Abstract
As the classical first trimester Down syndrome screening (FTS, combination test) has a false-negative rate of 20-25% and > 95% of the abnormal FTS results are false-positive, we evaluated the new Non-Invasive Prenatal Test (NIPT) in Belgium and the Netherlands.
The study population consisted of 3000 consecutive pregnancies in Belgium and the Netherlands in which NIPT was performed using the Harmony test. In 57 (1.9%) of the 3000 pregnancies an abnormal NIPT result was found. This included 51 fetuses with trisomy 21, 4 fetuses with trisomy 18 and 2 fetuses with trisomy 13. In 47 of the 57 the NIPT result was confirmed by genetic testing of material obtained by amniocentesis or chorionic biopsy, and no false-positive results were recorded. The false-negative rate as determined on more than 2000 women that had delivered at the time of reporting was low, and so far only 2 false-negative results were reported (one trisomy 18 and one trisomy 21). The failure rate where no NIPT result could be obtained after repeated sampling was 0.90%.
In this large clinical series, NIPT using the Harmony test proves to be a very reliable prenatal test to detect fetal trisomies 21, 18 and 13 in maternal blood in Belgium and the Netherlands.
Keywords: Combination test, Down Syndrome screening, first trimester screening, NIPT, non-invasive prenatal test, Triple test, Trisomy 13, Trisomy 18, Trisomy 21
Introduction
Currently, first trimester screening (FTS), also referred to as the combination test (CT), is the most widely used test in the prenatal screening for fetal aneuploidies including trisomy 21 (Down syndrome). Briefly, FTS is a calculated risk for fetal trisomy 21, 18 and 13 based on maternal age, nuchal translucency (NT), and two maternal serum markers, Pregnancy-Associated Placental Protein (PAPP-A) and free-beta HCG (fβHCG) (Wapner et al., 2003; Gyselaers et al., 2005; Nicolaides, 2005; Centraal Orgaan, 2012; Centrum voor Bevolkingsonderzoek van het Rijksinstituut voor Volksgezondheid en Milieu, 2012; Simpson, 2013). The cut off for an elevated FTS risk is 1/200 in the Netherlands (Centraal Orgaan, 2012; Centrum voor Bevolkingsonderzoek van het Rijksinstituut voor Volksgezondheid en Milieu, 2012) and 1/300 in Belgium (Gyselaers et al., 2005). Above this cut-off, patients traditionally have been offered an invasive test – amniocentesis (AC) or chorionic villus sampling (CVS) – to detect or exclude fetal trisomy 21, 18 and 13. In Belgium FTS and AC/CVS are available and reimbursed by the Social Security (RIZIV) for all pregnant women (Gyselaers et al., 2005). In the Netherlands only pregnant women of 36 years or older in week 18 of the pregnancy, or with an indication for invasive testing are reimbursed for these tests (Centraal Orgaan, 2012; Centrum voor Bevolkingsonderzoek van het Rijksinstituut voor Volksgezondheid en Milieu, 2012). Currently, about 70% of the pregnancies are screened by FTS in Belgium (Gyselaers et al., 2005), while in the Netherlands only 30% are screened (Centraal Orgaan, 2012; Centrum voor Bevolkingsonderzoek van het Rijksinstituut voor Volksgezondheid en Milieu, 2012). Despite such screening the number of babies born with trisomy 21 has increased over the last decade, mainly due to the increasing maternal age of pregnant women (Loane et al., 2013).
FTS has a detection rate (sensitivity) of 70-80% for trisomy 21, indicating a false-negative rate of 20-30%. This means that in one of 4 pregnancies with trisomy 21, a child with Down syndrome is born to parents that felt reassured by FTS. In addition, the high false-positive rate of FTS is a major drawback: in 5% of all women tested (95% of the women with increased FTS risk) FTS indicates an increased risk whereas there exists no fetal trisomy. This leads to anxiety and many unnecessary invasive procedures such as amniocentesis (AC) or chorionic villus sampling (CVS), which carry a risk of miscarriage of approximately 1% (Alfirevic et al., 2003). In Belgium alone invasive procedures performed because of an abnormal FTS test lead to miscarriage of approximately 50 healthy babies each year. The high percentage of false-positives and false-negatives of FTS, the associated fear and anxiety, and the relatively high risk of miscarriage after invasive procedures sparked further research towards alternative reliable but safe screening strategies for fetal trisomies on maternal blood.
