Abstract
Endovascular embolization with Onyx has been increasingly used to treat intracranial and spinal dural arteriovenous fistulas (DAVFs). Several case series have been published in recent years reporting high DAVF cure rates with this technique. Although it is seldom reported, DAVF recurrence may occur despite initial “cure.” The authors present 3 separate cases of a recurrent DAVF after successful transarterial Onyx embolization. Despite adequate Onyx penetration into the fistula and draining vein, these cases demonstrate that DAVF recanalization may reappear with filling from previous or newly recruited arterial feeders. Other published reports of DAVF recurrence are examined, and potential contributory factors are discussed. These cases highlight the need for awareness of this possible phenomenon and suggest that follow-up angiography should be considered in patients treated with catheter embolization.
Keywords: dural arteriovenous fistula, embolization, Onyx, recanalization, recurrence
Dural arteriovenous fistulas represent a group of arteriovenous shunts that account for 10%–15% of all intracranial AVMs and represent the most commonly encountered spinal vascular malformations.26,28 These lesions may demonstrate variable clinical features ranging from an asymptomatic presentation to neurological impairment and hemorrhage. The aggressiveness of DAVFs is primarily determined by the pattern of venous drainage with a higher hemorrhagic risk corresponding to retrograde leptomeningeal venous drainage. Borden et al.4 and Cognard et al.7 have offered 2 commonly used classification schemes to categorize fistula behavior largely based on the angiographic pattern of venous drainage.
Treatment options for DAVFs have included conservative management, radiosurgery, open surgery, and endovascular embolization, either alone or in combination. 19,34,41 Advancements in endovascular techniques have led to an increased use of this method to safely treat DAVFs using cyanoacrylate, ethyl alcohol, coils, and particles. Successful treatment requires occlusion of the venous recipient, as occlusion of the feeding arteries alone can lead to recruitment of other inputs and persistence of the fistula. For this reason, transvenous embolization has generally been favored in the past for intracranial DAVFs. However, in many cases, direct endovascular access to the draining vein is not possible, and transarterial embolization is necessary instead. In those circumstances, liquid embolic agents are preferred, and several endovascular techniques have been devised to enhance penetration into the draining vein.2
More recently, there has been an increasing number of endovascular embolizations performed using Onyx (ev3 Neurovascular), a novel ethylene vinyl alcohol copolymer preparation with low viscosity and delayed precipitation.30 While many authors have reported high curative rates, the long-term durability of such treatments remains uncertain as DAVF recurrence has been observed in patients after successful treatment.9,13,27 We report 3 cases of apparent cure of intracranial and spinal DAVFs that developed subsequent recanalization despite the documented presence of Onyx in the draining vein, and we review the literature for DAVF recurrence.
Case Reports
Case 1
This 63-year-old man presented with left homonymous hemianopia and hemiplegia due to spontaneous right occipital intracerebral hemorrhage with subarachnoid and intraventricular hemorrhages. Cerebral angiography revealed a midparietal Cognard Type IV DAVF fed by bilateral branches of the middle meningeal arteries, superficial temporal arteries, and occipital arteries. Venous drainage occurred primarily via a partially thrombosed ectatic cortical vein with multiple varices directed into the right transverse sinus (Fig. 1A). Partial thrombosis was noted in the superior sagittal sinus with abnormal delayed contrast filling (Fig. 1B). Embolization with Onyx-18 was performed via a posterior pedicle of the right middle meningeal artery, resulting in complete DAVF occlusion with permeation of Onyx into the draining vein as confirmed by angiography and subsequent head CT scanning (Fig. 1C and D). The patient was eventually able to return to home and remained clinically stable. However, additional angiography performed 8 months later demonstrated re-canalization of the DAVF fed by bilateral branches of the middle meningeal arteries, superficial temporal arteries, and occipital arteries (Fig. 1E). Partial thrombosis was still present in the superior sagittal sinus. Stage II Onyx embolization was performed via the anterior division of the right middle meningeal artery as well as the posterior division of the left middle meningeal artery. This resulted in complete obliteration of the fistula and draining vein once again with complete preservation of the transverse sinus (Fig. 1F). The patient has had no further symptoms and is awaiting surveillance angiographic follow-up.
Fig. 1. Case 1.
