Abstract
Effective navigation requires the ability to keep track of one’s location and maintain orientation during linear and angular displacements. Path integration is the process of updating the representation of body position by integrating internally-generated self-motion signals over time (e.g., walking in the dark). One major source of input to path integration is vestibular afference. We tested patients with reduced vestibular function (unilateral vestibular hypofunction, UVH), patients with aberrant vestibular function (benign paroxysmal positional vertigo, BPPV), and healthy participants (controls) on two linear path integration tasks: experimenter-guided walking and target-directed walking. The experimenter-guided walking task revealed a systematic underestimation of self-motion signals in UVH patients compared to the other groups. However, we did not find any difference in the distance walked between the UVH group and the control group for the target-directed walking task. Results from neuropsychological testing and clinical balance measures suggest that the errors in experimenter-guided walking were not attributable to cognitive and/or balance impairments. We conclude that impairment in linear path integration in UVH patients stem from deficits in self-motion perception. Importantly, our results also suggest that patients with a UVH deficit do not lose their ability to walk accurately without vision to a memorized target location.
Keywords: Vestibular navigation, vestibular hypofunction, path integration, spatial orientation
1. Introduction
Human spatial orientation and navigation rely on the crucial ability to sense self-motion during linear and angular displacements. The ability to keep track of our location and maintain orientation is so essential to our daily lives that those without this skill are significantly disabled. Vision provides multiple sources of information for determining self-position and orientation in external space. However, in the absence of vision, we retain the ability to remain oriented by relying upon internally-generated (idiothetic) self-motion signals such as proprioceptive cues from the musculature and acceleration signals from the vestibular end organs (Highstein, 1996; Israël and Berthoz, 1989). These signals are then referenced to a remembered spatial origin or target location in working memory, along with a method of updating one’s current position and orientation in space, known as path integration or dead reckoning (Etienne et al., 1996; Loomis et al., 1999; Mittelstaedt and Mittelstaedt, 1980).
There is extensive evidence from animal studies suggesting that the vestibular system is vital in path integration and spatial navigation (Barlow, 1964; Beritoff, 1965; Cullen and Roy, 2004; Etienne et al., 1996; Mittelstaedt and Mittelstaedt, 1980, Wallace et al., 2002). Rats with unilateral and bilateral vestibular lesions display deficits in spatial memory and navigation tasks, such as the water maze, radial arm maze, T-maze and foraging tasks (Russell et al., 2003; Stackman and Herbert, 2002; Wallace et al., 2002; Zheng et al., 2006, 2009). In these studies, the rats were tested months after labyrinthectomy, suggesting that any impairment in path integration was not related to acute vestibular or locomotor deficits. Interestingly, the spatial memory and navigational deficits were manifest differently depending on the type of lesion and type of task. Rats with unilateral vestibular deafferentation (UVD) initially showed impairments on a spatial navigation task (food foraging in the dark), which resolved six months after the onset of the lesion (Zheng et al., 2006). In contrast, although rats with bilateral vestibular deafferentation (BVD) were able to complete the same task in the light, they had chronic deficits when tested in darkness (Zheng et al., 2009). These data suggest that vestibular afference is essential for spatial orientation and navigation, likely by informing the brain about changes in head and body orientation in space during self-motion.
In humans, there is limited but emerging evidence for the role of vestibular afference in path integration. Behavioral testing using a ‘blind walking’ paradigm has been the main method for assessing path integration in humans. In a typical blind walking task, blindfolded participants attempt to walk without vision to a previously viewed target location. Using this approach it has been shown that healthy humans can estimate changes in body location and orientation at distances up to 20 m or more and precisely walk to the target location without vision (Loomis et al., 1992; Philbeck and Loomis, 1997; Thomson, 1983; for a review see Mittelstaedt and Mittelstaedt, 2001). In contrast, patients with vestibular deficits show veering and other impairments, during similar non-visually guided walking tasks (Borel et al., 2004; Cohen, 2000; Cohen and Kimball, 2002; Guidetti et al., 2008; Péruch et al., 1999, 2005). Of the studies that have been conducted, the majority have relied upon evaluating individuals with vestibular hypofunction, chronic vestibulopathy, and benign paroxysmal positional vertigo (BPPV) (e.g., Borel et al., 2004; Cohen, 2000; Cohen and Kimball, 2002; Guidetti et al., 2008). These individuals often complain of spatial disorientation in conjunction with the common symptoms of vertigo, imbalance, and gaze instability during head rotation (Herdman and Whitney, 2000; Hillier and Hollohan, 2007).
