Abstract
Background
Approximately 10–25% of patients treated with BRAF inhibitors develop cutaneous squamous cell carcinoma (SCC), but the mechanism responsible has not yet been determined. We report the first case in which Merkel cell polyomavirus (MCPyV) and HPV-17 are associated with cutaneous SCC which developed during treatment with BRAF inhibitor GSK2118436.
Observations
A 62 year-old woman with V600E BRAF-mutant metastatic melanoma enrolled on a phase I trial of GSK2118436, a selective inhibitor of V600-mutant BRAF kinase. During the first six weeks of treatment, the patient developed multiple skin lesions, including a 6-mm crusted papule on the left eyebrow, which was resected and revealed SCC. DNA extracted from paraffin-embedded tissue was amplified by polymerase chain reaction (PCR) for detection of MCPyV and epidermodysplasia verruciformis HPV (EV-HPV) types. Analysis of the cloned and sequenced PCR products revealed the presence of MCPyV and HPV-17 DNA. Other EV-HPV subtypes were not detected.
Conclusions
To our knowledge, this is the first report demonstrating the co-existence of MCPyV and HPV-17 in cutaneous SCC. Because both viruses have known oncogenic potential, the role of these viruses in the etiology of BRAF inhibitor-related SCC merits further investigation.
INTRODUCTION
BRAF inhibition is a promising therapeutic strategy for the treatment of patients with BRAF-mutant malignancies. Clinical trials of tyrosine kinase inhibitors that are highly selective for V600 BRAF mutations have demonstrated high response rates (50–80%) at therapeutic doses, as well as improvement in overall and progression-free survival.1–3 Approximately 10 – 30% of patients who are treated with BRAF inhibitors develop squamous cell carcinoma (SCC) of the skin.1–3 A prevailing hypothesis suggests that compensatory CRAF activation in the setting of BRAF inhibition and in the presence of a RAS mutation plays a role in the etiology of these squamous cell tumors, but the pathophysiology of these tumors is not definitively elucidated.4–9
Several viruses are known to be associated with the development of malignancies, including human papilloma virus (HPV), human herpes virus (HHV), Epstein-Barr virus (EBV), hepatitis B virus, hepatitis C virus, and human T-lymphotropic virus 1 (HTLV-1).10, 11 Merkel cell polyomavirus (MCPyV) was first described in 2008 and is present in approximately 80% of Merkel cell carcinoma tumors, which is a primary neuroendocrine tumor of the skin.12, 13 In addition, MCPyV is found in approximately 13–25% of cutaneous squamous cell carcinomas among immunocompetent individuals.14–16 HPV infection, usually subtypes 16 and 18, are associated with squamous cell carcinomas of the oropharynx, cervix, penis, anus, and skin.17–21 SCC of the skin associated with HPV-17 has been reported in epidermodysplasia verruciformis, a disorder in which flat wart-like liesions frequently evolve to squamous cell carcinoma, and HPV-17 is present in approximately 22% of cutaneous squamous cell carcinomas.22–24 Coexistence of HPV-17 and MCPyV has not been previously reported. We report a case in which coexisting MCPyV and HPV-17 were found in a cutaneous SCC that developed in a patient with melanoma during treatment with BRAF inhibitor GSK2118436.
REPORT OF CASE
A 62 year-old woman with V600E BRAF-mutant melanoma metastatic to the lungs and in-transit lesions involving the left thigh and lower leg enrolled on a phase I trial of GSK2118436 (200 mg twice daily), which is a selective inhibitor of V600 mutant BRAF kinase.2 During the first three weeks, the patient developed a mildly pruritic grade 2 maculopapular, folliculitis-like rash involving the face, scalp, chest, abdomen and axillary area. The rash improved after treatment with oral diphenhydramine and methylprednisolone. The patient also developed keratoderma on the soles of her feet bilaterally during this time period, which worsened during weeks four to six, and was treated with topical 20% carbamide cream.
Evaluation at the end of six weeks demonstrated a number of new skin lesions not present at the baseline skin examination, including a 6-mm crusted papule on the left eyebrow in the midline approximately 2 cm superior from the lid margin (Figure 1), a 4-mm crusted hyperkeratotic papule on the right scalp vertex, a 3-mm crusted papule on the anterior neck, several scattered pedunculated papules on the neck consistent with acrochordons, and many 2-3-mm pink hyperkeratotic papules scattered on the chest, back, upper and lower extremities. The lesions on the scalp vertex, left lateral arm, and left eyebrow were biopsied and reviewed by a U.T. MD Anderson Cancer Center dermatopathologist (VGP). The right scalp vertex biopsy contained verruca vulgaris. The left lateral arm biopsy contained hyperplastic actinic keratosis. Dermatopathology evaluation of the left eyebrow lesion demonstrated invasive squamous cell carcinoma, moderately differentiated, at least 3.3 mm in thickness, and present at the deep tissue edge (Figure 2). Perineural invasion was not identified. Due to the positive margin on the left eyebrow squamous cell carcinoma biopsy, the patient subsequently underwent Mohs surgery. Histologic tumor clearance was obtained after one stage and two sections.