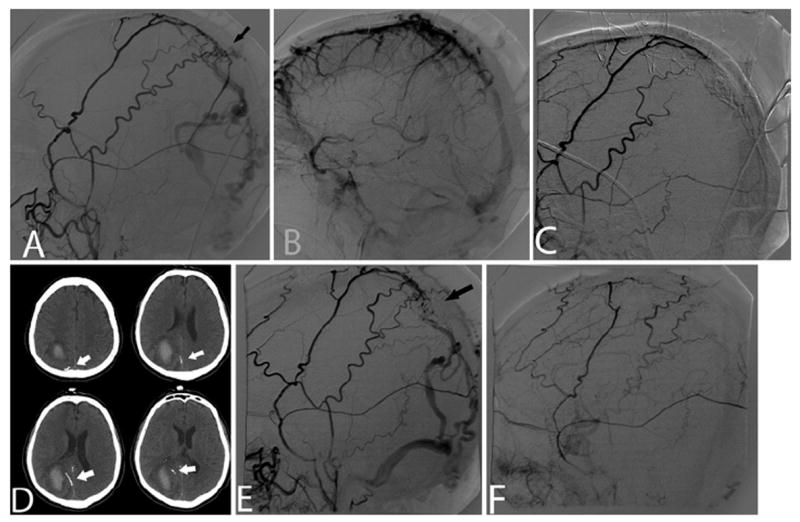
A: Right external carotid artery (ECA) angiogram (lateral projection) demonstrating a DAVF (arrow) supplied by branches of the right middle meningeal and superficial temporal arteries with venous drainage via an ectatic cortical vein with multiple varices. B: Right internal carotid artery angiogram (lateral projection) in venous phase demonstrating abnormal delayed filling of the superior sagittal sinus due to partial thrombosis. C: Right ECA angiogram (lateral projection) after transarterial Onyx embolization demonstrating complete DAVF occlusion with no evidence of early venous drainage. D: Multiple axial head CT slices obtained after embolization, revealing the presence of Onyx cast material (arrows) within the draining cortical vein. E: Repeat right ECA angiogram (lateral projection) demonstrating DAVF recurrence (arrow). F: Final right ECA angiogram (lateral projection) showing complete DAVF obliteration after repeat transarterial Onyx embolization.
Case 2
This 54-year-old woman presented with headache and tinnitus related to a DAVF in the left sphenoid wing. Cerebral angiography demonstrated that the DAVF was fed by the left middle meningeal artery with drainage via the superficial middle cerebral vein and vein of Labbé leading into the left transverse sinus (Fig. 2A). Embolization with Onyx-18 was performed 3 weeks later through a single pedicle in the left middle meningeal artery. Permeation of the Onyx into the fistula and an early component of the draining vein was noted with complete occlusion of the fistula on posttreatment angiography (Fig. 2B). The patient’s symptoms resolved immediately afterward, and she remained asymptomatic. However, follow-up MR angiography of the brain performed 1.5 years later suggested possible DAVF recurrence. Repeat angiography was subsequently performed, confirming the presence of the DAVF in the same location. The DAVF was again fed by the left middle meningeal artery with drainage via the same veins, distal to the previously placed embolic material (Fig. 2C). Repeat embolization was performed using N-butyl cyanoacrylate (nBCA) through a left middle meningeal pedicle with reduction of DAVF filling (Fig. 2D). Further angiography revealed alternate filling of the DAVF via small ethmoidal branches of the left ophthalmic artery (Fig. 2E). The remainder of the fistula was deemed unsuitable for embolization due to the significant risk of potentially incurring left vision loss. The patient remained asymptomatic and deferred additional treatment options.
Fig. 2. Case 2.
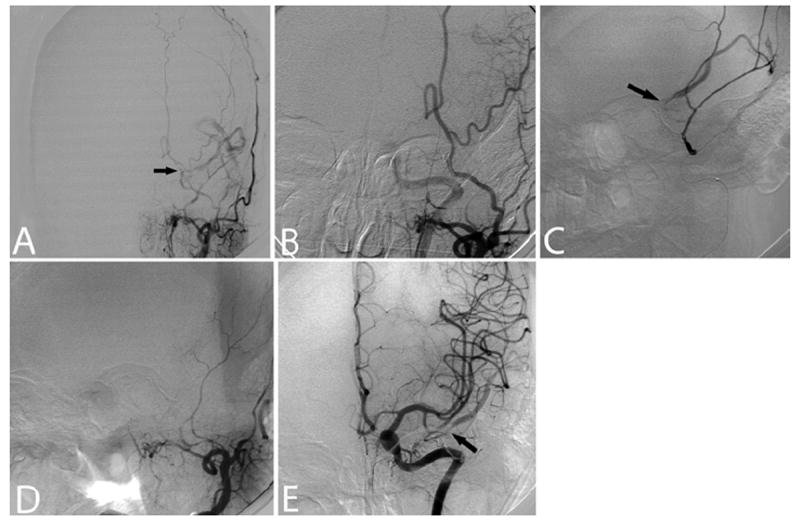
A: Left ECA angiogram (anteroposterior projection) demonstrating a DAVF (arrow) supplied by the left middle meningeal artery with venous drainage into the left superficial middle cerebral vein and vein of Labbé. B: Left ECA angiogram (anterior oblique projection) after transarterial Onyx embolization revealing occlusion of the fistula without early venous drainage. C: Selective microcatheter injection into a left middle meningeal pedicle revealing DAVF recanalization (arrow) with venous drainage into the left superficial middle cerebral vein. D: Left ECA angiogram (anterior oblique projection) obtained after repeat embolization, demonstrating DAVF occlusion. E: Left internal carotid artery angiogram (anteroposterior projection) revealing recruitment of new arterial feeders to the DAVF (arrow) via small ethmoidal branches of the left ophthalmic artery.
