Abstract
Background: Infertility care is one of the most neglected health care issues in developing countries (DC), affecting more than 50 million couples. The social stigma of childlessness still leads to isolation and abandonment. Bilateral tubal occlusion due to sexually transmitted diseases and pregnancy-related infections is the most common cause of infertility in DC. Consequently most cases of infertility are only treatable by using assisted reproductive technologies which are either unavailable or too costly. Lowering the laboratory costs associated with IVF is a crucial step to make IVF affordable for a larger part of the world population. We recently developed and described a new simplified method of IVF culturing, called the tWE lab method. Our initial results in fresh IVF cycles showed that IVF methodology can be significantly simplified and result in successful outcomes at levels that compare favourably to those obtained in high resource programs.
Case-reports: We report three pregnancies and four live births as a result of transferring five cryo/thawing embryos which were developed after using the simplified tWE lab system. The two singleton babies delivered vaginally, for the twin pregnancy a caesarean section was performed. All babies were healthy, the perinatal outcome was uneventful in all cases.
Conclusion: We provide proof-of-principle evidence that transferring cryopreserved/thawed embryos obtained with our tWE lab simplified culture system can lead to successful pregnancies and healthy live births.
Keywords: Accessible IVF, assisted reproduction, cryopreservation, developing countries, infertility care, low-cost IVF, simplified IVF, slow freezing, the walking egg project
Introduction
According to the literature on the worldwide prevalence of involuntary childlessness between 52.6 to 72.4 million couples can benefit from some form of medical intervention to achieve a pregnancy (Mascarenhas et al., 2012), most of them being residents of developing countries or resource-poor countries (Rutstein and Iqbal, 2004; Boivin et al., 2007). The social stigma of childlessness still leads to isolation and abandonment. Its implications are strongly related to and mediated by different socio-cultural factors. Especially in developing countries, most societies are organized such that children are necessary for care and maintenance of the older parents. Moreover, in the absence of social security systems, older people are economically completely dependent on their children (Dyer and Patel, 2012). Consequently, the inability to bear children is a tragedy and childlessness can be considered as an important and neglected reproductive health problem in developing countries (Bergstrom, 1992; Leek et al., 1993; Van Balen and Gerrits, 2001; Dyer et al., 2004; Fathalla et al., 2006; Van Balen and Bos, 2009; Gerrits and Shaw, 2010; Dhont, 2011a).
Bilateral tubal occlusion due to sexually transmitted diseases and pregnancy-related infections is the most common cause of infertility in DC and is only treatable by using IVF related procedures which are either unavailable or too costly (Ombelet et al., 2008).
During the past 35 years of IVF almost 5 million babies are born after using IVF and/or ICSI. This should be regarded as a great success story if a large part of the world population could benefit from these new technologies, which is currently not the case in most developing and even developed countries. To optimise infertility care in terms of availability, affordability and effectiveness the simplification of IVF procedures is urgently needed.
As part of the Walking Egg Project (Dhont, 2011b; Ombelet, 2013; 2014) and based on previous findings and experience (Van Blerkom and Manes, 1974; Swain, 2011) we developed a new simplified method of IVF culturing, called the tWE lab method. With this new system, specifically designed for low resource settings, we can avoid the complexity of the use of medical gases, high-tech incubation equipment and infrastructure typical of IVF laboratories in high resource settings.
In this closed system we only used 1000 - 5000 motile washed spermatozoa for insemination of the eggs, with very promising results, which makes this technique usable for a large part of the actual IVF/ICSI population, even if a moderate or severe male factor is involved (Van Blerkom et al., 2014).
Since development from insemination to transfer is undisturbed and in the same tube until embryo transfer, we can avoid many problems frequently occurring in regular IVF laboratories, such as unwanted temperature changes, air quality problems etc.
The birth of seven healthy babies from this simplified IVF system was recently reported (Van Blerkom et al., 2014). In this paper we describe the pregnancy outcome and birth of another four healthy babies born after cryo-thawing and embryo-transfer of embryos resulting from this tWE lab system.
