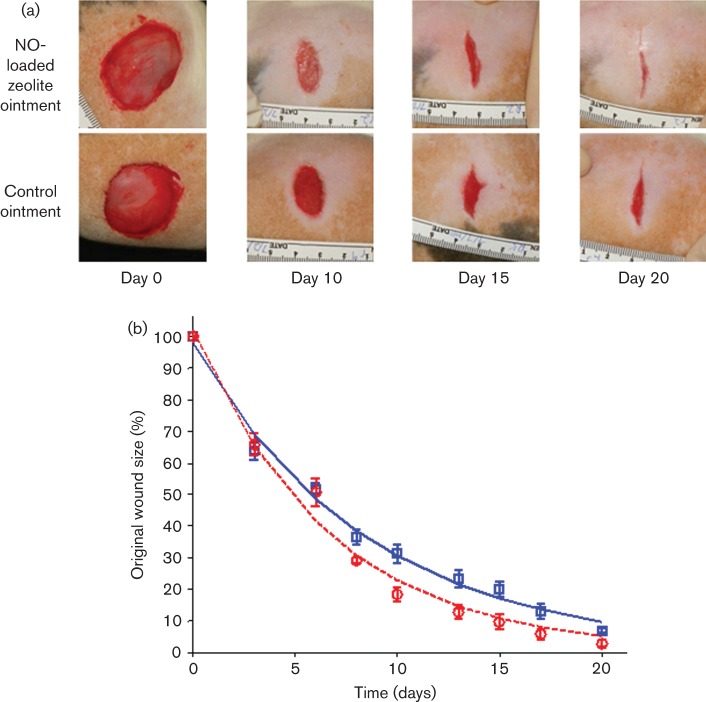
Representative images of wounds from each treatment group are shown in Fig. 3(a). Mean wound sizes at each measurement time point are shown in Fig. 3(b). Wound-healing trajectories are often represented as exponential functions (Steed, 2003). The rate of wound healing was calculated by fitting the wound size versus time data from each animal to an exponential function, S(t) = S0e−θt, where S(t) is the wound size at time t as a percentage of the initial wound size, S0 is the estimated initial wound size and θ is the wound-healing rate in units of per cent per day. The mean±sd healing rates of wounds treated with NO–zeolite ointment and control zeolite ointment were 15.1±1.6 and 11.7±1.7 % day−1, respectively. This difference was significant (P = 0.01, two-tailed Student t-test). These data demonstrate the beneficial effect of NO-loaded zeolite ointment on wound healing.
Fig. 3.
(a) Images of treated and control wounds on Zucker obese rats on the day of wound surgery (day 0), and at three different time points after wound surgery. (b) Mean wound size in each treatment group as a percentage of each wound’s original size.  , Rats treated with NO–zeolite ointment;
, Rats treated with NO–zeolite ointment;  , rats treated with control zeolite ointment. Error bars represent ses. Lines represent exponential curves of best fit.
, rats treated with control zeolite ointment. Error bars represent ses. Lines represent exponential curves of best fit.
Discussion
The present study demonstrates the antimicrobial efficacy of a topical ointment containing an NO-loaded zeolite powder that releases NO upon contact with moisture. The ointment formulation released measurable quantities of NO over at least 3 h. Studies of NO release from NO-loaded, zinc-exchanged zeolite powder have demonstrated that nearly all NO is released within 1 h of contact with moisture (Wheatley et al., 2008). Mixing the zeolite powder into a hydrophobic ointment base slows the transport of water to the zeolite molecules, reducing the rate at which NO is released. The ointment base also makes the powder easy to apply to skin or wounds.
The organisms used for microbicidal testing were selected to demonstrate effectiveness against a broad spectrum of common wound pathogens, including Gram-negative bacteria (E. coli and A. baumannii), Gram-positive bacteria (S. epidermidis and MRSA) and a fungus (C. albicans). Although the in vitro tests conducted in the present study do not represent the complexity of an infected wound, the results of MMC and log-reduction tests indicate that clinically relevant reductions in micro-organism concentrations can be achieved with NO–zeolite ointment. The control zeolite ointment did not impair the growth of any of the micro-organisms tested. These results are consistent with those of Fox et al. (2010), who demonstrated the antimicrobial effect of NO-loaded, zinc-exchanged zeolite mixed with a polytetrafluoroethylene polymer. This compound released most of its NO within 30 min of exposure to water and caused 1–2 log reductions in bacteria concentrations after 45 min. In the present study, 5–8 log reductions in bacteria concentrations were observed after 6–8 h of exposure to NO–zeolite ointment.