Case 3
This 68-year-old man presented with chronic progressive lower-extremity weakness and bladder dysfunction. Spinal angiography was notable for the presence of a spinal DAVF emanating from a radicular branch of the left L-3 segmental artery. Drainage of the fistula occurred superiorly up the spinal cord via a slow-flowing vein (Fig. 3A and B). Embolization of this fistula was performed using Onyx-18 with sufficient penetration and casting of Onyx into the fistula. Final angiography revealed no evidence of early venous drainage or filling of the fistula. The patient’s symptoms gradually improved, but repeat angiography performed 7 months later revealed DAVF recurrence. Catheterization of the L-2 lumbar segmental artery demonstrated filling of the fistula from collateralization into an L-3 radicular branch under the L-3 pedicle. Drainage of the fistula remained the same via a slow-flowing vein (Fig. 3C and D). Repeat embolization could not be performed due to the lack of microcatheter access to the fistulous site. Therefore, open neurosurgical ligation of the fistula was performed 2 months later. Exposure of the fistula during surgery established the continued presence of an Onyx cast into the proximal segment of the draining vein (Fig. 3E).
Fig. 3. Case 3.
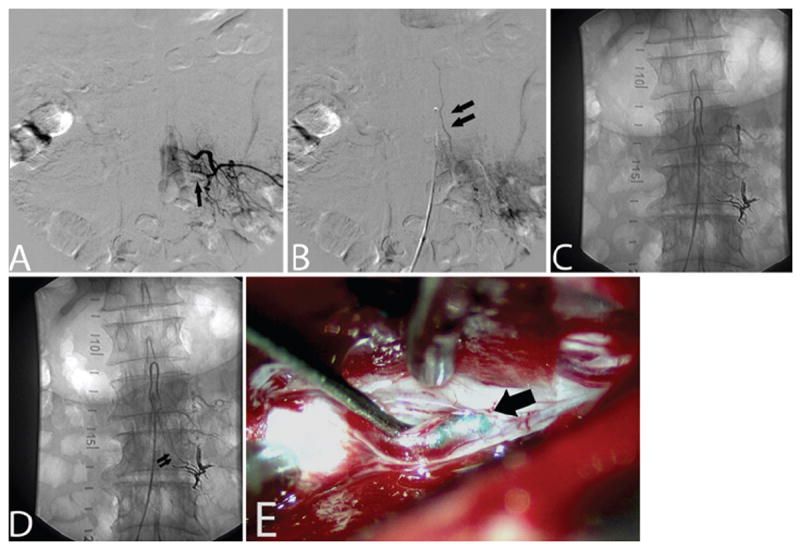
A and B: Left L-3 segmental artery injection on spinal angiography (anteroposterior projection) in early arterial (A) and late arterial (B) phases revealing a DAVF fed by a radicular branch at this level (single arrow) with drainage into a slow flowing vein (double arrows). C and D: Left L-2 segmental artery injection on spinal angiography (anteroposterior projection) in early arterial (C) and late arterial (D) phases, demonstrating DAVF recurrence from a collateralized radicular branch under the L-3 pedicle with similar drainage into a slow flowing vein (double arrows). E: Visual inspection during surgical ligation revealing the presence of Onyx cast material within the draining vein (arrow) of the fistula.
