Abstract
Background and Purpose
An increasing number of endovascular mechanical thrombectomy procedures are being performed for the treatment of acute ischemic stroke. This study examines variances in the allocation of these procedures in the United States at the hospital level. We investigate operative volume across centers performing mechanical revascularization and establish that procedural volume is independently associated with inpatient mortality.
Methods
Data was collected using the Nationwide Inpatient Sample database in the U.S. for 2008. Medical centers performing mechanical thrombectomy were identified using International Classification of Disease, 9th revision codes and procedural volumes were evaluated according to hospital size, location, control/ ownership, geographical characteristics and teaching status. Inpatient mortality was compared for hospitals performing ≥ 10 mechanical thrombectomy procedures versus those performing < 10 procedures yearly. After univariate analysis identified the factors that were significantly related to mortality, multivariable logistic regression was performed to compare mortality outcome by hospital procedure volume independent of covariates.
Results
Significant allocation differences existed for mechanical thrombectomy procedures according to hospital size (p<0.001), location (p<0.0001), control/ ownership (p<0.0001), geography (p<0.05) and teaching status (p<0.0001). Substantial procedural volume was independently associated with decreased mortality (p=0.0002, OR = 0.49) when adjusting for demographic covariates.
Conclusions
The number of mechanical thrombectomy procedures performed nationally remains relatively low, with a disproportionate distribution of neurointerventional centers in high volume, urban teaching hospitals. Procedural volume is associated with mortality in facilities performing mechanical thrombectomy for acute ischemic stroke patients. These results suggest a potential benefit for treatment centralization to facilities with substantial operative volume.
Keywords: Acute stroke, Neurointerventional Procedures, Mortality, Thrombectomy
INTRODUCTION
Stroke is the fourth leading cause of death and the most common source of permanent disability in the United States.1 An estimated 795,000 strokes occur each year.2 Thrombolysis with intravenous tissue plasminogen activator (IV tPA) is considered standard therapy for select patients presenting up to 4.5 hours after ischemic stroke onset.3-4 However, IV tPA treatment remains unavailable to the majority of ischemic stroke patients due to ineligibility, presentation outside of the prescribed treatment time window or provider reluctance. Additionally, the efficacy of IV tPA is often limited by low recanalization rates in acute large vessel occlusions, with recanalization rates as low as 18.9%.5
These limitations have led to the development of novel endovascular therapies for large vessel stroke, including intra-arterial thrombolysis and mechanical thrombectomy procedures. Prospective endovascular stroke therapy trials have demonstrated improved recanalization rates, ranging from 56%-81.6%.6-11 This promising data has resulted in approval of multiple mechanical revascularization devices by the United States Food and Drug Administration (FDA) since 2004.
Although thrombectomy devices have gained FDA approval, endovascular thrombectomy procedures are not universally accepted as standard of care for large vessel strokes presenting within applicable time windows. Utilization of this procedure remains at the discretion of the treating physician and may be subject to regional and demographic influences. As accreditation guidelines are being developed for stroke center certification12, it is critical to examine the current practice patterns of hospitals performing these procedures.
The Nationwide Inpatient Sample (NIS) is the largest publicly available inpatient care database representing 20% of admissions to nonfederal hospitals across the United States.13 The International Classification of Diseases, 9th Revision (ICD-9) procedure code 39.74, endovascular removal of obstruction from the head and neck, was introduced into the NIS database in 2006, enabling identification of patients treated by mechanical thrombectomy procedures. This study analyzes data from 2008 in order to minimize the impact of errors and information bias likely generated in the first years of coding.
This investigation determines the allocation of mechanical revascularization procedures in the United States at the hospital level in 2008. We examine operative volume across all hospitals performing mechanical revascularization procedures and establish that procedural volume is independently associated with inpatient mortality.