In summary, the overarching findings support the notion that the loss of vestibular afference impacts path integration during blind walking. However, the majority of these studies do not explicitly examine self-motion perception, per se. Instead, gait measures, such as lateral walking deviations, response time, and directional turn errors were reported (Borel et al., 2004; Cohen, 2000; Guidetti et al., 2008). For instance, Cohen (2000) asked patients with chronic peripheral vestibular hypofunction to walk a linear distance of 7.62 m, once with their eyes open and then three times with their eyes closed. The investigator measured the forward distanced walked prior to and after veering. Compared to healthy controls and patients with vestibular schwawanoma (pre-operative), the chronic vestibulopathy patients had significant errors in the mean distance walked prior to veering and angle of veering when walking a specified linear path with their eyes closed. While these impairments may be directly related to deficits in path integration as a result of the vestibular pathology, this particular study offers minimal insight into the underlying cause of linear path integration deficits due to methodological limitations: patients walked a straight course and were instructed when to stop when they crossed the finish line, which specified the target distance. This meant that participants received feedback as to when they arrived at the true target distance (7.62 m). Theoretically, participants may have responded without using path integration and simply walked the course until receiving the verbal command to stop. In addition, these participants completed an eyes-open condition prior to the eyes-closed condition, which may have confounded the results further by allowing participants to remember the path distance and to reproduce it during the eyes-closed condition. It may also be possible that the participants counted paces and/or estimated the temporal profile (i.e., duration). While path integration likely played a role in this study, the extent to which the monitoring and updating of non-visual self-motion signals contributed to the errors is unclear.
In addition to reduced vestibular afference, cognitive and sensorimotor dysfunction may also impact performance on non-visual walking tasks. Allen et al. (2004) examined the extent to which cognitive functions, specifically information-processing speed and working memory capacity, contribute to performance on a triangle completion task in healthy young and old participants. Results showed that these cognitive functions significantly accounted for age-declined performance. To date however, most studies on path integration in patients with vestibular disorders inadequately control for cognitive impairments or existing balance dysfunction (Borel et al., 2004; Cohen, 2000; Cohen and Kimball, 2002; Péruch et al., 1999, 2006). There is conflicting evidence of cognitive impairments in humans and rodents with vestibular dysfunction (Avni et al., 2009; Grimm et al., 1989; Guidetti et al., 2008; Zheng et al., 2006). Peripheral lesions in rats are known to cause deficits in attention and spatial memory (Zheng et al., 2006, 2009). Similarly, using a Corsi block test it has been shown that patients with unilateral vestibular hypofunction (UVH) exhibit spatial memory deficits (Guidetti et al., 2008). However, others have reported normal performance on tests of general memory, attention, and intelligence (for a review see Hanes and McCollum, 2006; see also Smith et al., 2005, 2010). One possible explanation for these conflicting results is a majority of patient studies employ self-report questionnaires that assess perceived handicap due to dizziness and imbalance (e.g., Dizziness Handicap Inventory) that are highly correlated with performance (such as posturography tests; see Jacobson et al., 1991), but do not assess general cognitive functions (e.g., visuospatial memory). As a result, it is difficult to ascertain the degree to which patients’ cognitive impairments (if any) contribute to self-ratings.
The primary goal of this study was to explore the parameters in which path integration deficits occur. In addition, we wished to glean insight into the underlying cause of linear path integration deficits in patients with abnormal vestibular afference. Our approach in the current study was to take an initial step in characterizing the effects of unilateral vestibular hypofunction (UVH). For comparison we also tested healthy controls and patients that were treated for BPPV. BPPV is a transient peripheral vestibular disorder caused when calcium carbonate material (otoconia) displaces into the semicircular canals. This addition renders the semicircular canals sensitive to gravity, when they should not normally be. Treatment involves physically repositioning the head and body to return the irritating otoconia back into the utricle (Hilton and Pinder, 2004). We chose to include the BPPV patients to further probe whether a loss of vestibular afference, instead of transient aberrant function, is truly detrimental to path integration.