Discussion
Among the different DAVF treatment modalities, Onyx embolization has gained increasing acceptance as an effective therapeutic option. Onyx is a liquid embolic agent that offers increased control and deep fistula penetration due to its lower viscosity and delayed precipitation compared with other embolic agents. Endovascular treatment of DAVFs with Onyx is typically performed via a transarterial approach with the aim of obliterating the fistulous site to prohibit abnormal venous outflow. Upon occlusion of the fistulous site, elevated outflow resistance may permit preferential backfilling of Onyx into multiple feeding vessels from a single arterial embolic injection. This unique feature allows curative treatment of DAVFs and potential embolization of contributory vessels that may otherwise be too small or tortuous for individual catheterization.6 Adequate positioning of the microcatheter at, or immediately adjacent to, the fistula during injection is suggested as a determining factor for complete DAVF obliteration.5 Increasing experience with this technique has resulted in the successful treatment of many DAVFs primarily using Onyx, with reported occlusion rates ranging from 62.5% to 100%.8,20,25,27,40
While Onyx embolization remains a promising method of achieving DAVF occlusion, our cases demonstrate that recanalization may occur after a period of time, despite apparent filling of the venous elements. The durability of occlusion is presumably important in preventing symptom recurrence and hemorrhage. Recurrence of a DAVF after successful primary transarterial Onyx embolization is uncommonly reported but has been noted in a review of case series that include long-term angiography results (Table 1).1,3,5,6,8-10,13,14,20,22,25,27,28,31,35,40,43 To our knowledge, there are 3 such case series in the literature that have noted DAVF recurrence, but the true prevalence of this phenomenon remains uncertain as repeat angiographic results are not uniformly reported in the literature.9,13,27 Additionally, the time period for recurrence is unclear as the angiographic follow-up period in this review varies significantly from less than 1 month to 40 months.
TABLE 1.
Published case series with angiographic follow-up results of DAVFs treated with primary Onyx embolization*
| Authors & Year | Fistula Type | No. of Patients | Initial Cure Rate (%) | % Patients w/Follow-Up | Follow-Up Period (mos) | No. of Patients w/DAVF Recurrence |
|---|---|---|---|---|---|---|
| Abud et al., 2011 | DAVF | 42 | 81.8 | 95.2 | 6 | 0 |
| Amiridze & Darwish, 2009 | DAVF/CCF | 9 | 100 | 100 | 6 | 0 |
| Carlson et al., 2007 | DAVF | 6 | 83.3 | 100 | 3–9 | 0 |
| Chew et al., 2009 | DAVF | 12 | 75.0 | 75.0 | 0.2–12 | 0 |
| Cognard et al., 2008 | DAVF | 30 | 80.0 | 83.3 | 3 | 0 |
| De Keukeleire et al., 2011 | DAVF | 20 | 85.0 | 70.0 | 4–14 | 1 |
| Elhammady et al., 2010 | CCF | 12 | 100 | 50.0 | 4–35 | 0 |
| Hu et al., 2011 | DAVF | 50 | 82.0† | 92.0 | 3–25 | 5 |
| Huang et al., 2009 | DAVF | 14 | 85.7 | 57.1 | 3–12 | 0 |
| Lv et al., 2009 | DAVF | 40 | 62.5 | 72.5 | 3–8 | 0 |
| Maimon et al., 2011 | DAVF | 17 | 94.0 | 47.1 | 3–12 | 0 |
| Natarajan et al., 2010 | DAVF/CCF | 30 | 70.0 | 90.0 | 2–40 | 0 |
| Nogueira et al., 2008 | DAVF | 12 | 83.3 | 75.0 | 1–6 | 1 |
| Nogueira et al., 2009 | sDAVF | 3 | 100 | 100 | 10–15 | 0 |
| Saraf et al., 2010 | DAVF | 25 | 84.0 | 80.0 | 6 | 0 |
| Stiefel et al., 2009 | DAVF | 28 | 72.4 | 28.6 | 1–15 | 0 |
| van Rooj et al., 2010 | DAVF | 8 | 100 | 100 | 1.5–3 | 0 |
| Zenteno et al., 2010 | CCF | 5 | 80.0 | 80.0 | 6 | 0 |
CCF = carotid-cavernous fistula; sDAVF = spinal DAVF.
Reported cure rate at follow-up.
De Keukeleire et al.9 described a patient in their series who presented with a Cognard Type II DAVF and experienced fistula recurrence upon 6-month follow-up angiography despite symptomatic improvement. In all of our cases, the patients also demonstrated improvement despite DAVF recanalization, suggesting that clinical symptoms cannot be reliably used to discern for potential recurrence. Nogueira et al.27 described a patient with a Cognard Type IV DAVF who underwent successful transarterial Onyx embolization; however, the DAVF recurred 4.6 months later with interval recruitment of alternate feeders. This case is similar to our Case 2 in which the presence of new arterial feeders was observed via the ophthalmic artery subsequent to embolization. In the cases published in the literature, it is not clear whether occlusion of the venous recipient pouch was achieved during the initial embolization. In a large case series of 50 patients, Hu et al.13 noted that several patients developed new fistulous connections at other sinuses upon follow-up despite initial angiographic success. One of these patients initially underwent treatment of a DAVF draining into the left transverse-sigmoid sinus, but the patient was found to have a new DAVF draining into the confluence of sinuses with subsequent drainage into the right sigmoid sinus and superior sagittal sinus.