The simplified tWE lab system
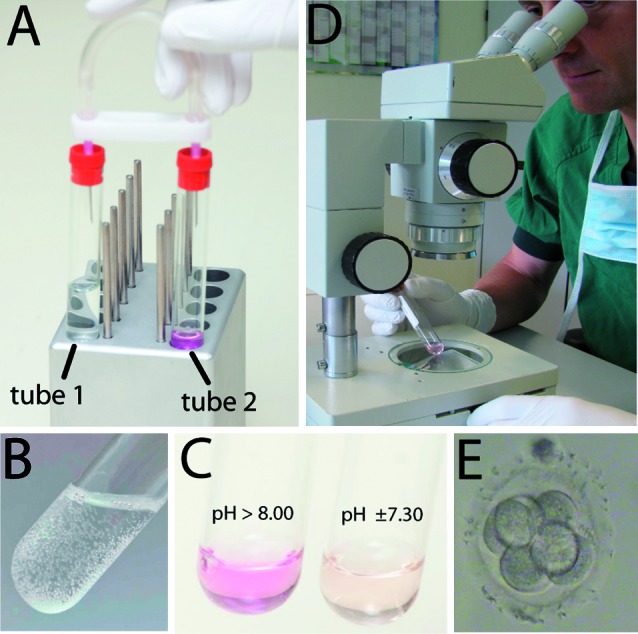
A simple chemical reaction was used to produce CO2 de novo, namely the combination of a weak base (sodium bicarbonate), a weak acid (citric acid) and water. The result is a defined atmosphere that is stable for prolonged periods such that culture medium could be equilibrated in advance of an IVF cycle. Consequently the possibility of changes in temperature, atmosphere or pH that could have adverse developmental effects are minimized or eliminated. We only need a uniform temperature which can be maintained quite simply and without the need for conventional and more expensive incubators (Fig. 1).
Fertilization and embryo development are observed through the glass vacutainer. Pre-selection of the embryo’s to be used for transfer can be done without opening the vacutainers.
The tWE lab system is a closed system and intended to enable fertilization and embryo development to occur undisturbed in the same tube until day 3, when uterine transfers are done (Fig. 1).
Fig. 1. The tWE-lab system: (A) connection between tube 1 with citric acid and sodium bicarbonate in water and tube 2 with IVF culture medium transfers enough CO2 gas (B) produced by the chemical reaction between the acid and the base to equilibrate the pH of the medium to +/- 7.30. Fertilization and embryo scoring by looking through the glass tube (D,E).
The patient cohort
The patients described in this paper are part of a prospective non-inferiority study performed at the ZOL Hospitals in Genk, Belgium. The methodology of this study was described before (Van Blerkom et al., 2014). Couples enrolled in this study required IVF as assessed by the standard protocol of infertility evaluation used at ZOL Hospitals. In all patients it was the first IVF attempt, all women were below 36 years of age and only IVF cycles with at least 8 oocytes recovered at follicular aspiration were included. Participation in this clinical trial required informed consent documentation and this study was approved by the local Ethical Committee of Genk and the Ethical Committee of the Free University of Brussels, and registered as B.U.N. 143201110348. In the fresh IVF cycle and according to the Belgian law for this specific patient population, single embryo transfer (SET) was obliged (Ombelet et al., 2005). Therefore a reasonable number of embryos, resulting from the tWE lab simplified IVF system, were cryopreserved.
Cryopreservation methodology
Surplus embryos were cryopreserved using a controlled rate programmable freezer (Planer KRYO 360 1.7, T.S. Scientific, Perkasie, PA, USA) and Sydney IVF cryopreservation kit SICS-5000. Embryos were frozen according to the manufacturer’s protocol. Briefly, embryos were exposed for 10 minutes to each of the freezing solutions with increasing concentrations of PROH, supplemented with human serum albumin (up to 12mg/mL) and gentamicin (0.01 mg/mL). Solution one was equilibrated to 37°C in air whilst solutions 2 and 3 were used at room temperature. The embryos were loaded into a 0.3ml CBSTM high security embryo straw (CryoBioSystem) and both ends of the straw were sealed. The straws were places into the controlled rate programmed freezing machine (precooled to 20°C) and kept at 20°C for 10 minutes. The straws were cooled to -6°C at a rate of -2°C per minute, held at this temperature for 4 minutes and then seeded manually and held at -6°C for another 15 minutes. It was then cooled to -30°C at a rate of -0, 3°C per minute and subsequently cooled to -160°C at a rate of -10°C per minute and held there for 10 minutes. The straws were plunged in liquid nitrogen and stored in liquid nitrogen vessels. For thawing of the frozen embryos, the straws were removed from the liquid nitrogen vessel and warmed to 30°C for 1 minute. The cryoprotectant was removed by transferring the embryos to stepwise dilutions of the thawing solutions from the Sydney IVF thawing kit SITS-5000 at room temperature. The embryos were warmed to 37°C, evaluated on their survival and cleavage stage and cultured in embryo culture medium. The embryos were evaluated again after 24 hours and transferred to the uterus.