To address these concerns, we tested participants’ performance on two blind-walking tasks: target-directed and experimenter-guided walking. Target-directed walking is a goal-directed task that requires the participant to walk to a previously viewed target location in the absence of direct visual input (Philbeck and Loomis, 1997; Philbeck et al., 2004). Experimenter-guided walking is also a blind-walking task; however, a target location is not specified before walking. Instead, participants estimated distances walked without vision while guided along a linear trajectory by a sighted experimenter. This task provides an opportunity to assess path integration without drawing upon visual perception and spatial memory of a target. In contrast with prior studies (Borel et al., 2004; Cohen, 2000; Cohen and Kimball, 2002), our walking tasks are direct measures of self-motion sensing during non-visual locomotion — with no prior trials that allowed walking with vision.
We tested the hypothesis that vestibular afference plays a role in path integration, particularly self-motion updating, and we predict that only patients with missing vestibular afference would exhibit abnormal path integration. We restricted our focus to self-motion updating on linear trajectories using two tasks designed to evaluate path integration separately (described in more detail below). Our focus on two particular vestibular populations (UVH and BPPV) further allowed us to conduct more comprehensive neuropsychological testing in conjunction with the main behavioral walking tasks. Given that cognitive factors such as visual perception, verbal and visual memory, and visuospatial processing are likely to be implicated in our blind-walking tasks, we chose neuropsychological tests to rule out the possibility that specific cognitive impairments could account for potential errors in self-motion updating. Finally, the severity of ataxia was rated using a qualitative scale in order to assess patients’ imbalance during gait, stance, and sitting.
2. Method
2.1. Participants
The study was approved by the Johns Hopkins Medical Institution’s Institutional Review Board and was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki. Three groups of participants provided written informed consent prior to their participation. Data from n = 6 patients with UVH and n = 4 patients with BPPV were separately compared to those of five healthy controls (Table 1). Patients were identified from the vestibular rehabilitation clinic at Johns Hopkins Hospital. Patients were included based on clinical and laboratory testing. Testing was performed by one of the authors. Diagnosis of UVH was based on: (1) a history of imbalance, (2) non-positional vertigo, (3) physical examination showing a positive head impulse test result toward the affected ear, (4) absence of a mass-enhancing lesion within the internal auditory canals or cerebellopontine angle and (5) abnormal electronystagmography (ENG) examination establishing >20% asymmetry; the ENG battery included oculomotor testing, the caloric test, and positional testing. Posterior semicircular canal canalithiasis (BPPV) was diagnosed based on: (1) normal caloric examination within the ENG testing suite and (2) upbeating nystagmus with a torsional component beating geotropically elicited during the Dix Hallpike test. Healthy control group participants were identified from hospital staff and students and by use of IRB-approved fliers. Inclusion criteria for the healthy controls included: (1) negative Romberg test, (2) absent history of neurological disorder, and (3) absent orthopedic impairment. All participants had normal or corrected-to-normal vision. Exclusion criteria included: (1) non-consentable age (i.e., under 18), (2) over 85 years of age, due to age-related (peripheral and central) decline of vestibular function, (3) specific motor or visuomotor disorder (e.g., ataxia) that affected posture or dynamic gait control that required use of an assistive device, (4) legal blindness, (5) use of neuroleptics or vestibular-suppressant medications and/or (6) inability to tolerate or cooperate with study protocols. We did not test our healthy controls for abnormal vestibulo-ocular reflex (VOR) function or BPPV; we had no reason to expect any pathology of their inner ear.
Table 1.
Patient and control participants demographics
| Participant | Group | Sex | Age |
|---|---|---|---|
| 1 | BPPV | Male | 63 |
| 2 | BPPV | Male | 66 |
| 3 | BPPV | Female | 47 |
| 4 | BPPV | Male | 60 |
| 5 | UVH | Female | 63 |
| 6 | UVH | Female | 54 |
| 7 | UVH | Male | 38 |
| 8 | UVH | Female | 80 |
| 9 | UVH | Female | 59 |
| 10 | UVH | Male | 46 |
| 11 | Healthy | Female | 47 |
| 12 | Healthy | Male | 42 |
| 13 | Healthy | Female | 35 |
| 14 | Healthy | Female | 45 |
| 15 | Healthy | Male | 53 |
2.2. Design
Participants were individually tested. The experimental trials were completed in a well-lit, carpeted hallway approximately 15 m long and consisted of three tasks which were blocked and presented in the following order: verbal distance estimation, target-directed walking and experimenter-guided walking. The order of trials within each block was randomized. Distances of 3 and 6 m were presented three times within each task. To increase the range of targets, three ‘dummy’ trials were randomly interspersed within the experimental trials. During the dummy trials the target was place at 1.5, 3.5 and 5.5 m or 2.5, 3.5 and 4.5 m, measured one apiece. The dummy trials were also randomly determined. These trials were intended to discourage the participant from pre-determining the two stimulus target distances (i.e., ‘near’ and ‘far’) and attempting to produce the same response on each trial; data from these trials were not included in analyses. Participants were asked to perform each trial as accurately as possible. There were no time constraints imposed. Walking duration was measured with an electronic stopwatch. A tape measure was used to record distance walked. No feedback concerning results was provided.