The appearance of a new DAVF distal to the site of the initial fistula was not found in our cases, and it is unknown whether this relates to the same underlying pathophysiology. The principal mechanism of DAVF recanalization remains elusive, and it is uncertain whether a DAVF recurs from development of a new fistula or from maturation of an angiographically occult fistula that already existed in the vasculature. One hypothesis purports that small arterial feeders are not initially opacified due to small-caliber flow limitation or from restricted outflow during the initial curative stage. During the time that elapses after treatment, these arterial feeders may enlarge with or without stimulation from endovascular treatment. 12,13 Furthermore, poor penetration of Onyx into the fistulous component and draining vein may slowly allow for recruitment of nonvisualized arterial supply into the low-pressure system.27
Empirical evidence suggests that the presence of a dense Onyx cast at the fistulous site is a determining factor in ensuring adequate penetration by angiography. However, no definitive angiographic methods are available to establish sufficient Onyx penetration beyond subjective visualizations of cast density with contrast stasis proximal to the occlusion. In all 3 reported cases, a permanent cast was noted at follow-up evaluation both radiographically, as well as upon visual inspection in Case 3 during surgical ligation. Despite sufficient Onyx injections, it has been suggested that DAVF recanalization may occur due to incomplete durability of the Onyx agent.13 In cerebral AVMs, recanalization was noted in 18% of resected specimens previously embolized with Onyx.24 Relating these findings to the treatment of DAVFs should also be taken with caution, as achievement of a durable preoperative AVM embolization is often a less crucial aim. The possible inadequacy of Onyx embolization is unlikely to be the principal reason for DAVF recanalization, as several cases have reported DAVF embolization using alternate embolization methods such as transvenous coiling. 16,17,22,23,42 Due to the low number of cases reporting DAVF recanalization, it is difficult to compare Onyx embolization with other forms of endovascular treatments.
Additional proposed hypotheses for DAVF recurrence have been extrapolated from the current understanding of initial DAVF pathogenesis, which also remains poorly established. Dural AVFs are generally thought to be acquired and are associated with several conditions including intracranial surgery, ear infection, tumor, hypercoagulability, puerperium, and trauma. A common predisposing factor appears to be the presence of venous sinus thrombosis or venous flow obstruction.11,18,32 One hypothesis postulates that venous flow obstruction promotes venous hypertension, which may initiate proliferation of microscopic vascular connections within the dura.39 Another hypothesis purports that these vascular channels may be already be naturally present as confirmed by radiographic and histological findings in normal individuals.15 Ultimately, the maturation of these channels results in the development of direct shunts between the arteries and dural veins.
It is possible that the continued presence of venous thrombosis in Case 1 may have contributed to promoting DAVF recurrence, but the presence of venous hypertension is suggested to be more critical to the development of fistulous channels than the actual finding of venous thrombosis.37 Because venous hypertension seems contributory to DAVF development, some have speculated that DAVF recurrence may occur through induction of venous hypertension from embolic occlusion at the fistulous site or potentially from injury and thrombosis in the sinus, especially during any transvenous microcatheter manipulation.12,16
Other hypotheses for fistula development have focused on inflammatory markers, maintaining that an elevated expression of inflammatory mediators such as cytokines from sinusitis or local trauma (including catheter intervention) may induce neovascularization.33 Additional theories have centered on the promotion of dural neovascularization by angiogenic factors. The release of angiogenic growth factors such as vascular endothelial growth factor and basic fibroblast growth factor may occur in response to hypoxia or increased venous pressure. This hypothesis has been validated in both histological and animal studies with excessive expression of these factors observed in the wall of DAVFs in humans.29,38 Embolization may mediate neovascularization as a result of local tissue hypoxia created from occlusion of the fistula. An increased expression of vascular endothelial growth factor has been noted in the endothelium of patients who underwent endovascular embolization for AVMs.36
Conclusions
Transarterial Onyx embolization is an emerging form of treatment for DAVFs, with impressive immediate posttreatment cure rates. However, the long-term durability of embolization may be overestimated, as cases of DAVF recanalization have been noted in studies with angiographic follow-up. The underlying mechanism for recurrence remains unclear, but hypothesized mediators include hemodynamic flow diversion with venous hypertension, as well as angiogenic factors from hypoxic microenvironments possibly initiated by catheter embolization. Predictors for recurrence are uncertain, although poor Onyx penetration into the fistula and draining vein is believed to be contributory. Further investigation is needed to discern additional features that make DAVF embolization more susceptible to recanalization. Although uncommon, the possibility for DAVF recanalization should also prompt consideration for performing follow-up angiography for all patients undergoing catheter embolization.