Case reports
The outcome of three pregnancies resulting in four live births are described in Figure 2.
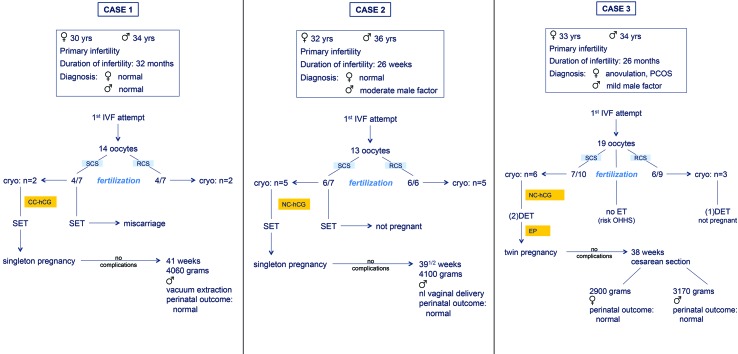
Fig. 2. Description of three cases of live births after freezing/thawing of embryos obtained with the tWE lab simplified IVF procedure (SCS = simplified culture system or tWE lab system, RCS = regular culture system, SET = single embryo transfer, DET = double embryo transfer, PCOS = polycystic ovary syndrome, CC = clomiphene citrate, NC = natural cycle, EP = estrogen / progesterone scheme).
The first couple (case 1) was referred to our infertility clinic with a history of primary infertility for more than two years. An infertility work-up of both partners couldn’t detect any abnormality. The diagnosis of unexplained infertility was made and three intrauterine inseminations were unsuccessful. The couple agreed to be included in the prospective study in which our simplified IVF culture system (SCS) was compared with regular IVF culture system (RCS), described by Van Blerkom et al. (2014). In the first (fresh) IVF attempt 14 oocytes were retrieved, a single embryo transfer (SET) from SCS was performed according to the protocol of the study (Figure 1a). The patient became pregnant but miscarried at 7 weeks of gestation. Four embryos were cryopreserved and two months later one embryo originating from the SCS was thawed and transferred in a CC (clomiphene citrate) stimulated cycle. The patient became pregnant and delivered at 41 weeks of gestation of a boy weighing 4060 grams. The perinatal outcome was uneventful.
The second couple (case 2) had a history of primary infertility for 26 months. A moderate male factor could be detected, an investigation showed no female abnormalities. The couple also volunteered to be included in the prospective study, as mentioned above. 13 oocytes were retrieved and a SET from SCS was performed, without success (Fig. 2b). Ten embryos were cryopreserved. A few months later a SET was performed after thawing of two embryos, one embryo didn’t survive. Both embryos originated from the SCS. The transfer was done in a natural cycle. She became pregnant and delivered at 39 1/2 weeks of gestation of a boy weighing 4100 grams. The perinatal outcome was normal.
The third couple (case 3) had a history of primary infertility for 28 months. A moderate male factor could be detected, an investigation of the women revealed the diagnosis of anovulation due to PCOS (Polycystic Ovary Syndrome). The couple agreed to be included in the prospective study and 19 oocytes were retrieved during the first IVF attempt. Because of a high risk for OHSS (Ovarian Hyperstimulation Syndrome) it was decided to trigger ovulation with an agonist (Decapeptyl 0.2 mg IM, Ipsen, Belgium) instead of using hCG (Pregnyl 5000, MSD, Belgium). All embryos (n = 9) were cryopreserved (Figure 2c). A few months later a DET (double embryo transfer) was performed after thawing of three embryos originating of the RCS, without success. During the second attempt two embryos originating of the SCS were transferred. This resulted in a twin pregnancy. The gestation was uneventful, she delivered at 38 weeks of gestation of a boy weighing 3170 grams and a girl of 2900 grams. A caesarean section was performed, the perinatal outcome was normal.