Figure 1.
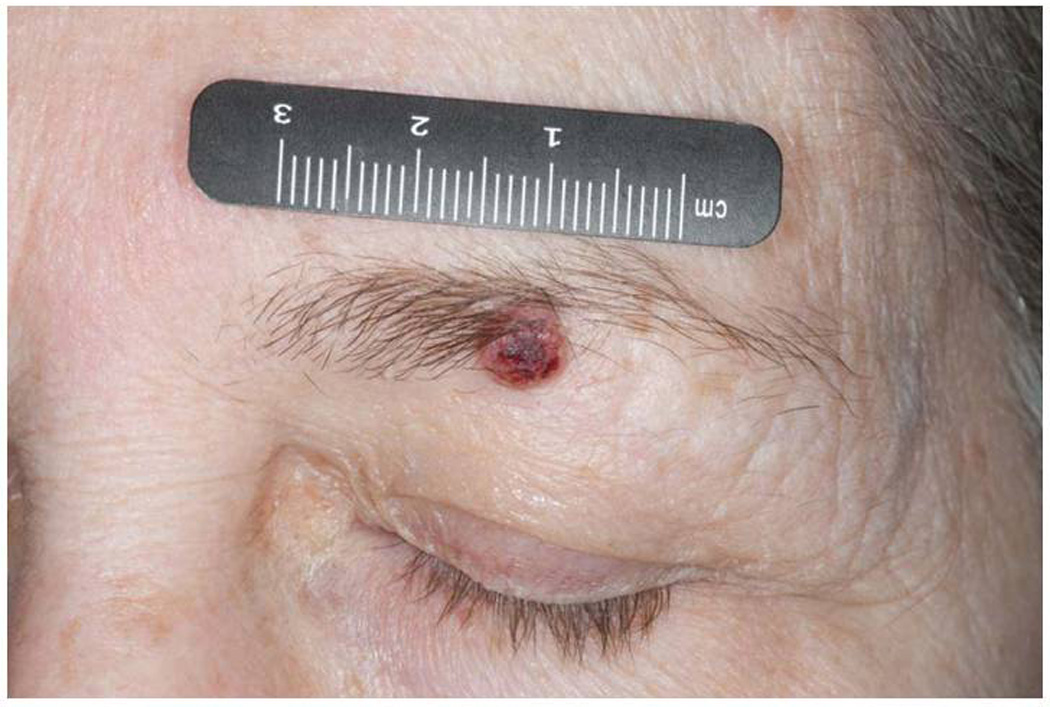
Left eyebrow squamous cell carcinoma before resection
Figure 2.
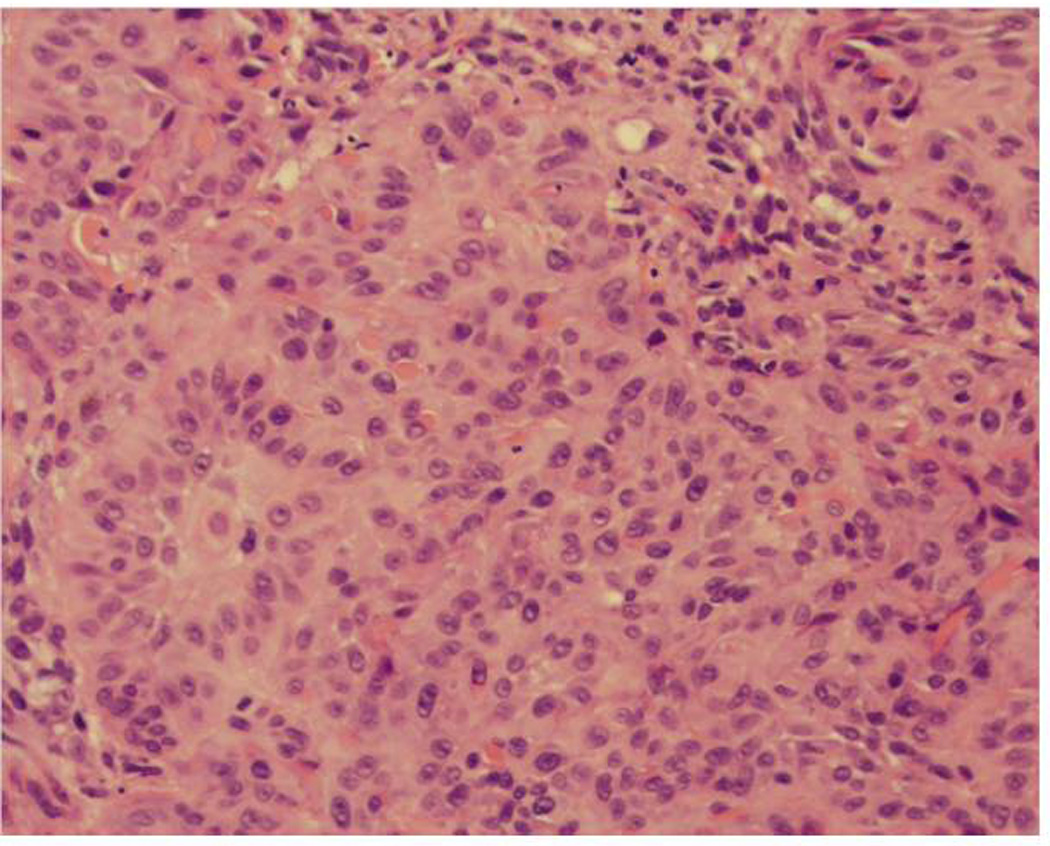
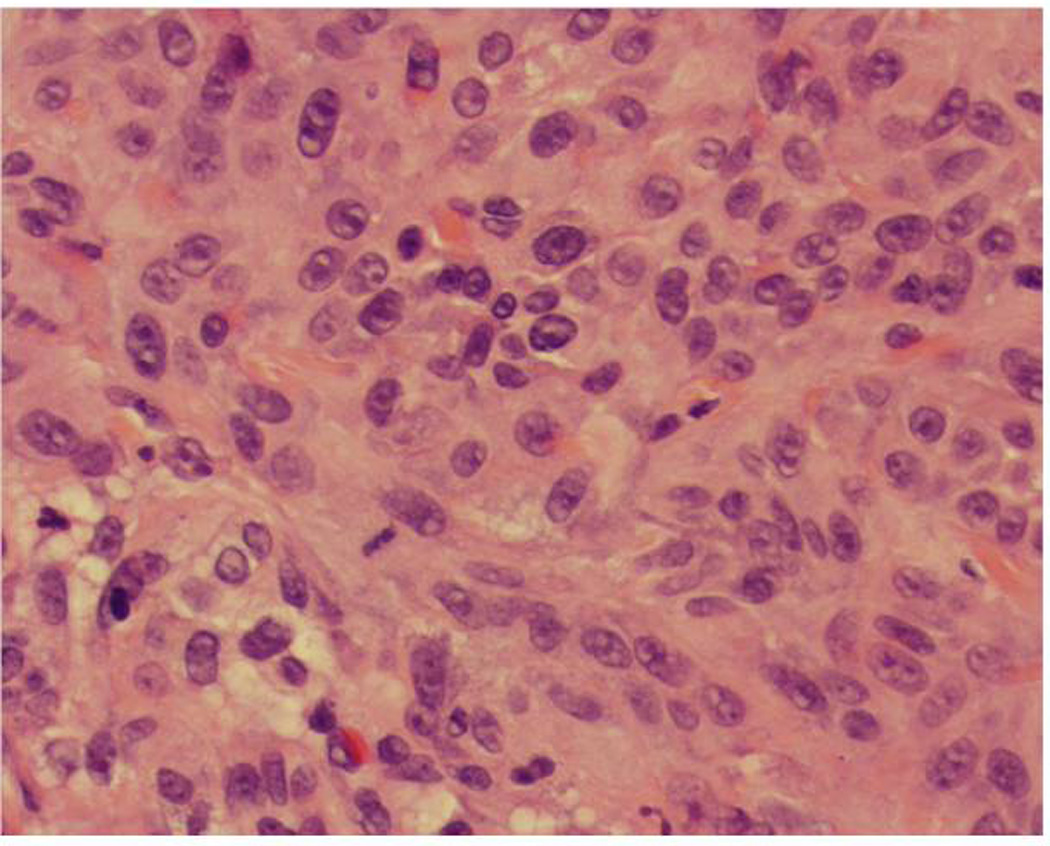
Invasive squamous cell carcinoma, moderately differentiated, 3.3 mm in thickness
(A) Marked cytologic atypia at the interface between the tumor and the dermis (×20)
(B) Notice the presence of large nuclei, prominent nucleoli and mitotic figures (×40)
The patient’s multiple hypertrophic actinic keratoses and verruca vulgaris lesions were treated with cryotherapy. At the end of cycle 5 (each cycle being three weeks), a biopsy of a right forearm lesion revealed verruca vulgaris with focal cytologic atypia of keratinocytes, and a biopsy of a right temple lesion demonstrated actinic keratosis, present at tissue edges, with focal cytologic atypia of keratinocytes. To date, the patient’s melanoma lesions have decreased in size by 22% by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1.25 The patient has completed 22 cycles (66 weeks) and remains on treatment with GSK2118436 at the time this report was compiled.