Abbreviations used in this paper
- AVM
arteriovenous malformation
- DAVF
dural arteriovenous fistula
Footnotes
Author contributions to the study and manuscript preparation include the following. Conception and design: Adamczyk, Amar. Acquisition of data: Adamczyk, Amar. Analysis and interpretation of data: Adamczyk, Amar. Drafting the article: Adamczyk. Critically revising the article: all authors. Reviewed submitted version of manuscript: Adamczyk.
Disclosure
Dr. Amar was the chair of the Clinical Events Committee for the Solitaire with Intention for Thombectomy (SWIFT) trial sponsored by ev3/Covidien. This was a multicenter randomized trial of a stroke device made by ev3/Covidien. He was compensated for the time he spent performing the adjudication of adverse events for submission of data to the FDA. ev3/Covidien also manufactures Onyx, one of the products mentioned in the manuscript, but that product is unrelated to his activities on the Clinical Events Committee.
References
- 1.Abud TG, Nguyen A, Saint-Maurice JP, Abud DG, Bresson D, Chiumarulo L, et al. The use of Onyx in different types of intracranial dural arteriovenous fistula. AJNR Am J Neuroradiol. 2011;32:2185–2191. doi: 10.3174/ajnr.A2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amar AP, Teitelbaum GP, Larsen DW. A novel technique and new grading scale for the embolization of cerebral vascular malformations. Neurosurgery. 2006;59(5 Suppl 3):S158–S162. S3–S13. doi: 10.1227/01.NEU.0000237518.36683.6A. [DOI] [PubMed] [Google Scholar]
- 3.Amiridze N, Darwish R. Hemodynamic instability during treatment of intracranial dural arteriovenous fistula and carotid cavernous fistula with Onyx: preliminary results and anesthesia considerations. J Neurointerv Surg. 2009;1:146–150. doi: 10.1136/jnis.2009.000042. [DOI] [PubMed] [Google Scholar]
- 4.Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995;82:166–179. doi: 10.3171/jns.1995.82.2.0166. [DOI] [PubMed] [Google Scholar]
- 5.Carlson AP, Taylor CL, Yonas H. Treatment of dural arteriovenous fistula using ethylene vinyl alcohol (Onyx) arterial embolization as the primary modality: short-term results. J Neurosurg. 2007;107:1120–1125. doi: 10.3171/JNS-07/12/1120. [DOI] [PubMed] [Google Scholar]
- 6.Chew J, Weill A, Guilbert F, Raymond J, Audet ME, Roy D. Arterial Onyx embolisation of intracranial DAVFs with cortical venous drainage. Can J Neurol Sci. 2009;36:168–175. doi: 10.1017/s0317167100006521. [DOI] [PubMed] [Google Scholar]
- 7.Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671–680. doi: 10.1148/radiology.194.3.7862961. [DOI] [PubMed] [Google Scholar]
- 8.Cognard C, Januel AC, Silva NA, Jr, Tall P. Endovascular treatment of intracranial dural arteriovenous fistulas with cortical venous drainage: new management using Onyx. AJNR Am J Neuroradiol. 2008;29:235–241. doi: 10.3174/ajnr.A0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Keukeleire K, Vanlangenhove P, Kalala Okito JP, Hallaert G, Van Roost D, Defreyne L. Transarterial embolization with ONYX for treatment of intracranial non-cavernous dural arteriovenous fistula with or without cortical venous reflux. J Neurointerv Surg. 2011;3:224–228. doi: 10.1136/jnis.2010.004119. [DOI] [PubMed] [Google Scholar]
- 10.Elhammady MS, Wolfe SQ, Farhat H, Moftakhar R, Aziz-Sultan MA. Onyx embolization of carotid-cavernous fistulas. Clinical article. J Neurosurg. 2010;112:589–594. doi: 10.3171/2009.6.JNS09132. [DOI] [PubMed] [Google Scholar]
- 11.Graeb DA, Dolman CL. Radiological and pathological aspects of dural arteriovenous fistulas. Case report. J Neurosurg. 1986;64:962–967. doi: 10.3171/jns.1986.64.6.0962. [DOI] [PubMed] [Google Scholar]
- 12.Hiu T, Horie N, Hayashi K, Kitagawa N, Morikawa M, Kawakubo J, et al. Recurrence of the cavernous sinus dural arteriovenous fistula at adjacent sinuses following repeated transvenous embolizations: case report and literature review. Radiat Med. 2008;26:431–437. doi: 10.1007/s11604-008-0245-8. [DOI] [PubMed] [Google Scholar]