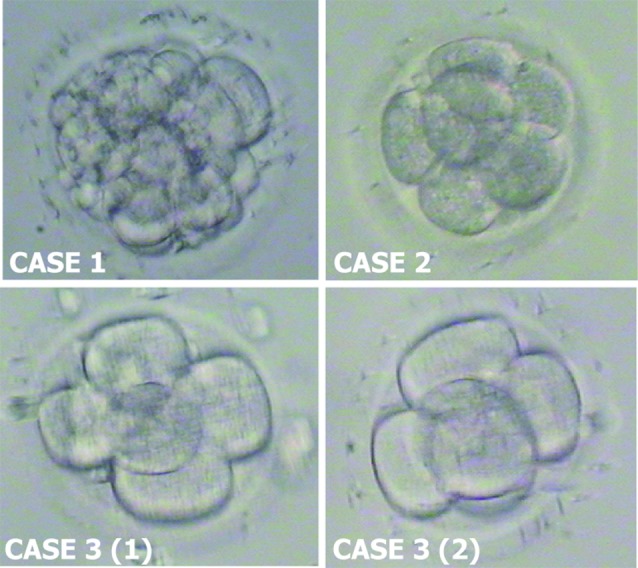
Fig. 3. Frozen-thawed embryos at time of embryo transfer. Case 1: embryo with 15 blastomeres compacting, Case 2: embryo with 6 blastomeres, Case 3: 2 embryos with 5 blastomeres each.
Discussion
Infertility is a universal health issue and the large majority of childless couples are residents of developing countries. It is a silent population of more than 50 million couples facing the consequences of childlessness day by day. These consequences of involuntary childlessness are usually much more dramatic in developing countries and can create broader problems compared to Western societies, particularly for women (Van Balen and Gerrits, 2001; Dyer et al., 2004; Van Balen and Bos, 2009; Dhont, 2011a). Despite its relatively high prevalence and the cultural values associated with childbearing, infertility remains a low priority area for local health care providers and community leaders (Ombelet et al., 2008; Dhont, 2013). Most health care providers argue that the limited recourses should only be given to programs focussing on reducing sexually transmitted diseases (STDs), postpartum and post abortion complications rather than offering high-technology treatments to infertile couples, the so-called limited resources argument. Severe male infertility due to STDs and female infertility due to tubal block can only be treated by “expensive” assisted reproductive technologies (ART) which are not available at all or only within reach of those who can afford it, mostly in a private setting. Lowering the costs associated with IVF laboratory procedures, namely fertilization and culture of eggs and embryos, was the first aim of the Walking Egg project (Ombelet, 2013; 2014).
In a recent paper we described that using a very simplified and inexpensive method of IVF culturing good results can be obtained in terms of clinical pregnancy rate per cycle and the birth of healthy babies (Van Blerkom et al., 2014). The results with the simplified IVF method were comparable with the outcome results after regular high-tech IVF.
The next question might be: do the surplus embryos obtained with this new method survive cryopreservation and thawing with reasonable outcome results. In this paper we report the birth of four healthy babies, the result of two singletons and one twin pregnancy. No gestational complications occurred and the perinatal outcome was uneventful for all babies.
Our preliminary outcome results in fresh IVF cycles and after cryopreservation and thawing of surplus embryos clearly show that this simplified system may make advanced infertility treatment in DC practical, affordable and effective.
If the results of the initial study can be reproduced in other centres we believe that the simplified tWE lab procedure will open a new era in the history of IVF as the method not only offers affordable and successful access to IVF, it will make assisted reproductive techniques available to a much larger part of the worlds’ infertile population, an important breakthrough in terms of human rights, equity and social justice for infertile couples in resource-poor countries (Johnson et al., 2014; Fathalla et al., 2006; Pennings et al., 2009; Vayena et al., 2009; Ombelet, 2011; Dhont, 2013).