RESULTS
This study and treatment were performed in accordance with the guidelines of the U.T. MD Anderson Cancer Center Institutional Review Board. The biopsied eyebrow lesion was reviewed by a U.T. MD Anderson Cancer Center dermatopathologist (VGP). DNA was extracted from two paraffin-embedded tissue biopsies (SCC of the left eyebrow and actinic keratosis of the left arm) using Gentra Puregene Genomic DNA Purification Kit (Qiagen Inc, Valencia, CA). DNA quality was assessed by beta-globin reference gene polymerase chain reaction (PCR).26
HPV testing by polymerase chain reaction (PCR) and sequencing
HPV-DNA was amplified by PCR utilizing a nested primer system designed for detection of epidermodysplasia verruciformis HPV (EV-HPV) types, including HPV-5b, -8, -9, -12, -14a, -15, -17, -19, -20, -21, -22, -23, -24, -36, -37, -38, -46, and -49.27 EV-HPV includes the HPV subtypes that are associated with epidermodysplasia verruciformis.23 Isolation, cloning and sequencing of putative HPV-PCR products were carried out as described earlier.28 Briefly, the obtained putative HPV sequence information were aligned and compared to known HPV types available through the GenBank database using the BLAST program (National Center for Biotechnology Information, National Institutes of Health, Bethesda, MD). The PCR mixtures and products were analyzed on 2.0% agarose gel electrophoresis and visualized on a UV transilluminator.
NCBI-BLAST analysis of the cloned and sequenced HPV-PCR product determined the SCC sample had a single infection of HPV-17 (Figure 3, Panels A and B). Other EV-HPV subtypes (HPV-5b, -8, -9, -12, -14a, -15, -17, -19, -20, -21, -22, -23, -24, -36, -37, -38, -46, and -49) were not detected. HPV-17, a beta-2-HPV, has been associated with development of SCC in epidermodysplasia verruciformis (EV).29 No EV-HPV was detected in the actinic keratosis lesion.
Figure 3.

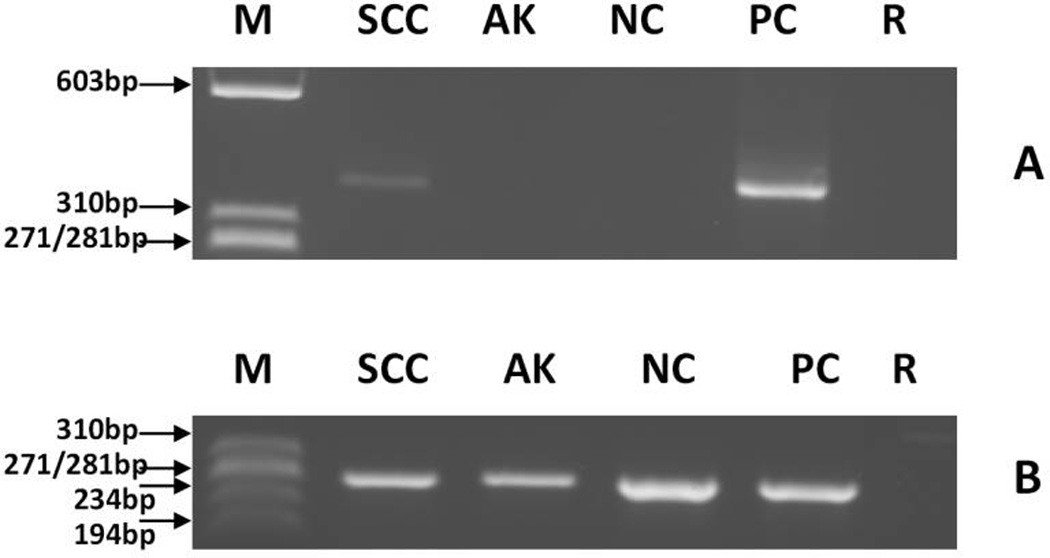
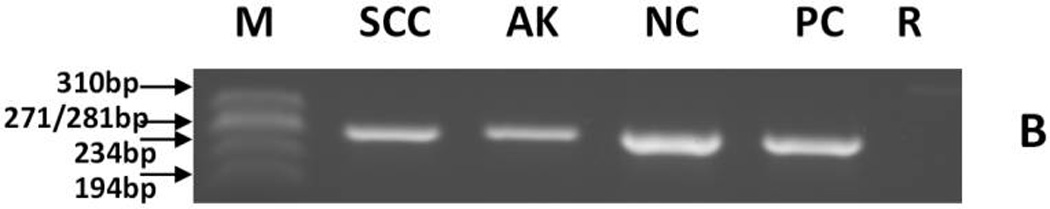
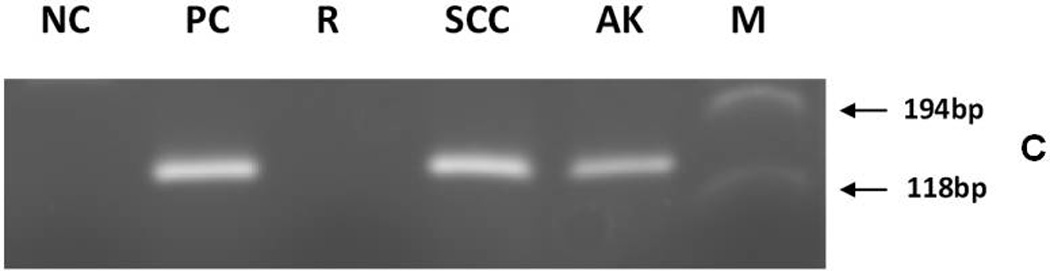
Detection of EV-HPV- and β-globin reference gene-DNA and MCPyV DNA by PCR in skin squamous cell carcinoma from a melanoma patient treated with a BRAF inhibitor.