In vitro cytotoxicity testing demonstrated that more than 90 % of fibroblasts treated with control zeolite ointment were viable, while approximately one-third of cells treated with NO–zeolite ointment were viable after 24 h. These data indicate that the materials in the zeolite ointment are not toxic to fibroblasts, but that the amount of NO released by the ointment impairs fibroblast viability in vitro. However, significant fibroblast toxicity in vitro is unsurprising in light of the reported toxicity of other antimicrobial treatments. For example, in vitro cytotoxicity testing of some commercially available silver wound dressings resulted in less than 25 % fibroblast viability (Burd et al., 2007). Fibroblast exposure to 1 % povidone–iodine resulted in approximately 5 % viability after 24 h (Balin & Pratt, 2002), yet a systematic review of the use of iodine in wound-treatment clinical trials concluded that iodine does not impair wound healing (Vermeulen et al., 2010). A recent review of iodine and chlorhexidine in acute wound management highlights the apparent discrepancy among the results of preclinical in vitro cytotoxicity testing and those of clinical trials of these antiseptic agents (Eardley et al., 2012). Therefore, the results from NO–zeolite ointment in vitro cytotoxicity testing are presented in order to demonstrate the similarity of NO–zeolite ointment to other broad-spectrum antimicrobial treatments. Moreover, the present study demonstrated the ability of NO-loaded zeolite ointment to enhance healing in full-thickness cutaneous wounds in obese Zucker rats. Wounds that were treated with NO–zeolite ointment did not show external signs of inflammation such as erythema or swelling in comparison with wounds that were treated with control dressings. Treatment with NO–zeolite ointment resulted in earlier wound closure when compared with wounds treated with control ointment, suggesting that, in contrast to the in vitro studies, NO–zeolite ointment does not have a cytotoxic effect on healing wounds and could potentially be used as a treatment for infected wounds.
Acknowledgements
Part of this research was presented at the annual meeting of the Wound Healing Society, Denver, CO, 1–5 May 2013. This work was supported by the US Army Medical Research and Materiel Command (contract no. W81XWH-11-C-0031). The views, opinions and/or findings contained in this report are those of the authors and should not be construed as an official Department of the Army position, policy or decision unless so designated by other documentation. The authors thank Abraham Araya at PQ Silicas UK for supplying NO-loaded zeolite powder, Dr Thomas Edlind, Drexel University College of Medicine, Philadelphia, PA, USA, for donating C. albicans and Richard Huneke and Janet Schulenberg at the Drexel University College of Medicine University Laboratory Animal Resources for their assistance with animal research. The contribution of the prematurely departed Dr Elisabeth Papazoglou, who was instrumental in initiating this research, is respectfully acknowledged. M. N. is an employee of and receives financial compensation from Zeomedix, a private company that has licensed intellectual property involving NO-loaded zeolite technology. The remaining authors are employees of Drexel University, which received funding from Zeomedix for portions of this research.
Abbreviations:
- ATCC
American Type Culture Collection
- MMC
minimum microbicidal concentration
- MRSA
meticillin-resistant Staphylococcus aureus
- NO
nitric oxide
References
- Balin A. K., Pratt L. (2002). Dilute povidone-iodine solutions inhibit human skin fibroblast growth. Dermatol Surg 28, 210–214 10.1046/j.1524-4725.2002.01161.x [DOI] [PubMed] [Google Scholar]
- Burd A., Kwok C. H., Hung S. C., Chan H. S., Gu H., Lam W. K., Huang L. (2007). A comparative study of the cytotoxicity of silver-based dressings in monolayer cell, tissue explant, and animal models. Wound Repair Regen 15, 94–104 10.1111/j.1524-475X.2006.00190.x [DOI] [PubMed] [Google Scholar]
- Eardley W. G., Watts S. A., Clasper J. C. (2012). Limb wounding and antisepsis: iodine and chlorhexidine in the early management of extremity injury. Int J Low Extrem Wounds 11, 213–223 10.1177/1534734612450589 [DOI] [PubMed] [Google Scholar]
- Fox S., Wilkinson T. S., Wheatley P. S., Xiao B., Morris R. E., Sutherland A., Simpson A. J., Barlow P. G., Butler A. R., Megson I. L. (2010). NO-loaded Zn2+-exchanged zeolite materials: a potential bifunctional anti-bacterial strategy. Acta Biomater 6, 1515–1521 10.1016/j.actbio.2009.10.038 [DOI] [PubMed] [Google Scholar]