2.3. Procedure
2.3.1. Objective and Subjective Measures
2.3.1.1. Path Integration
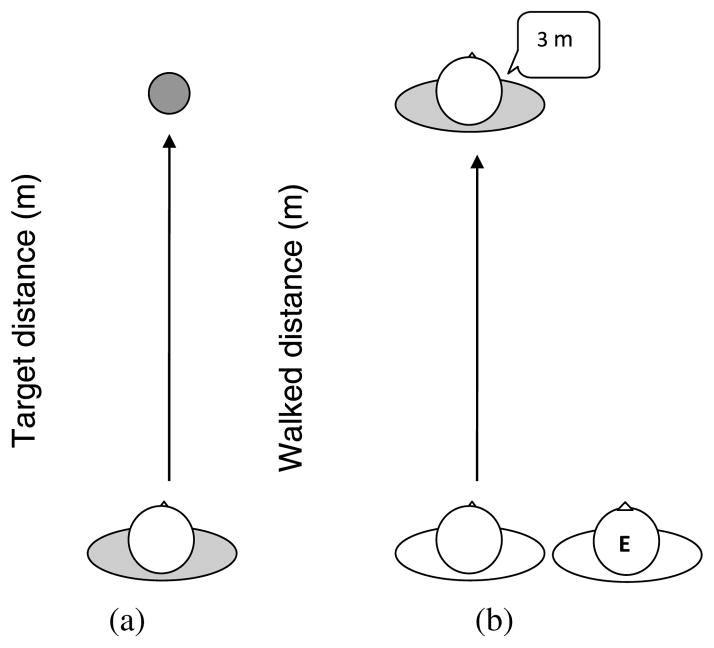
Using a blind-walking paradigm, two tasks, target-directed walking and experimenter-guided walking, were used to assess path integration (Philbeck and Loomis, 1997; Philbeck et al., 2004). Target-directed walking requires the participant to walk to a previously viewed target location in the absence of direct visual input (Fig. 1(a)). Experimenter-guided walking requires participants to keep track of their displacement from a known starting point while being led to an unknown location and to then generate a verbal estimate of the distance traveled (Fig. 1(b)). Participants were allowed to veer slightly since intrinsic veering tendencies exist in blind and blindfolded navigators (Kallie et al., 2007). No feedback was given.
Figure 1.
Overhead depiction of the two blind-walking tasks: (a) target-direct and (b) experimenter-guided walking.
2.3.1.2. Target-Directed Walking
Participants viewed the target (generally, for several seconds), then lowered a blindfold and donned hearing protectors (overall noise reduction rating: −30 dB) to minimize auditory cues. They then attempted to walk directly to the estimated target location. The target was removed prior to blind walking for safety reasons and so that participants did not receive haptic feedback. Participants were instructed to walk until they felt they had reached the remembered target location. We did not control for walking speed; instead, participants were instructed to walk at their normal speed (preferred walking velocity) as they would in everyday life. For safety, on all walking trials a sighted experimenter shadowed the participant to avoid collisions with any environmental objects (e.g., walls, etc.). In addition, to prevent the use of pace counting and other verbalization strategies, participants were given a random four-digit number to memorize. At the end of the trial, participants were asked to recall the four-digit memory number given at the beginning of the trial. Participants were led back to the origin by the sighted experimenter, still without vision, to prevent error feedback. The experimenter measured the walked distance from the origin to the terminal location.