References
- Bergstrom S. Reproductive failure as a health priority in the Third World: a review. East Afr Med J. 1992;69:174–180. [PubMed] [Google Scholar]
- Boivin J, Bunting L, Collins JA, et al. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506–1512. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- Dhont N. Clinical, epidemiological and socio-cultural aspects of infertility in resource-poor settings. FV&V in ObGyn. 2011;3:77–88. [PMC free article] [PubMed] [Google Scholar]
- Dhont N. The Walking Egg non-profit organisation. FV&V in ObGyn. 2011;3:253–255. [PMC free article] [PubMed] [Google Scholar]
- Dhont N. The importance of being fertile. A call for a more balanced approach towards reproductive health. FV&V in ObGyn. 2013;5:243–246. [PMC free article] [PubMed] [Google Scholar]
- Dyer SJ, Abrahams N, Mokoena NE, et al. you are a man because you have children”: experiences, reproductive health knowledge and treatment-seeking behaviour among men suffering from couple infertility in South Africa. Hum Reprod. 2004:960–967. doi: 10.1093/humrep/deh195. [DOI] [PubMed] [Google Scholar]
- Dyer SJ, Patel M. The economic impact of infertility on women in developing countries – a systematic review. FV&V in ObGyn. 2012;4:102–109. [PMC free article] [PubMed] [Google Scholar]
- Fathalla MF, Sinding SW, Rosenfield A, et al. Sexual and reproductive health for all: a call for action. Lancet. 2006;368:2095–2100. doi: 10.1016/S0140-6736(06)69483-X. [DOI] [PubMed] [Google Scholar]
- Gerrits T, Shaw M. Biomedical infertility care in sub-Saharan Africa: a social science review of current practices, experiences and viewpoints. FV&V in ObGyn. 2010;2:194–207. [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Cohen J, Grudzinskas G. Accessible and affordable IVF: is Bob Edwards’ dream about to become reality? Reprod Biomed Online. 2014;28:267–272. doi: 10.1016/j.rbmo.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Leek RJ, Oduma JA, Mayagoitia S, et al. Regional and geographical variations in infertility: effects on environmental, cultural, and socioeconomic factors. Environ Health Perspect. 1993;101(Suppl 2):73–80. doi: 10.1289/ehp.93101s273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas M, Flaxman S, Boerma T, et al. National, regional, and global trends in infertility prevalence since 1990: A systematic analysis of 277 health surveys. PLOS Medicine. 9(e1001356) doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ombelet W, De Sutter P, Van der Elst J, et al. Multiple gestation and infertility treatment: registration, reflection and reaction--the Belgian project. Hum Reprod Update. 2005;11:3–14. doi: 10.1093/humupd/dmh048. [DOI] [PubMed] [Google Scholar]
- Ombelet W, Cooke I, Dyer S, et al. Infertility and the provision of infertility medical services in developing countries. Hum Reprod Update. 2008;14:605–621. doi: 10.1093/humupd/dmn042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ombelet W. Global access to infertility care in developing countries: a case of human rights, equity and social justice. FV&V in ObGyn. 2011;3:257–266. [PMC free article] [PubMed] [Google Scholar]
- Ombelet W. The Walking Egg Project: Universal access to infertility care – from dream to reality. FV&V in ObGyn. 2013;5:161–175. [PMC free article] [PubMed] [Google Scholar]
- Ombelet W. Is global access to infertility care realistic? The Walking Egg Project. Reprod Biomed Online. 2014;28:267–272. doi: 10.1016/j.rbmo.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Pennings G, de Wert G, Shenfield F, et al. ESHRE Task Force on Ethics and Law. Providing infertility treatment in resource-poor countries. Hum Reprod. 2009;24:1008–1011. doi: 10.1093/humrep/den503. [DOI] [PubMed] [Google Scholar]
- Rutstein SO, Iqbal HS. Infecundity, Infertility, and Childlessness in Developing Countries. DHS Comparative Reports, WHO. 2004 [Google Scholar]
- Swain JE. A self-Contained Culture Platform Using Carbon Dioxide Produced from a Chemical Reaction Supports Mouse Blastocyst Development. In Vitro J Reprod Dev. 2011;57:551–555. doi: 10.1262/jrd.11-022m. [DOI] [PubMed] [Google Scholar]
- Van Balen F, Gerrits T. Quality of infertility care in poor-resource areas and the introduction of new reproductive technologies. Hum Reprod. 2001;16:215–219. doi: 10.1093/humrep/16.2.215. [DOI] [PubMed] [Google Scholar]
- Van Balen F, Bos HMW. The social and cultural consequences of being childless in poor-resource areas. FV&V in ObGyn. 2009;1:106–121. [PMC free article] [PubMed] [Google Scholar]
- Van Blerkom J, Manes C. Development of preimplantation rabbit embryos in vivo and in vitro. II. A comparison of qualitative aspects of protein synthesis. Devel Biol. 1974;40:40–51. doi: 10.1016/0012-1606(74)90105-5. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Ombelet W, Klerkx E, et al. First Births with a Simplified Culture System for Clinical IVF and ET. 2014;28:310–320. doi: 10.1016/j.rbmo.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Vayena E, Peterson HB, Adamson D, et al. Assisted reproductive technologies in developing countries: are we caring yet? Fertil Steril. 2009;92:413–416. doi: 10.1016/j.fertnstert.2009.02.011. [DOI] [PubMed] [Google Scholar]