In 1997 Dennis Lo discovered that the cell-free DNA (cfDNA) fraction of blood (plasma) of pregnant women contains a much higher fetal/maternal ratio of DNA than total maternal blood (Lo et al., 1997). In 2012 it became possible to analyze cell-free fetal DNA (cffDNA) in the maternal blood with the use of Next Generation Sequencing (NGS) (Behjati and Tarpey, 2013). This led to the introduction of Non-Invasive Prenatal Testing (NIPT) for fetal trisomies on maternal blood (Sparks et al., 2012; Gil et al., 2013; Fairbrother et al., 2013; Morain et al., 2013; Ohno and Caughey, 2013).
We report the results of the first 3,000 consecutive NIPT tests performed in pregnant women from Belgium and The Netherlands.
Materials and Methods
Between March 2013 and December 2013, more than 4,000 NIPT tests were performed by GENDIA (Genetic Diagnostic Network, Antwerp, Belgium). The first 3,000 NIPT tests in pregnancies from Belgium and the Netherlands were selected for this study. All patients received detailed information on the value and limitations of NIPT before and during the consultation, were given reference to a specific patient consent and informational materials including but not limited to the website created by GENDIA (www.DownsyndromeNIPT.info), and were asked to fill out an application form which the following data were recorded: maternal age, duration of pregnancy, mode of conception, single or multiple pregnancy, vanishing twins, indication for NIPT, results of the first trimester screening (FTS) and familial genetic diseases. All patient data was entered in the NIPT database of the GENDIA lab.
In all pregnancies the Harmony prenatal test from Ariosa Diagnostics (San Jose, California, USA) was performed. Streck DNA BCT blood tubes (2 × 10 ml) were used for blood sampling during this study. Blood was collected from the 10th week of gestation on, and sent out for testing with express mail carrier to Ariosa Diagnostics. When a fetal/maternal cfDNA ratio of < 4% was observed, no NIPT analysis was performed and a redraw was offered at no additional cost to the patient. Both low risk and high risk test results were communicated to the pregnant woman and her referring doctor. When a trisomy was detected, the couple was counseled by a clinical geneticist (PW) or gynecologist, and confirmation of the NIPT diagnosis after chorionic biopsy (CVS) or amniocentesis (AC) was suggested.
Results
Patients
We collected more than 4,000 blood samples from pregnant women from March 2013 until December 2013 for non-invasive prenatal testing (NIPT). The first 3,000 consecutive NIPT tests from women living in Belgium (21.24%) or the Netherlands (78.76%) are reported here.
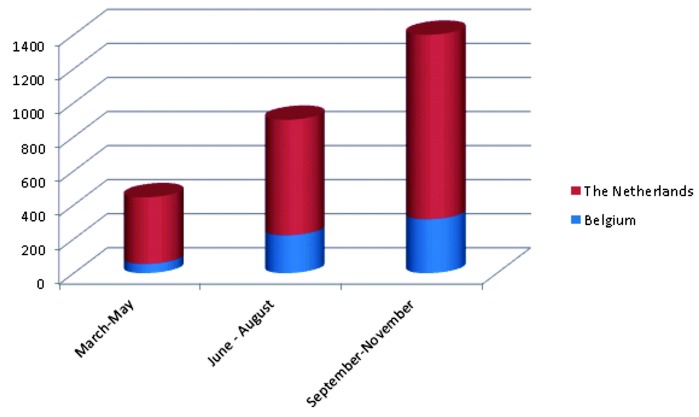
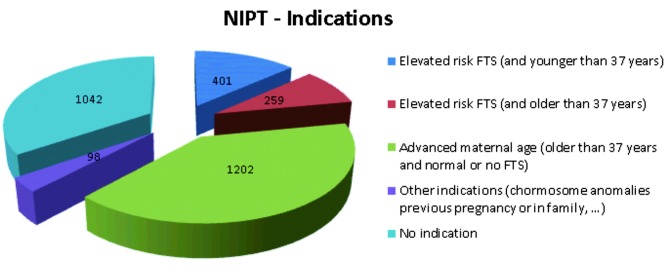
Since March 2013 the number of patients increased significantly during the 9-month study period from < 100 to > 400 per month (Fig. 1). The mean maternal age was 36 ± 3 yrs (range: 18-49 yrs), whereas the mean gestational age was 13 ± 2 wks (range: 10-30 weeks). Indications for testing (Fig. 2) were diverse: i) an elevated FTS risk (> 1/200 in the Netherlands and > 1/300 in Belgium) was the indication in 660 women (22%), with 259 of these women being 37 years or older at the expected day of delivery, ii) advanced maternal age (> 37 yrs at expected day of delivery) without increased risk on FTS or no FTS done was present in 1202 women (40.06%), iii) other indications for NIPT, including previous pregnancies with chromosome anomalies and chromosome anomalies in one or both parents or family, were present in 98 pregnancies (3.27%); iv) none of these indications were present in 1042 (34.73%) of the pregnancies (Fig. 2). Anxiety for a baby with trisomy or miscarriage after invasive procedures (CVS/AC) was often mentioned, but not recorded due to its subjective nature. Of the 944 pregnancies with known first trimester serum screening, 660 had an elevated risk for trisomy 21, 18 or 13.