2.3.1.3. Experimenter-Guided Walking
Participants viewed their environment for several seconds (no target present), then donned a blindfold and hearing protectors. They were then led by a sighted experimenter along a straight path to a predetermined stimulus distance; participants grasped the experimenter’s upper arm and walked along a straight path between 1.5 to 6 m long. Upon stopping, participants were required to provide a verbal estimate of the walked distance from the origin (in feet or meters). The four-digit number manipulation was also used in this task to discourage use of pace-counting strategies.
2.3.1.4. Visual Distance Perception
Verbal estimates of target distance were gathered to assess participants’ distance perception. Participants binocularly viewed a single target (an orange cone approximately 31 cm tall) under full-cue viewing conditions and verbally estimated its distance from their location (in feet or meters) after a short delay (less than 5 s) with their eyes closed. This evaluated participants’ ability to visually perceive the target and verbally report its location, without drawing on participant’s path integration abilities (Philbeck et al., 2004).
2.3.1.5. Neuropsychological Tests
To test for specific cognitive impairments and to characterize the sample populations, each participant performed a battery of neuropsychological tests. This included subjective reports and objective measures to allow for comparison between the samples tested in our study with those described in previous studies. Neuropsychological and visuomotor tests were administered prior to the walking task. To minimize the risk of boredom and/or fatigue, breaks were given between each test, and between the neuropsychological testing and experiment proper.
2.3.1.6. Verbal Memory
Verbal memory deficits can impact the aforementioned walking tasks, as well as performance on other neuropsychological tests administered; specifically, patients may have deficits in the acquisition, encoding and retention of experimental instructions. To control for this, we assessed verbal memory using the Hopkins Verbal Learning Test-Revised (HVLT-R) (Brandt and Benedict, 2001). The HVLT-R examines the ability of patients to learn and remember a verbally presented list of 12 target words. The test consists of three immediate recall trials, one delayed recall trial, and one recognition test. Scores from the three recall trials were combined to calculate the total recall score. The percentage of words retained after the delay was also calculated (number of words recalled after delay divided by the greatest number of words recalled on any of the three learning trials). Finally, the recognition discrimination index was calculated by taking the number of true positives minus the number of false positives. Higher scores on all three measures indicate better verbal learning and memory performance. The HVLT-R has been shown to have high test–retest reliability (see Woods et al., 2005) and construct validity (see Shapiro et al., 1999).
2.3.1.7. Visual Memory
Deficits in encoding and retrieving the spatial image of the target may impact the target-directed walking tasks used to assess path integration, as well as the visual distance perception task. To control for this, we used the Brief Visuospatial Memory Test-Revised (BVMT-R; Benedict, 1997) to examine visuospatial learning and memory. Patients were presented with an 8 × 11 inch page containing six geometric visual designs in a 2 × 3 array. The stimulus page was viewed for 10 s, after which the patient was instructed to reproduce as many designs in the correct locations as precisely possible. The total recall score, percentage retained, and discrimination index were calculated in the same manner as the HVLT-R. Higher scores on all three measures indicate better visual learning and memory performance. Prior research has shown that the BVMT-R has good interform reliability and supports the construct and criterion-related validity (see Benedict et al., 1996).
2.3.1.8. Visual Perception
Deficits in perceiving the initial target location may affect performance on the target-directed walking task. The Judgment of Line Orientation (JLOT; Benton et al., 1978) was used to control for deficits in visual spatial processing. The JLOT requires little motor skill to complete, and is regarded as a “pure” measure of visual perception. This task required patients to match a pair of visually-presented angled lines to two of eleven numbered lines appearing as a semicircle. The number of correct responses out of the thirty items was totaled. Good performance on this test reflects good visual acuity, visual attention, and visuospatial processing. The JLOT has high test–retest reliability and demonstrable construct validity (see Benton et al., 1978; see also Riccio and Hybd, 1992).
2.3.1.9. Working Memory
Our walking tasks required participants to attend to the stimuli (i.e., distance walked) while at the same time performing other mental tasks (i.e., maintain a four-digit number and the target location). Therefore, deficits in working memory and attentional capabilities may significantly decrease performance on the walking tasks. We used two subtests of the Wechsler Memory Scale-III (WMS-III; Wechsler, 1997), digit span and spatial span, to examine working memory capacity. We specifically addressed the ability to concentrate on, hold, and manipulate both simple and complex information. The digit span subtest tests auditory working memory by requiring patients to repeat sequences of numbers forward and backward. The spatial span subtest tests spatial sequence learning by requiring patients to reproduce a tapped sequence of block locations on a board both forward and backwards. The total number of items completed correctly on digit span and spatial span were determined. High test scores reflect good auditory and visual working memory capacity. Both subtests are reliable and valid (see WMS III Technical Manual, 1997).