(A) EV-HPV detection. Putative EV-HPV PCR products can be seen in lanes SCC and PC.
(B) β-globin detection. β-globin PCR fragments can be seen in lanes SCC, AK, NC, and PC.
(C) MCPyV detection. PCR products can be seen in lanes SCC, AK, and PC.
MCPyV detection by PCR and sequencing
PCR was utilized for MCPyV detection. The assay was performed twice on tissue acquired from different tissue blocks of the left eyebrow lesion. The forward PCR primer sequence was 5’GCGCTTGTATTAGCTGTAAGTTGT3’, the reverse primer sequence was 5’ACCAGTCAAAACTTTCCCAAGTAG3’. These primers were derived from small T antigen viral gene region of MCPyV and were expected to generate a 150bp MCPyV-PCR product.12 The PCR steps were 1min at 94C°, followed by 40 cycles each 94 C° for 1min, 63 C° for 1 min and 72° C for 1 min, and final extension of 10 min at 72 C°. The PCR products were run on 2.0% agarose gel electrophoresis and visualized on a UV transilluminator. The obtained MCPyV-PCR fragment was excised from agarose gel, cloned (TOPO TA cloning kit for sequencing, Invitrogen Co. Carlsbad, CA) and sequenced. The obtained DNA sequences were subjected to computer assisted alignment by NCBI-BLAST program using sequence information from NCBI-GenBank.
Expected 150 bp sized putative MCPyV PCR fragments were generated in the SCC and actinic keratoses samples (Figure 3, Panel C). The cloning and sequencing of PCR products as well as the computer analysis of the obtained sequencing data confirmed the presence of MCPyV DNA in these samples. Repeat testing at a different time point and performed on a second distinct piece of the SCC sample again demonstrated the presence of MCPyV DNA.
COMMENT
BRAF inhibitors have demonstrated significant antitumor activity in clinical trials and are potential candidates for approval by the Food and Drug Adminstration (FDA).1–3 Therefore, further investigation is needed to determine why 10–30% of patients receiving these treatments develop cutaneous SCC.1–3 One hypothesis suggests that, in BRAF wild-type cells, binding of the BRAF inhibitor to BRAF induces RAS-dependent BRAF/CRAF dimerization, resulting in CRAF activation.4–9
In addition to SCC, a spectrum of keratinizing squamous cell neoplasms has been observed during treatment with BRAF inhibitors, including ketatoacanthomas, actinic keratoses, and verruca vulgaris, and many of these lesions demonstrate focal cytologic atypia of keratinocytes.1–3 Indeed our patient developed actinic keratosis and verruca vulgaris in addition to the SCC of the skin. Similar findings have been reported among patients receiving sorafenib, a multi-kinase inhibitor that inhibits intracellular RAF kinases (CRAF, BRAF, mutant BRAF) and cell surface kinase receptors (VEGFR-2, VEGFR-3, PDGFR-beta, cKIT, and FLT-3).30–32
We report the first case of HPV-17 and MCPyV co-infection associated with SCC of the skin in a patient with melanoma treated with a BRAF inhibitor. It is conceivable that some cells have some degree of transformation due to HPV or MCPyV, and that the transformation processes are progressed and/or accelerated by paradoxical CRAF activation.33 Another possibility is that viral reactivation caused by BRAF inhibition occurs. Indeed, it is known that beta-HPVs occur in normal skin, including HPV-17 in 13–31% of normal eyebrow hair samples, and that MCPyV is found in 0–17% of normal skin tissue samples.12, 16, 34–36 Of interest in this regard, a recent large study demonstrated a correlation between genus β human papillomavirus infection (including HPV-17) and the incidence of squamous cell carcinoma of the skin in the general population, as well as potential enhancement of risk by immunosuppression.37 Furthermore, the eruption of verruca vulgaris, also known to be associated with HPV, in this BRAF inhibitor-treated patient further supports a viral role for the simultaneous squamous cell carcinoma of the skin. MCPyV has also been frequently detected in SCCs, from both immunocompetent and immunocompromised patients.14, 16 In the general population, ultraviolet radiation is the major risk factor for squamous cell carcinoma of the skin, and HPV may be a co-factor, working by disturbing cellular DNA repair and/or apoptosis in sun-exposed keratinocytes.33 Preclinical models show that activation of the MAPK pathway by RAF inhibitors requires concurrent RAS activation, and one potential cause of RAS activation is occult viral infection of keratinocytes.8 Therefore, BRAF inhibitor-mediated CRAF activation could potentially accelerate transformation of previously infected cells. Preliminary results of a trial combining BRAF inhibitor GSK2118436 with MEK inhibitor GSK1120212 demonstrated a markedly lower incidence of cutaneous squamous cell carcinoma (<1%) and other hyperproliferative skin lesions, further supporting a role for activated CRAF in the development of cutaneous squamous cell carcinoma.38
If further studies demonstrate evidence of a viral role in such patients, then therapeutic strategies to prevent cell transformation could be investigated. However, since existing vaccines such as Cervarix and Gardasil do not cross-react with HPV-17 and do not eliminate HPV in patients with latent infections, it seems unlikely that a vaccination strategy with these vaccines would be effective.