Fig. 1. Increase of the amount of NIPT tests in Belgium and the Netherlands during the initial 9-month period since the start of NIPT in March 2013.
Fig. 2. Indications for 3,000 pregnant woman in Belgium and the Netherlands to request NIPT (FTS = first trimester screening) .
The turnaround time (TAT) between blood sampling and result (including the transit time to the USA from Belgium) was 11 ± 3 days (range: 7-21 days).
Trisomies
In a total of 3,000 NIPT pregnancies 2968 results were obtained, of which 2907 were normal, whereas 57 (1.9%) fetal trisomies were found (Table 1). These consisted of 51 fetuses with trisomy 21 (42 Dutch, 9 Belgian pregnancies), 4 with trisomy 18 (4 Dutch pregnancies), and 2 with trisomy 13 (2 Dutch pregnancies). All women were counseled to have NIPT results confirmed by genetic testing using an invasive diagnostic procedure (CVS/AC). Of these 57 trisomies detected by NIPT, 47 were confirmed by genetic analysis of fetal material obtained by chorionic biopsy or amniocentesis. Three women had termination in an abortion clinic without confirmation, 6 women had a spontaneous miscarriage in the week before or after the NIPT result without confirmation of the abnormal NIPT result, and in 1 pregnancy there was no follow up. The mean age of the women with a trisomic fetus was 38,18 ± 2,74 yrs (range: 27- 46 yrs).
Table I. Theoretical comparison of classical Down syndrome screening (FTS/ invasive testing) versus NIPT in Belgium.
| FTS/Invasive | NIPT | |
| Number screenings* | 100.000 | 100.000 |
| Expected T21** | 200 | 200 |
| Detection rate T21 | 73% | > 99% |
| Diagnosed T21 | 146 | 199 |
| False-negatives T21 | 54 | 2 |
| False-positives*** | 4990 | < 100 |
| Iatrogenic miscarriage**** | 50 | 1 |
* In Belgium about 35% of the population is screened
** Population risk set at 1/500
***False-positive rate of FTS: 4.8%
***False-positive rate of NIPT: < 0.1%
**** Iatrogenic miscarriage: miscarriage due to invasive procedures: 1%
False-positives
Of the 57 positive NIPT results, 47 were confirmed by follow-up genetic testing. In the other 10 no confirmation was obtained, as outlined above. Thus, no false-positives were reported.
False-negatives
So far, only 2 false-negatives have been recorded: a trisomy 18 not diagnosed by NIPT was found after amniocentesis initiated due to ultrasound anomalies. Karyotype analysis of amniotic fluid and post partum sampling showed trisomy 18 in all mitoses. A baby with trisomy 21 was born after normal NIPT with trisomy 21 in all 10 mitoses studied after birth. At the time of writing, more than 2000 of the 3000 women included in this series had delivered. Therefore, the false-negative rate is 2/ > 2.000 NIPT tests.
Failures
Ariosa Diagnostics measures and analyses fetal fraction in every NIPT sample, but only issues a report if the fetal cfDNA fraction is > 4% of the total cfDNA in the sample received. In 55 (1,83%) cases, the fetal fraction was < 4%, and NIPT analysis was not performed. This figure is in line with previous papers published measuring the fetal fraction of pregnancies in > 22,000 pregnant patients by Ariosa Diagnostics. In all of these cases, a repeat sampling was offered. The cffDNA was also low in the second sample in 27 (0,90%) of pregnancies after the repeat sampling. In 5 pregnancies with failure on the first sample, no second attempt was made. An invasive test (amniocentesis) showed a triploidy in one of the pregnancies were NIPT failed twice due to low fetal fraction.