METHODS
Patient population
Variables were retrieved from the Nationwide Inpatient Sample (NIS) hospital discharge database for 2008. This database is sponsored by the Agency for Healthcare Research and Quality and was developed as part of the Healthcare Cost and Utilization Project (HCUP).13 Inpatients with a diagnosis of ischemic stroke were identified with ICD-9 codes 433, 434, 436, 437.0, and 437.1. Patients undergoing endovascular clot retrieval were extracted from the aforementioned stroke cohort using the ICD-9 procedure code 39.74, “endovascular removal of obstruction from the head and neck,” which was first introduced into the NIS database in 2006. Selection criteria was modeled after that described by Brinjikji et al.14
Statistical Analysis
To obtain national estimates, proper weights were applied as indicated in the HCUP–NIS Calculating NIS Variances Guide.13 All statistical analysis was performed using SAS software 9.2 (SAS Institute, Inc., Cary, NC) at the 0.05 significance level.
Demographic data
The universe of hospitals that treated patients for ischemic stroke according to the above ICD-9 codes was examined. Hospitals were stratified according to those that offered mechanical thrombectomy (performed ≥ 1 mechanical thrombectomy procedure during 2008) and those that did not. Patient age was described as a continuous variable (AGE). Patient race was expressed as a discrete variable (RACE). Frequency of hospitals offering mechanical thrombectomy was determined according to hospital size (HOSP_BEDSIZE: small, medium, large), geographic region (HOSP_REGION: West, South, Midwest, Northeast), teaching status (HOSP_TEACH: teaching/non-teaching), location (H_LOC: urban/rural), and hospital control/ownership (HOSP_CONTROL: government - nonfederal public, private – non-profit, private – investor owned, government/private collapsed category, private collapsed category). Urban hospital designations were based on American Hospital Association survey results defining medical centers located in core areas with a population ≥50,000. Hospital ownership was defined using the American Hospital Association stratifications for government and private hospitals. When sample sizes were not sufficiently large, stratification was collapsed to include all private hospitals (HOSP_CONTROL: private collapsed) and when no stratification could be provided, hospitals were defined in another collapsed category (HOSP_CONTROL: government/private). Each was expressed as a categorical variable. Chi-square testing was used to compare categorical variables and the Student t test was used to compare continuous variables.
Procedural Volume
The number of mechanical thrombectomy procedures performed at each hospital was calculated and displayed in scatter plot and frequency histogram figures. Hospitals were dichotomously categorized according to procedural volume (≥10 vs. <10).
Mortality
The association between hospital procedural volume and mortality was examined. Factors hypothesized or previously demonstrated to affect mortality in this dataset were included in the model as covariates. Univariable logistic regression was performed with mortality as the dependant variable (binary HCUP variable DIED). Independent variables assessed were age (AGE), race (RACE), hospital bed size (HOSP_BEDSIZE), geographic region (HOSP_REGION), urban/rural location (HOSP_LOC), teaching status (HOSP_TEACH) control/ownership of hospital (HOSP_CONTROL), and procedural volume (hospitals performing ≥ 10 mechanical thrombectomy procedures vs. those that performed < 10 procedure). For this analysis, age was represented as a continuous variable. Variables reaching at least marginal significance (p<0.10) in univariable analysis were considered candidates for subsequent forward stepwise multivariate logistic regression modeling. A final model was determined to compare mortality outcome by hospital procedure volume independent of covariates.
RESULTS
Patient and Hospital Demographics
In 2008, a total of 2749 patients underwent endovascular mechanical revascularization procedures in the setting of acute stroke at facilities tracked by the NIS dataset. Age of treated patients was 65.2 ±35.3 (mean± standard deviation). Patient age ranged from 7 to 94. Of the treated population, 52.2% were female. 77.0% of treated patients were White, 8.2% Black, 8.0% Hispanic, 3.2% Asian/pacific islander, 0.46% Native American and 3.1% other.