In conclusion, our study demonstrates MCPyV in an actinic keratosis, and co-existing HPV-17 and MCPyV in a cutaneous squamous cell carcinoma, developing within three weeks after starting a BRAF inhibitor in a patient in whom verruca vulgaris also simultaneously appeared. To our knowledge, this is the first report demonstrating the co-existence of these viruses in a these lesions, and taken together with their potentially oncogenic nature, suggests that their role in the etiology of BRAF related SCC merits further investigation. Still, the active role of MCPyV in SCC needs to be proven by study of expression of the potential viral oncoproteins, specifically T antigens.39 In addition, larger quantitative viral copy number determination studies are needed to better evaluate the role of HPV-17 and other beta HPVs, as well as MCPyV, in squamous cell carcinoma of the skin that develops during treatment with a BRAF inhibitor, and to determine definitively if these viruses play a driver rather than a passenger role.
Acknowledgment
We are indebted to Dr. Samuel Blackman and Dr. Vicki Goodman of GlaxoSmithKline for their comments on this manuscript, and Sarah Nester of Rice University for her assistance with the HPV testing.
We would like to thank Dr. Samuel Blackman and Dr. Vicki Goodman of GlaxoSmithKline for their comments on this manuscript, and Sarah Nester of Rice University for her assistance with the HPV testing.
Abbreviations
- AK
Actinic keratosis
- M
ΦX174RF DNA marker (Promega Co.)
- NC
viral negative control was HPV-negative DNA (panels A and B) or MCPyV-negative DNA (panel C) extracted from PBMC (Promega Co.)
- PC
viral positive control was EV-HPV DNA (panels A and B) or MCPyV positive DNA (plasmid with MCPyV DNA insert derived from the small T viral gene) (panel C)
- R
Reagent control
- SCC
Squamous cell carcinoma
Footnotes
Author Contributions:
Dr (s) Falchook, Tyring, Hong, and Kurzrock, and Mr. Nguyen had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dr (s) Falchook, Hymes, Tyring, and Kurzrock.
Acquisition of data: Dr (s) Falchook, Rady, Hymes, Tyring, Prieto, and Hong, and Mr. Nguyen.
Analysis and interpretation of data: Dr (s) Falchook, Rady, Tyring, and Prieto.
Drafting of the manuscript: Dr (s) Falchook and Rady.
Critical revision of the manuscript for important intellectual content: Dr (s) Falchook, Hymes, Tyring, Prieto, Hong, and Kurzrock and Mr. Nguyen.
Statistical analysis: Dr. Falchook.
Obtained funding: None.
Administrative, technical, or material support: Dr (s) Rady and Tyring, and Mr. Nguyen.
Study supervision: Dr (s) Falchook, Hymes, Tyring, and Kurzrock.
- Relationships relevant to this manuscript
- All other relationships
Funding /Support: None.
Role of the Sponsors: The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; or in the preparation, review, or approval of the manuscript.
Financial Disclosure:
Dr. Falchook and Dr. Kurzrock received grant research support from GlaxoSmithKline. Dr. Falchook also received travel reimbrusement from GlaxoSmithKline for ESMO 2010.
- Employment - none
- Consultancies - none
- Honoraria – Travel reimbursement from GlaxoSmithKline for oral presentation at ESMO 2010.
- Speakers bureau - none
- Stock ownership or options - none
- Expert testimony - none
- Grants – Dr. Kurzrock received grant research support from GlaxoSmithKline. Dr. Falchook received grant research support from GlaxoSmithKline.
- Patents filed, received, pending, or in preparation - none
- Royalties - none
Contributor Information
Gerald S. Falchook, Email: gfalchoo@mdanderson.org.