Discussion
The first 3,000 NIPT results with the Harmony prenatal test in Belgium and the Netherlands confirm the clinical value of this new test for the detection of fetal trisomies 21, 18 and 13.
In these 3000 pregnancies 57 trisomies were detected, 2911 NIPT results were normal, and in 32 pregnancies no NIPT result was obtained. Of the 57 trisomies 51 were trisomy 21, 4 were trisomy 18 and 2 were trisomy 13 (Fig. 3). No false-positive results were recorded, with 47 (82.25%) of the trisomies being confirmed after invasive testing. This very high specificity and ratio between true positives and false-positives in our series of 3000 NIPT pregnancies is in accordance with the reported specificity of > 99.9% in the much larger series of ARIOSA (Fairbrother et al., 2013). The sensitivity in the full set of 3000 pregnancies could not yet been determined as not all participating women had yet delivered at the time of publication. However, the sensitivity is high as only 2 false-negative results (one pregnancy with trisomy 18, one pregnancy with trisomy 21) were reported so far on a total of more than 2.000 women that have delivered by now.
Fig. 3. NIPT testing in 3000 pregnancies in Belgium and the Netherlands .
The failure rate was low in our series: in 1.83% of the first blood samples cffDNA was below the threshold to analyse (< 4% of total cfDNA ratio), and in 0,90% of the pregnancies the cffDNA was too low also after repeated sampling, and no NIPT result could be obtained. Remarkably, in one of the pregnancies where NIPT failed twice and invasive testing was performed, triploidy was found. As we know of another similar case (Jani, personal communication) it is possible that triploidy is associated with decreased cffDNA, as is known for trisomy 18. Also IVF pregnancies were associated with a higher incidence of failures : in 16 of the 306 IVF pregnancies (5,23%) the cffDNA was below the 4% threshold, and in 1,96% of the IVF pregnancies (6 woman) there was also a low cffDNA after the repeated sampling.
The main indication for NIPT (Fig. 2) was increased maternal age (40%). In only 22% elevated FTS risk was the indication, and in 35% of the pregnancies there was no specific indication (apart from fear for Down syndrome and/or fear for invasive procedures and/or fear for false-negative FTS). Although 660 pregnancies had an increased FTS risk, a fetus with trisomy 21,18 or 13 was found in only 22 of these; consequently, NIPT avoided 638 invasive procedures. Given a miscarriage rate of 0.5-2% of invasive procedures, NIPT saved the life of 5-10 healthy fetuses in this series of 3,000 pregnancies. If we would extrapolate this to Down syndrome screening in the whole of Belgium (Table I), the classical screening (FTS with invasive procedure) leads to the birth of about 50 Down syndrome babies in pregnancies without increased FTS risk (false-negatives) and miscarriage of about 50 fetuses without Down syndrome due to invasive procedures.
Replacing of invasive procedures (AC/CVS) by NIPT will save the lives of many babies. Relaxing the threshold of increased risk on FTS (1/200 in the Netherlands and 1/300 in Belgium) to a lower threshold (eg. 1/1,000) will reduce the false-negative screening tests, but increase the rate of false-positives. Moreover as elevated FTS risk was the indication for NIPT in only 22% of the 3000 pregnancies described here (Fig. 2), the expected policies of regulatory bodies or insurance companies in Belgium and the Netherlands to allow or reimburse NIPT only in case of increased FTS risk, will most likely not be very successful. With the expected further decrease in cost of NIPT, and expected reimbursement it might be expected that NIPT will soon become the first line screening test for fetal trisomy.
Conclusion
NIPT is a new non-invasive prenatal test that proves to be reliable with very few false-positives, false-negatives and failures in a diagnostic setting in Belgium and the Netherlands. Consequently, NIPT will most likely replace the classical first trimester screening (FTS) followed by invasive procedures (AC/CVS) in the coming years. The relatively high cost of NIPT versus FTS and the absence of reimbursement of NIPT in Belgium and the Netherlands are the only drawbacks for pregnant women to opt for NIPT at the moment.