Endovascular clot retrieval was performed in the setting of acute stroke in 296 hospitals and was not performed in 5002 hospitals. Significant allocation differences existed by geographic region (p<0.0001). The procedure was offered at 7.4% (53/718) of hospitals in the Northeast, 3.1% (46/1487) in the Midwest, 5.8% (119/2075) in the South, and 7.6% (78/1017) in the West. Difference in procedure frequency was noted among hospitals set in urban and rural areas (p<0.0001). 9.5% (296/ 3121) of hospitals in urban settings offered mechanical revascularization, whereas no rural hospitals performed the procedure (0/2161). The difference in procedure performance rate by hospital size was significant (p<0.0001). Mechanical revascularization was offered in 15.0% (244/1628) of large size hospitals, 3.7% (47/1280) of medium size hospitals and 0.2% (5/2375) of small size hospitals. Teaching facility designation was associated with a higher proportion of hospitals performing mechanical revascularization procedures (p<0.0001). The procedure was offered at 27.2% (242/888) of teaching hospitals and 1.2% (54/4395) of non-teaching hospitals. Procedure allocation also differed by hospital control/ ownership (p<0.0001). Mechanical thrombectomy was performed at 13.5% (246/1829) of government / private (collapsed) hospitals, 0.85% (8/930) of government, non-federal public hospitals, 3.83% (36/930) of private, non-profit voluntary hospitals, 0.65% (6/900) of private, investor-owned hospitals and 0% (0/694) of private (collapsed) hospitals. Facilities offering endovascular mechanical revascularization procedures are represented in Tables 1 and 2.
Table 1.
| Variable | Total Hospitals | Hospitals offering endovascular clot retrieval (% of hospitals) | p-value * |
|---|---|---|---|
| Regional Location | |||
| Northeast | 718 | 53 (7.4%) | <0.0001 |
| Midwest | 1487 | 46 (3.1%) | |
| South | 2075 | 119 (5.8%) | |
| West | 1017 | 78 (7.6%) | |
| Urban/Rural Location | |||
| Urban | 3122 | 296 (9.5%) | <0.0001 |
| Rural | 2161 | 0 (0%) | |
| Hospital Bedsize | |||
| Small | 2375 | 5 (0.2%) | <0.0001 |
| Medium | 1280 | 47 (3.7%) | |
| Large | 1628 | 244 (15.0%) | |
| Teaching Status | |||
| Teaching | 888 | 242 (27.2%) | <0.0001 |
| Non-teaching | 4395 | 54 (1.2%) | |
| Hospital Control | |||
| Government/Private (Collapsed) | 1829 | 246 (13.5%) | <0.0001 |
| Government, Nonfederal | 930 | 8 (0.9%) | |
| Private, Non-profit | 930 | 36 (3.8%) | |
| Private, Investor-Owned | 900 | 6 (0.7%) | |
| Private (Collapsed) | 694 | 0 (0%) | |
P-values represent overall comparisons among groups. P-values for post hoc, between group, analyses are included in table 2
Table 2.