Peter Rady, Email: Peter.Rady@uth.tmc.edu.
Sharon Hymes, Email: srhymes@mdanderson.org.
Harrison P. Nguyen, Email: harrison.p.nguyen@gmail.com, harrison.nguyen@rice.edu.
Stephen K. Tyring, Email: stephen.k.tyring@uth.tmc.edu.
Victor G. Prieto, Email: vprieto@mdanderson.org.
David S. Hong, Email: dshong@mdanderson.org.
Razelle Kurzrock, Email: rkurzroc@mdanderson.org.
REFERENCES
- 1.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010 Aug;26363(9):809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kefford R, Arkenau H, Brown M, et al. Phase I/II study of GSK2118436, a selective inhibitor of oncogenic mutant BRAF kinase, in patients with metastatic melanoma and other solid tumors. J Clin Oncol, 2010 ASCO Annual Meeting Proceedings. 2010;28(15S):611s. [Google Scholar]
- 3.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011 Jun 30;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012 Jan 19;366(3):207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010 Mar 18;464(7287):427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010 Mar 18;464(7287):431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 7.Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010 Jan 22;140(2):209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robert C, Arnault JP, Mateus C. RAF inhibition and induction of cutaneous squamous cell carcinoma. Curr Opin Oncol. 2011 Mar;23(2):177–182. doi: 10.1097/CCO.0b013e3283436e8c. [DOI] [PubMed] [Google Scholar]
- 9.Cichowski K, Janne PA. Drug discovery: inhibitors that activate. Nature. 2010 Mar 18;464(7287):358–359. doi: 10.1038/464358a. [DOI] [PubMed] [Google Scholar]
- 10.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006 Jun 15;118(12):3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 11.Thompson MP, Kurzrock R. Epstein-Barr virus and cancer. Clin Cancer Res. 2004 Feb 1;10(3):803–821. doi: 10.1158/1078-0432.ccr-0670-3. [DOI] [PubMed] [Google Scholar]
- 12.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008 Feb 22;319(5866):1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Group RMCC. Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis, and clinical management. J Clin Oncol. 2009 Aug 20;27(24):4021–4026. doi: 10.1200/JCO.2009.22.6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dworkin AM, Tseng SY, Allain DC, Iwenofu OH, Peters SB, Toland AE. Merkel cell polyomavirus in cutaneous squamous cell carcinoma of immunocompetent individuals. J Invest Dermatol. 2009 Dec;129(12):2868–2874. doi: 10.1038/jid.2009.183. [DOI] [PubMed] [Google Scholar]
- 15.Murakami M, Imajoh M, Ikawa T, et al. Presence of Merkel cell polyomavirus in Japanese cutaneous squamous cell carcinoma. J Clin Virol. 2011 Jan;50(1):37–41. doi: 10.1016/j.jcv.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Kassem A, Technau K, Kurz AK, et al. Merkel cell polyomavirus sequences are frequently detected in nonmelanoma skin cancer of immunosuppressed patients. Int J Cancer. 2009 Jul 15;125(2):356–361. doi: 10.1002/ijc.24323. [DOI] [PubMed] [Google Scholar]
- 17.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007 May 10;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 18.Bosch FX, Manos MM, Munoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995 Jun 7;87(11):796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 19.Varma VA, Sanchez-Lanier M, Unger ER, et al. Association of human papillomavirus with penile carcinoma: a study using polymerase chain reaction and in situ hybridization. Hum Pathol. 1991 Sep;22(9):908–913. doi: 10.1016/0046-8177(91)90181-n. [DOI] [PubMed] [Google Scholar]
- 20.Palefsky JM, Holly EA, Ralston ML, Jay N. Prevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus (HIV)-positive and HIV-negative homosexual men. J Infect Dis. 1998 Feb;177(2):361–367. doi: 10.1086/514194. [DOI] [PubMed] [Google Scholar]
- 21.Karagas MR, Nelson HH, Sehr P, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006 Mar 15;98(6):389–395. doi: 10.1093/jnci/djj092. [DOI] [PubMed] [Google Scholar]
- 22.Yutsudo M, Shimakage T, Hakura A. Human papillomavirus type 17 DNA in skin carcinoma tissue of a patient with epidermodysplasia verruciformis. Virology. 1985 Jul 15;144(1):295–298. doi: 10.1016/0042-6822(85)90328-9. [DOI] [PubMed] [Google Scholar]