Acknowledgments
The authors thank the Ariosa Diagnostics team for the excellent service and NIPT analyses. We also thank Dr Lieve Page-Christiaens for suggestions and comments on this paper.
Footnotes
Conflict of interest
Two authors (E.S. Vandenakker and D. Bekedam) are employed by the Onze Lieve Vrouwe Gasthuis, that offers the NIPT test.
Five authors (P.J. Willems, H. Dierickx, N. Segers, K. Deboulle, and A. Vereecken) are employed by or hold shares of the GENDIA Lab that offers the NIPT test.
References
- Alfirevic Z, Sundberg K, Brigham S. Amniocentesis and chorionic villus sampling for prenatal diagnosis. Cochrane Database Syst Review. 2003;3(CD003252) doi: 10.1002/14651858.CD003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behjati S, Tarpey PS. What is next generation sequencing? . Arch Dis Child Educ Pract Ed. 2013;98:236–238. doi: 10.1136/archdischild-2013-304340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centraal Orgaan, Rijksinstituut voor Volksgezondheid en Milieu. Informatie over de screening op downsyndroom. 2012;(Versie 2012)
- Centrum voor Bevolkingsonderzoek van het Rijksinstituut voor Volksgezondheid en Milieu. Rotterdam: RIVM; 2012. Monitoring 2010 van gerapporteerde verrichtingen van het screeningsprogramma Downsyndroom /Structureel Echoscopisch Onderzoek. Eindrapport, juli 2012. [Google Scholar]
- Dennis Lo YM, Corbetta N, Chamberlain PF, et al. Presence of fetal DNA in maternal plasma and serum. The Lancet. 1997;350:485–487. doi: 10.1016/S0140-6736(97)02174-0. [DOI] [PubMed] [Google Scholar]
- Fairbrother G, Johnson J, Musci TJ, et al. Clinical experience of noninvasive prenatal testing with cell-free DNA for fetal trisomies 21, 18 and 13, in a general screening population. Prenat Diagn. 2013;33:580–583. doi: 10.1002/pd.4092. [DOI] [PubMed] [Google Scholar]
- Gil MM, Quezada MS, Bregant, et al. Implementation of maternal blood cell-free DNA testing in early screening for aneuploidies. Ultrasound Obstet Gynecol. 2013;42:34–40. doi: 10.1002/uog.12504. [DOI] [PubMed] [Google Scholar]
- Gyselaers WJ, Vereecken AJ, Van Herck EJ, et al. Population screening for fetal trisomy 21: easy access to screening should be balanced against a uniform ultrasound protocol. Prenat Diagn. 2005;11:984–990. doi: 10.1002/pd.1217. [DOI] [PubMed] [Google Scholar]
- Loane M, Morris JK, Addor MC, et al. Twenty-year trends in the prevalence of Down syndrome and other trisomies in Europe: impact of maternal age and prenatal screening. Eur J Hum Genet. 2013;21:27–33. doi: 10.1038/ejhg.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morain S, Greene MF, Mello MM. A new era in noninvasive prenatal testing. N Engl J Med. 2013;369:499–501. doi: 10.1056/NEJMp1304843. [DOI] [PubMed] [Google Scholar]
- Nicolaides KH. First-trimester screening for chromosomal abnormalities. Sem Perinat. 2005;29:190–194. doi: 10.1053/j.semperi.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Ohno M, Caughey A. The role of noninvasive prenatal testing as a diagnostic versus a screening tool-a cost-effectiveness analysis. Prenat Diagn. 2013;33:630–635. doi: 10.1002/pd.4156. [DOI] [PubMed] [Google Scholar]
- Simpson JL. Cell-free fetal DNA and maternal serum analytes for monitoring embryonic and fetal status. Fertil Steril. 2013;99:1124–1134. doi: 10.1016/j.fertnstert.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Sparks AB, Wang ET, Struble CA, et al. Selective analysis of cell-free DNA in maternal blood for evaluation of fetal trisomy. Prenat Diagn. 2012;32:3–9. doi: 10.1002/pd.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wapner R, Thom E, Simpson JL, et al. First-trimester screening for trisomies 21 and 18. N Engl J Med. 2003;349:1405–1413. doi: 10.1056/NEJMoa025273. [DOI] [PubMed] [Google Scholar]