Availability of endovascular clot retrieval in hospitals. Comparison between subgroups from Table 1 analyzed using chi-square tests with Bonferroni correction.
| Category | Groups Compared | P- value |
|---|---|---|
| Regional Location | Midwest vs. Northeast Midwest vs. South Midwest vs. West Northeast vs. South Northeast vs. West South vs. West |
<0.001 <0.001 <0.001 <0.001 <0.001 <0.001 |
| Hospital Bedsize | Large vs. Medium Large vs. Small Medium vs. Small |
<0.001 <0.001 <0.001 |
| Hospital Control | Government, Nonfederal vs. Government/Private (Collapsed) Government, Nonfederal vs. Private (Collapsed) Government, Nonfederal vs. Private, Investor-Owned Government, Nonfederal vs. Private, Non-Profit Government/Private (Collapsed) vs. Private (Collapsed) Government/Private (Collapsed) vs. Private, Investor-Owned Government/Private (Collapsed) vs. Private, Non-Profit Private (Collapsed) vs. Private, Investor-Owned Private (Collapsed) vs. Private, Non-Profit Private, Investor-Owned vs. Private, Non-Profit |
<0.001 <0.001 1.00 1.00 <0.001 <0.001 <0.001 0.001 <0.001 1.00 |
Procedural Volume
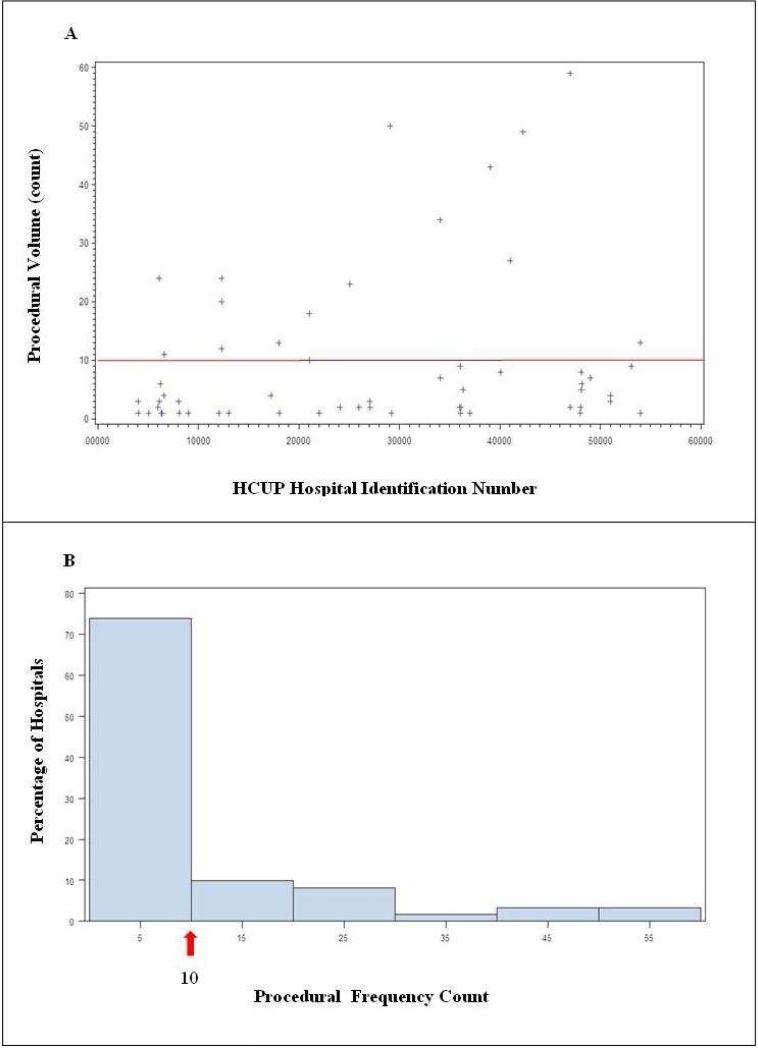
Based on scatterplot and histogram data generated for procedural volume across all hospitals represented (Figure 1), performance of greater than or equal to 10 procedures over the course of the year was designated as “substantial volume.” Based on this criterion, 26.4% (78/296) of hospitals performed substantial volume of mechanical thrombectomy procedures in the setting of acute stroke.
Figure 1.
Procedural volume by hospital. (A) Scatterplot demonstrating frequency of patients treated with endovascular mechanical revascularization at represented hospitals and (B) Histogram demonstrating percentage of hospitals performing mechanical thrombectomy by procedural volume. A natural segregation between centers performing ≥10 procedures (substantial volume) and those performing <10 procedures was derived from scatterplot results. Total substantial volume centers (≥ 10 patients treated/year) 78 (26.4%) and total lower volume centers were 218 (73.6%)
Mortality by Volume
In univariable analysis, a significant decrease in mortality was noted in substantial volume centers (502/2098, 24.0%) when compared to lower volume centers (184/651, 28.3%; p=0.027, odds ratio 0.80). Age (p<0.001), race (p<0.0001), hospital region (p=0.0003), teaching status (p=0.0004), and hospital ownership/ control (p=0.0004) were also associated with mortality. Hospital bedsize was not associated with mortality (p=0.42). In multivariable analysis, high volume centers remained independently associated with decreased mortality (p<0.0001, odds ratio = 0.49, CI [0.37,0.63]) when adjusting for the above covariates. Values are represented in Table 3
Table 3.