- 13.Hu YC, Newman CB, Dashti SR, Albuquerque FC, McDougall CG. Cranial dural arteriovenous fistula: transarterial Onyx embolization experience and technical nuances. J Neurointerv Surg. 2011;3:5–13. doi: 10.1136/jnis.2010.003707. [DOI] [PubMed] [Google Scholar]
- 14.Huang Q, Xu Y, Hong B, Li Q, Zhao W, Liu J. Use of onyx in the management of tentorial dural arteriovenous fistulae. Neurosurgery. 2009;65:287–293. doi: 10.1227/01.NEU.0000348298.75128.D0. [DOI] [PubMed] [Google Scholar]
- 15.Kerber CW, Newton TH. The macro and microvasculature of the dura mater. Neuroradiology. 1973;6:175–179. doi: 10.1007/BF00335317. [DOI] [PubMed] [Google Scholar]
- 16.Kiyosue H, Tanoue S, Okahara M, Yamashita M, Nagatomi H, Mori H. Recurrence of dural arteriovenous fistula in another location after selective transvenous coil embolization: report of two cases. AJNR Am J Neuroradiol. 2002;23:689–692. [PMC free article] [PubMed] [Google Scholar]
- 17.Kubota Y, Ueda T, Kaku Y, Sakai N. Development of a dural arteriovenous fistula around the jugular valve after transvenous embolization of cavernous dural arteriovenous fistula. Surg Neurol. 1999;51:174–176. doi: 10.1016/s0090-3019(97)00420-5. [DOI] [PubMed] [Google Scholar]
- 18.Kwon BJ, Han MH, Kang HS, Chang KH. MR imaging findings of intracranial dural arteriovenous fistulas: relations with venous drainage patterns. AJNR Am J Neuroradiol. 2005;26:2500–2507. [PMC free article] [PubMed] [Google Scholar]
- 19.Luciani A, Houdart E, Mounayer C, Saint Maurice JP, Merland JJ. Spontaneous closure of dural arteriovenous fistulas: report of three cases and review of the literature. AJNR Am J Neuroradiol. 2001;22:992–996. [PMC free article] [PubMed] [Google Scholar]
- 20.Lv X, Jiang C, Zhang J, Li Y, Wu Z. Complications related to percutaneous transarterial embolization of intracranial dural arteriovenous fistulas in 40 patients. AJNR Am J Neuroradiol. 2009;30:462–468. doi: 10.3174/ajnr.A1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maimon S, Nossek E, Strauss I, Blumenthal D, Frolov V, Ram Z. Transarterial treatment with Onyx of intracranial dural arteriovenous fistula with cortical drainage in 17 patients. AJNR Am J Neuroradiol. 2011;32:2180–2184. doi: 10.3174/ajnr.A2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makiuchi T, Takasaki K, Yamagami M, Oda H, Todoroki K, Atsuchi M, et al. A case of sigmoid sinus dural arteriovenous fistula after treated cavernous dural arteriovenous fistula. Interv Neuroradiol. 1998;4(Suppl 1):219–222. doi: 10.1177/15910199980040S145. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa H, Kudo S, Nakajima Y, Izumoto S, Fujita T. Shifting of dural arteriovenous malformation from the cavernous sinus to the sigmoid sinus to the transverse sinus after transvenous embolization. A case of left spontaneous carotid-cavernous. sinus fistula Surg Neurol. 1992;37:30–38. doi: 10.1016/0090-3019(92)90062-r. [DOI] [PubMed] [Google Scholar]
- 24.Natarajan SK, Born D, Ghodke B, Britz GW, Sekhar LN. Histopathological changes in brain arteriovenous malformations after embolization using Onyx or N-butyl cyanoacrylate. Laboratory investigation. J Neurosurg. 2009;111:105–113. doi: 10.3171/2008.12.JNS08441. [DOI] [PubMed] [Google Scholar]
- 25.Natarajan SK, Ghodke B, Kim LJ, Hallam DK, Britz GW, Sekhar LN. Multimodality treatment of intracranial dural arteriovenous fistulas in the Onyx era: a single center experience. World Neurosurg. 2010;73:365–379. doi: 10.1016/j.wneu.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Newton TH, Cronqvist S. Involvement of dural arteries in intracranial arteriovenous malformations. Radiology. 1969;93:1071–1078. doi: 10.1148/93.5.1071. [DOI] [PubMed] [Google Scholar]
- 27.Nogueira RG, Dabus G, Rabinov JD, Eskey CJ, Ogilvy CS, Hirsch JA, et al. Preliminary experience with onyx embolization for the treatment of intracranial dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2008;29:91–97. doi: 10.3174/ajnr.A0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nogueira RG, Dabus G, Rabinov JD, Ogilvy CS, Hirsch JA, Pryor JC. Onyx embolization for the treatment of spinal dural arteriovenous fistulae: initial experience with long-term follow-up. Technical case report. Neurosurgery. 2009;64:E197–E198. doi: 10.1227/01.NEU.0000335157.90249.97. [DOI] [PubMed] [Google Scholar]