- 23.Nindl I, Gottschling M, Stockfleth E. Human papillomaviruses and non-melanoma skin cancer: basic virology and clinical manifestations. Dis Markers. 2007;23(4):247–259. doi: 10.1155/2007/942650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rollison DE, Pawlita M, Giuliano AR, et al. Measures of cutaneous human papillomavirus infection in normal tissues as biomarkers of HPV in corresponding nonmelanoma skin cancers. Int J Cancer. 2008 Nov 15;123(10):2337–2342. doi: 10.1002/ijc.23795. [DOI] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009 Jan;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Resnick RM, Cornelissen MT, Wright DK, et al. Detection and typing of human papillomavirus in archival cervical cancer specimens by DNA amplification with consensus primers. J Natl Cancer Inst. 1990 Sep 19;82(18):1477–1484. doi: 10.1093/jnci/82.18.1477. [DOI] [PubMed] [Google Scholar]
- 27.Berkhout RJ, Tieben LM, Smits HL, Bavinck JN, Vermeer BJ, ter Schegget J. Nested PCR approach for detection and typing of epidermodysplasia verruciformis-associated human papillomavirus types in cutaneous cancers from renal transplant recipients. J Clin Microbiol. 1995 Mar;33(3):690–695. doi: 10.1128/jcm.33.3.690-695.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Oliveira WR, He Q, Rady PL, et al. HPV typing in Brazilian patients with epidermodysplasia verruciformis: high prevalence of EV-HPV 25. J Cutan Med Surg. 2004 Mar-Apr;8(2):110–115. doi: 10.1007/s10227-003-0100-6. [DOI] [PubMed] [Google Scholar]
- 29.Yutsudo M, Hakura A. Human papillomavirus type 17 transcripts expressed in skin carcinoma tissue of a patient with epidermodysplasia verruciformis. Int J Cancer. 1987 May 15;39(5):586–589. doi: 10.1002/ijc.2910390507. [DOI] [PubMed] [Google Scholar]
- 30.Hong DS, Reddy SB, Prieto VG, et al. Multiple squamous cell carcinomas of the skin after therapy with sorafenib combined with tipifarnib. Arch Dermatol. 2008 Jun;144(6):779–782. doi: 10.1001/archderm.144.6.779. [DOI] [PubMed] [Google Scholar]
- 31.Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009 Jan;7(1):20–23. doi: 10.3816/CGC.2009.n.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnault JP, Wechsler J, Escudier B, et al. Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib. J Clin Oncol. 2009 Aug 10;27(23):e59–e61. doi: 10.1200/JCO.2009.23.4823. [DOI] [PubMed] [Google Scholar]
- 33.Rust A, McGovern RM, Gostout BS, Persing DH, Pittelkow MR. Human papillomavirus in cutaneous squamous cell carcinoma and cervix of a patient with psoriasis and extensive ultraviolet radiation exposure. J Am Acad Dermatol. 2001 Apr;44(4):681–686. doi: 10.1067/mjd.2001.112359. [DOI] [PubMed] [Google Scholar]
- 34.de Koning MN, Weissenborn SJ, Abeni D, et al. Prevalence and associated factors of betapapillomavirus infections in individuals without cutaneous squamous cell carcinoma. J Gen Virol. 2009 Jul;90(Pt 7):1611–1621. doi: 10.1099/vir.0.010017-0. [DOI] [PubMed] [Google Scholar]
- 35.Feltkamp MC, de Koning MN, Bavinck JN, Ter Schegget J. Betapapillomaviruses: innocent bystanders or causes of skin cancer. J Clin Virol. 2008 Dec;43(4):353–360. doi: 10.1016/j.jcv.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Foulongne V, Dereure O, Kluger N, Moles JP, Guillot B, Segondy M. Merkel cell polyomavirus DNA detection in lesional and nonlesional skin from patients with Merkel cell carcinoma or other skin diseases. Br J Dermatol. 2010 Jan;162(1):59–63. doi: 10.1111/j.1365-2133.2009.09381.x. [DOI] [PubMed] [Google Scholar]
- 37.Karagas MR, Waterboer T, Li Z, et al. Genus beta human papillomaviruses and incidence of basal cell and squamous cell carcinomas of skin: population based case-control study. BMJ. 2010;341:c2986. doi: 10.1136/bmj.c2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Infante FGLD, JR, Weber JS, Kefford RF, Bendell RC, Kurzrock R, Shapiro G, Kudchadkar RR, Long GV, Burris HA, Kim KB, Clements A, Peng S, Yi B, Allred AJ, Ouellet D, Patel K, Lebowitz PF, Flaherty KT . Phase I/II study to assess safety, pharmacokinetics, and efficacy of the oral MEK 1/2 inhibitor GSK1120212 (GSK212) dosed in combination with the oral BRAF inhibitor GSK2118436 (GSK436). J Clin Oncol (Meeting Abstract) 2011;29(18 suppl):CRA8503. [Google Scholar]
- 39.Reisinger DM, Shiffer JD, Cognetta AB, Jr, Chang Y, Moore PS. Lack of evidence for basal or squamous cell carcinoma infection with Merkel cell polyomavirus in immunocompetent patients with Merkel cell carcinoma. J Am Acad Dermatol. 2010 Sep;63(3):400–403. doi: 10.1016/j.jaad.2009.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]