In hospital mortality following mechanical revascularization procedures. P values for significant differences for in-hospital mortality as a function of hospital procedural volume, patient age, race, hospital region, bedsize, control and teaching status.
| Variable | Univariate p-value, odds ratio [CI] | Multivariate p-value, odds ratio [CI] |
|---|---|---|
| Substantial vs. lower volume | 0.027 , 0.80 [0.66,0.98] | <0.0001, 0.55 [0.43, 0.72] |
| Age | <0.001, 1.02 [1.02,1.03] | <0.0001, 1.02 [1.01,1.03] |
| Race | <0.0001 * | <0.0001* |
| Hospital Region | 0.0003* | <0.0001* |
| Hospital Bedsize | 0.418* | |
| Hospital Control | 0.0004* | |
| Teaching status | 0.0004, 0.47 [0.31,0.72] | <0.0001, 0.32 [0.20,0.52] |
Represents statistic for overall comparison among multiple categories
#Blank spaces in the multivariate column represent variables not included in multivariate analysis
DISCUSSION
This is the first study to document patterns in the allocation of mechanical revascularization procedures for acute ischemic stroke at the national level. The utilization of mechanical thrombectomy procedures is of interest for several unique reasons. It is a relatively new procedure that requires a significant investment in personnel and equipment. While FDA approved for vessel recanalization, mechanical thrombectomy has not gained universal acceptance as “standard of care” across disciplines and specialties. Therefore performance of the procedure is highly dependent upon physician and hospital preference, and is likely subject to demographic trends.
Facilities offering mechanical revascularization procedures in 2008 treated a broad age range of patients, indicating that these procedures are occasionally performed outside the inclusion criteria prescribed by large investigations and clinical trials.6-11 Overall, the number of centers performing endovascular stroke procedures remains low relative to the universe of hospitals treating patients for ischemic stroke. This likely relates to the specialized equipment, infrastructure and expertise required in offering such treatments, limiting widespread availability. Our data analysis also reveals a seemingly low number of endovascular acute stroke treatments for the vast majority of individual facilities. This figure may be impacted by a large proportion of hospital admissions secondary to small vessel disease, transient ischemic attacks, mild strokes, or onset times that exceed typically accepted treatment time windows. Nonetheless, the overall number of acute strokes treated with endovascular therapy remains far below the estimated 7-15% that may potentially benefit from acute intervention.15 These small procedural volumes may represent difficulty in effectively screening and transporting patients to endovascular centers in a timely manner as well as a continued lack of urgency among the general public in seeking treatment for stroke symptoms. This public awareness deficiency is highlighted by the continued low national rates of intravenous alteplase administration.16 Additional steps are needed to address these gaps in public knowledge, and raise awareness of potential endovascular treatment options in the setting of acute stroke.
Hospital size is strongly correlated with performance of mechanical thrombectomy procedures. Further, the vast majority of these operations are undertaken at teaching institutions. Larger academic, facilities often have the resources and support necessary to provide continuous high level care to critically ill patients. The need to recognize facilities that perform therapeutic endovascular procedures amongst local hospitals and primary stroke centers has led to growing advocacy in distinguishing these facilities as comprehensive stroke centers. These hospitals may then serve to provide a higher level of care for surrounding centers in a “hub-and-spoke” type model. According to our data set, mechanical revascularization procedures are performed exclusively at hospitals in urban settings, potentially limiting access to patients in more remote, rural areas. This disproportionate distribution of centers may partially account for low individual procedural volumes due to redundancy in services within a small geographical area. As hospitals begin moving towards comprehensive stroke center certification, coordinated efforts must be made to recognize and address this possible disparity and design an effective triage system to provide care for all potential acute stroke patients. Regional geographic differences exist, with a greater proportion of hospitals in the Northeast and West performing mechanical thrombectomy procedures. This may reflect a higher density of subspecialization in these areas or a greater propensity for the “hub-and-spoke” type of model in the South and Midwest. Greater geographic distances exist between large, academic centers in these regions.