- Friedman A. J., Han G., Navati M. S., Chacko M., Gunther L., Alfieri A., Friedman J. M. (2008). Sustained release nitric oxide releasing nanoparticles: characterization of a novel delivery platform based on nitrite containing hydrogel/glass composites. Nitric Oxide 19, 12–20 10.1016/j.niox.2008.04.003 [DOI] [PubMed] [Google Scholar]
- Ghaffari A., Miller C. C., McMullin B., Ghahary A. (2006). Potential application of gaseous nitric oxide as a topical antimicrobial agent. Nitric Oxide 14, 21–29 10.1016/j.niox.2005.08.003 [DOI] [PubMed] [Google Scholar]
- Ghaffari A., Jalili R., Ghaffari M., Miller C., Ghahary A. (2007). Efficacy of gaseous nitric oxide in the treatment of skin and soft tissue infections. Wound Repair Regen 15, 368–377 10.1111/j.1524-475X.2007.00239.x [DOI] [PubMed] [Google Scholar]
- Jones M. L., Ganopolsky J. G., Labbé A., Wahl C., Prakash S. (2010). Antimicrobial properties of nitric oxide and its application in antimicrobial formulations and medical devices. Appl Microbiol Biotechnol 88, 401–407 10.1007/s00253-010-2733-x [DOI] [PubMed] [Google Scholar]
- Keefer L. K. (2005). Nitric oxide (NO)- and nitroxyl (HNO)-generating diazeniumdiolates (NONOates): emerging commercial opportunities. Curr Top Med Chem 5, 625–636 10.2174/1568026054679380 [DOI] [PubMed] [Google Scholar]
- Kröncke K. D., Suschek C. V. (2008). Adulterated effects of nitric oxide-generating donors. J Invest Dermatol 128, 258–260 10.1038/sj.jid.5701162 [DOI] [PubMed] [Google Scholar]
- Li Y., Lee P. I. (2010). Controlled nitric oxide delivery platform based on S-nitrosothiol conjugated interpolymer complexes for diabetic wound healing. Mol Pharm 7, 254–266 10.1021/mp900237f [DOI] [PubMed] [Google Scholar]
- Liu H. A., Balkus K. J., Jr (2009). Novel delivery system for the bioregulatory agent nitric oxide. Chem Mater 21, 5032–5041 10.1021/cm901358z [DOI] [Google Scholar]
- Miller C. C., Miller M. K., Ghaffari A., Kunimoto B. (2004). Treatment of chronic nonhealing leg ulceration with gaseous nitric oxide: a case study. J Cutan Med Surg 8, 233–238 10.1007/s10227-004-0106-8 [DOI] [PubMed] [Google Scholar]
- Morris R. E., Wheatley P. S. (2008). Gas storage in nanoporous materials. Angew Chem Int Ed Engl 47, 4966–4981 10.1002/anie.200703934 [DOI] [PubMed] [Google Scholar]
- Mowbray M., Tan X., Wheatley P. S., Rossi A. G., Morris R. E., Weller R. B. (2008). Topically applied nitric oxide induces T-lymphocyte infiltration in human skin, but minimal inflammation. J Invest Dermatol 128, 352–360 10.1038/sj.jid.5701096 [DOI] [PubMed] [Google Scholar]
- Ormerod A. D., Copeland P., Hay I., Husain A., Ewen S. W. (1999). The inflammatory and cytotoxic effects of a nitric oxide releasing cream on normal skin. J Invest Dermatol 113, 392–397 10.1046/j.1523-1747.1999.00692.x [DOI] [PubMed] [Google Scholar]
- Rizk M., Witte M. B., Barbul A. (2004). Nitric oxide and wound healing. World J Surg 28, 301–306 10.1007/s00268-003-7396-7 [DOI] [PubMed] [Google Scholar]
- Shin J. H., Metzger S. K., Schoenfisch M. H. (2007). Synthesis of nitric oxide-releasing silica nanoparticles. J Am Chem Soc 129, 4612–4619 10.1021/ja0674338 [DOI] [PubMed] [Google Scholar]
- Steed D. L. (2003). Wound-healing trajectories. Surg Clin North Am 83, 547–555, vi–vii 10.1016/S0039-6109(02)00208-6 [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Liu X., Kantrow S. P., Lancaster J. R., Jr (2001). The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc Natl Acad Sci U S A 98, 355–360 10.1073/pnas.98.1.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen H., Westerbos S. J., Ubbink D. T. (2010). Benefit and harm of iodine in wound care: a systematic review. J Hosp Infect 76, 191–199 10.1016/j.jhin.2010.04.026 [DOI] [PubMed] [Google Scholar]
- Weller R., Finnen M. J. (2006). The effects of topical treatment with acidified nitrite on wound healing in normal and diabetic mice. Nitric Oxide 15, 395–399 10.1016/j.niox.2006.04.002 [DOI] [PubMed] [Google Scholar]
- Wheatley P. S., Butler A. R., Crane M. S., Fox S., Xiao B., Rossi A. G., Megson I. L., Morris R. E. (2006). NO-releasing zeolites and their antithrombotic properties. J Am Chem Soc 128, 502–509 10.1021/ja0503579 [DOI] [PubMed] [Google Scholar]
- Wheatley P. S., McKinlay A. C., Morris R. E. (2008). A comparison of zeolites and metal organic frameworks as storage and delivery vehicles for biologically active nitric oxide. In Zeolites and related materials: trends, targets and challenges, Proceedings of the 4th International FEZA Conference, Paris, France, 2–6 September 2008. Edited by Gédéon A., Massiani P., Babonneau F. Stud. Surface Sci Catalysis 174, 441–446 10.1021/ja0503579 [DOI] [Google Scholar]
- Zacharia I. G., Deen W. M. (2005). Diffusivity and solubility of nitric oxide in water and saline. Ann Biomed Eng 33, 214–222 10.1007/s10439-005-8980-9 [DOI] [PubMed] [Google Scholar]