- 29.Rothbart D, Awad IA, Lee J, Kim J, Harbaugh R, Criscuolo GR. Expression of angiogenic factors and structural proteins in central nervous system vascular malformations. Neurosurgery. 1996;38:915–925. doi: 10.1097/00006123-199605000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Roy D, Raymond J. The role of transvenous embolization in the treatment of intracranial dural arteriovenous fistulas. Neurosurgery. 1997;40:1133–1144. doi: 10.1097/00006123-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Saraf R, Shrivastava M, Kumar N, Limaye U. Embolization of cranial dural arteriovenous fistulae with ONYX: indications, techniques, and outcomes. Indian J Radiol Imaging. 2010;20:26–33. doi: 10.4103/0971-3026.59748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarma D, ter Brugge K. Management of intracranial dural arteriovenous shunts in adults. Eur J Radiol. 2003;46:206–220. doi: 10.1016/s0720-048x(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 33.S Miyachi E, Izumi T, Matsubara N, Naito T, Haraguchi K, Wakabayashi T. Mechanism of the formation of dural arteriovenous fistula: the role of the emissary vein. Interv Neuroradiol. 2011;17:195–202. doi: 10.1177/159101991101700209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Söderman M, Edner G, Ericson K, Karlsson B, Rähn T, Ulfarsson E, et al. Gamma knife surgery for dural arteriovenous shunts: 25 years of experience. J Neurosurg. 2006;104:867–875. doi: 10.3171/jns.2006.104.6.867. [DOI] [PubMed] [Google Scholar]
- 35.Stiefel MF, Albuquerque FC, Park MS, Dashti SR, McDougall CG. Endovascular treatment of intracranial dural arteriovenous fistulae using Onyx: a case series. Neurosurgery. 2009;65(6 Suppl):132–140. doi: 10.1227/01.NEU.0000345949.41138.01. [DOI] [PubMed] [Google Scholar]
- 36.Sure U, Butz N, Schlegel J, Siegel AM, Wakat JP, Mennel HD, et al. Endothelial proliferation, neoangiogenesis, and potential de novo generation of cerebrovascular malformations. J Neurosurg. 2001;94:972–977. doi: 10.3171/jns.2001.94.6.0972. [DOI] [PubMed] [Google Scholar]
- 37.Terada T, Higashida RT, Halbach VV, Dowd CF, Tsuura M, Komai N, et al. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg. 1994;80:884–889. doi: 10.3171/jns.1994.80.5.0884. [DOI] [PubMed] [Google Scholar]
- 38.Uranishi R, Nakase H, Sakaki T. Expression of angiogenic growth factors in dural arteriovenous fistula. J Neurosurg. 1999;91:781–786. doi: 10.3171/jns.1999.91.5.0781. [DOI] [PubMed] [Google Scholar]
- 39.van Dijk JM, Willinsky RA. Venous congestive encephalopathy related to cranial dural arteriovenous fistulas. Neuroimaging Clin N Am. 2003;13:55–72. doi: 10.1016/s1052-5149(02)00063-1. [DOI] [PubMed] [Google Scholar]
- 40.van Rooij WJ, Sluzewski M. Curative embolization with Onyx of dural arteriovenous fistulas with cortical venous drainage. AJNR Am J Neuroradiol. 2010;31:1516–1520. doi: 10.3174/ajnr.A2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Rooij WJ, Sluzewski M, Beute GN. Dural arteriovenous fistulas with cortical venous drainage: incidence, clinical presentation, and treatment. AJNR Am J Neuroradiol. 2007;28:651–655. [PMC free article] [PubMed] [Google Scholar]
- 42.Yamashita K, Taki W, Nakahara I, Nishi S, Sadato A, Kikuchi H. Development of sigmoid dural arteriovenous fistulas after transvenous embolization of cavernous dural arteriovenous fistulas. AJNR Am J Neuroradiol. 1993;14:1106–1108. [PMC free article] [PubMed] [Google Scholar]
- 43.Zenteno M, Santos-Franco J, Rodríguez-Parra V, Balderrama J, Aburto-Murrieta Y, Vega-Montesinos S, et al. Management of direct carotid-cavernous sinus fistulas with the use of ethylene-vinyl alcohol (Onyx) only: preliminary results. Clinical article. J Neurosurg. 2010;112:595–602. doi: 10.3171/2009.6.JNS09440. [DOI] [PubMed] [Google Scholar]