Recommended guidelines have been established for comprehensive stroke center designation, but while many hospitals provide neuroendovascular services, few hospitals in the U.S. meet the suggested procedural volume criteria.17 Examination of our frequency histograms and scatter plots demonstrate a clustering of procedural volume by hospital. A natural segregation occurs at a yearly rate of 10 mechanical thrombectomy procedures. Hospitals that perform 10 or more procedures likely evaluate a significantly greater number of acute stroke patients for potential therapy. Categorization of hospitals that perform 10 or more yearly procedures as “substantial volume centers” appears to be both statistically and clinically judicious. This rate may reflect an important threshold for both technical and clinical experience required by neurointerventionalists. Prior studies have reported a learning curve for all endovascular procedures and have exhibited a reduction in complication rates with increased experience.18-20 Acute endovascular stroke therapy represents a unique procedure with a distinct set of challenges. Even seasoned neurointerventional surgeons require practice and experience with the technical aspects of the procedure.
Our study is the first to demonstrate that operative volume is correlated with inpatient mortality following mechanical revascularization procedures for acute stroke. Substantial procedural volume is associated with decreased mortality, independent of covariates such as patient characteristics and hospital demographics. In addition to technical experience, increased endovascular stroke volume may lead to improvements in organized delivery of acute stroke care in the higher volume centers through quality control measures. This parallels trends demonstrated in prior studies examining utilization of intravenous tPA. Stroke centers providing organized care with more frequent use of IV tPA also demonstrate reduced mortality.21 If transport time is not prohibitive, transfer of patients with large vessel occlusions to centers with substantial operative experience may improve aggregate outcomes following mechanical thrombectomy procedures. The inpatient mortality rates in our study were high, but appear to remain within the 90 day rates reported in published trials. Monitoring future outcome trends will be important as hospitals gain further experience with acute endovascular stroke care.
This study has several limitations based on the retrospective analysis of the NIS database. The data set is reliant upon accurate ICD-9 coding for diagnoses and procedures. Therefore, limitations may be present due to potential coding errors or omission of data. Additionally, the data captured and tracked within the NIS does not allow for assessment of many important related factors. Recanalization rates are not documented and, therefore, cannot be correlated with operative volume. Likewise, procedural details, such as time interval from symptom onset to treatment, specific devices utilized, and appropriateness of interventions cannot be determined. Outcome is assessed by mortality rate, which does not serve as an accurate surrogate for functional capacity. However, National Institutes of Health stroke scale (NIHSS) score at presentation and premorbid clinical status are not available for analysis. An inability to control for, or segregate according to, these parameters renders functional status a somewhat subjective, and potentially misleading, outcome measure. The definitive and objective nature of mortality allows for relatively straightforward analysis of a large and complex data set. Distinguishing causes of mortality such as withdrawal of care or procedure-related mortality cannot be performed. This hinders the ability to offer position statements about the appropriate utilization of these devices from a societal standpoint.
Utilization of data from 2008 presents inherent limitations with regard to external validity and generalizability. Although the Penumbra aspiration system was approved in January 2008, many centers did not have access to the device until later that year. Endovascular devices and techniques utilized to treat acute stroke continue to evolve rapidly. Newer generations of existing devices and the advent of novel devices such as stentrievers have substantially changed the landscape of interventional stroke treatment since 2008. Nonetheless, this analysis documents important cross sectional variances in procedural utilization and establishes a benchmark to which future studies may be compared
The treatment of acute stroke is evolving rapidly. Clinical and technological advances may outpace organizational efforts focused on optimal resource allocation and design of stroke networks. Policymakers and physicians are formulating metrics while medical centers are developing infrastructure to accommodate the demand of acute stroke. In designing stroke networks, examination of existing trends and aggregate data is essential. This study describes the demographics of hospitals offering mechanical thrombectomy procedures for acute stroke in 2008. Further, the data advocates a potential threshold for “substantial volume”. Analyses suggest that hospital volume impacts mortality following mechanical thrombectomy for acute stroke. These results imply, under optimal conditions, a potential benefit of treatment centralization to centers that perform substantial volume
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES:
WJM serves on the Clinical Events Committee for the Penumbra Therapy Trial
REFERENCES
- 1.Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke. 2011;42:2351–2355. doi: 10.1161/STROKEAHA.111.621904. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NINDS rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 4.Del Zoppo GJ, Saver JL, Jauch EC, et al. American Heart Association Stroke Council. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40:2945–2948. doi: 10.1161/STROKEAHA.109.192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rha JH, Saver JL. “The impact of recanalization on ischemic stroke outcome: a meta-analysis,”. Stroke. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 6.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 7.Penumbra Pivotal Stroke Trial Investigators The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761–2768. doi: 10.1161/STROKEAHA.108.544957. [DOI] [PubMed] [Google Scholar]
- 8.IMS Study Investigators Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke. 2004;35:904–911. doi: 10.1161/01.STR.0000121641.77121.98. [DOI] [PubMed] [Google Scholar]
- 9.IMS II Trial Investigators The Interventional Management of Stroke (IMS) II Study. Stroke. 2007;38:2127–2135. doi: 10.1161/STROKEAHA.107.483131. [DOI] [PubMed] [Google Scholar]
- 10.Gobin YP, Starkman S, Duckwiler GR, et al. MERCI 1: a phase 1 study of Mechanical Embolus Removal in Cerebral Ischemia. Stroke. 2004;35:2848–2854. doi: 10.1161/01.STR.0000147718.12954.60. [DOI] [PubMed] [Google Scholar]
- 11.Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39:1205–1212. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 12.Leifer D, Bravata DM, Connors JJ, 3rd, et al. Metrics for measuring quality of care in comprehensive stroke centers: detailed follow-up to Brain Attack Coalition comprehensive stroke center recommendations: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:849–877. doi: 10.1161/STR.0b013e318208eb99. [DOI] [PubMed] [Google Scholar]
- 13.HCUP Overview. Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality. Rockville, MD: Nov, 2009. http://www.hcup-us.ahrq.gov/overview.jsp. Accessed March 2, 2012. [PubMed] [Google Scholar]
- 14.Brinjikji W, Rabinstein AA, Kallmes DF, et al. Patient outcomes with endovascular embolectomy therapy for acute ischemic stroke: a study of the national inpatient sample: 2006 to 2008. Stroke. 2011;42:1648–1652. doi: 10.1161/STROKEAHA.110.607952. [DOI] [PubMed] [Google Scholar]
- 15.Meyers PM, Schumacher HC, Connolly ES, Jr, et al. Current status of endovascular stroke treatment. Circulation. 2011;123:2591–2601. doi: 10.1161/CIRCULATIONAHA.110.971564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adeoye O, Hornung R, Khatri P, et al. Recombinant tissue-type plasminogen activator us for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke. 2011;42:1952–1955. doi: 10.1161/STROKEAHA.110.612358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grigoryan M, Chaudhry SA, Hassan AE, et al. Neurointerventional Procedural Volume per Hospital in United States: Implications for Comprehensive Stroke Center Designation. Stroke. 2012;43:1309–1314. doi: 10.1161/STROKEAHA.111.636076. [DOI] [PubMed] [Google Scholar]
- 18.Vitek JJ, Roubin GS, Al-Mubarek N, et al. Carotid artery stenting: technical considerations. AJNR. 2000;21:1736–1743. [PMC free article] [PubMed] [Google Scholar]
- 19.Singh V, Gress DR, Higashida RT, et al. The learning curve for coil embolization of unruptured intracranial aneurysms. AJNR. 2002;23:768–771. [PMC free article] [PubMed] [Google Scholar]
- 20.Verzini F, Cao P, De Rango P, et al. Appropriateness of learning curve for carotid artery stenting: An analysis of periprocedural complications. J Vasc Surg. 2006;44:1205–1211. doi: 10.1016/j.jvs.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 21.Xian Y, Holloway RG, Chan PS, et al. Association between stroke center hospitalization for acute ischemic stroke and mortality. JAMA. 2011;305:373–380. doi: 10.1001/jama.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]