2.3.1.10. Visual–Spatial Integration
The Hooper-Visual Organization Test (VOT; Hooper, 1958) was used to assess mental rotation, visual analysis and integration. Patients were presented with pictures of thirty common objects that had been cut into several pieces and placed in a random order on the card. Patients were required to identify and write the name of the original objects as if the pieces were assembled into one image. The number of correct responses out of the thirty items was totaled. Higher scores on this test reflect good visual spatial and perceptual abilities. The VOT possesses high interrater reliability and validity (see Lopez et al., 2003).
2.3.1.11. Ataxia
A subset of the Scale for the Assessment and Rating of Ataxia (SARA; Schmitz-Hübsch et al., 2006) and the Romberg Test Suite were used to quantify balance and visuomotor function. Four functional items from the SARA were assessed: gait, stance, sitting, and speech. To assess gait, participants were asked to: (1) walk at a safe distance parallel to a wall, (2) turn around to face the opposite direction of gait and (3) walk in tandem without support. To assess stance, participants were asked to stand: (1) in a natural position, (2) with feet together in parallel and (3) in tandem. For the stance test, three trials are allowed for each condition and the best trial was rated. On average, participants completed each condition in one trial. SARA scores are known to increase with the disease stage of ataxia (Schmitz-Hübsch et al., 2006). The SARA has high interrater and test–retest reliability and high validity (Schmitz-Hübsch et al., 2006). For the Romberg Test Suite, the three stance positions (as above) were repeated with eyes closed. Participants were screened for truncal ataxia by sitting on an examination bed without the support of their feet, with their eyes open and arms stretched out in front of them. The experimenter stood nearby and observed if any difficulties were present in each task (i.e., staggering, intermittent or continuous sway and/or support). Speech was also assessed during normal conversation for signs of dysarthria. Behavior on each task was rated with a qualitative scale, where a low score (i.e., ‘0’) indicates no observable difficulties or irregularities during performance (‘normal’) and a high score indicating a greater disturbances (e.g., a ‘6’ on the stance scale indicates ‘unable to stand for >10 s even with the constant support of one arm’).
2.3.1.12. Self-Reported Handicap from Dizziness
The Dizziness Handicap Inventory (DHI; Jacobson and Newman, 1990) is frequently used to evaluate the general ability of patients to cope with activities of daily life and quantify the self-perceived handicap (e.g., avoiding social situations, quitting their jobs) caused by vestibular symptoms. The DHI consists of 25 items with 3 subscales pertaining to the physical, functional, and emotional aspects associated with vertigo. The total score quantifies patients’ perception of disablement in everyday function. Scores range between 0 and 100: 0–30 suggests a mild handicap, 31–60 suggests a moderate handicap and 61–100 suggests severe handicap (physical, functional and emotional). The DHI has high concurrent validity and discriminate validity, and is able to detect statistically significant changes following interventions (Cattaneo et al., 2006; Enloe and Shields, 1997; Jacobson and Newman, 1990). The DHI also has high test–retest reliability and moderate to high internal consistency (Cattaneo et al., 2006; Enloe and Shields, 1997).
2.3.1.13. Level of Independence
The Vestibular Disorders Activities of Daily Living (VADL; Cohen and Kimball, 2000), assesses patients’ perception of independence on a much broader range of activities of daily life than the DHI (e.g., putting on shoes, reaching overhead, standing up, driving a car, etc.). The VADL consists of 28 items and a multilevel scale to rate self-efficacy on functional, ambulatory, and instrumental tasks. Scale ratings range from 1 (independent) to 10 (disabled). The VADL has good face validity, high internal consistency, and high test–retest reliability.
2.4. Statistical Analysis
The walking and verbal data were analyzed in terms of three types of error: (1) variable error, as measured by the within-subject standard deviations (this provides a measure of response consistency, with high error indicating low consistency); (2) constant error (or ‘bias’), calculated as response value minus the physical target location (this provides a measure of the overall tendency to overshoot or undershoot the physically accurate value); (3) absolute (unsigned) error, calculated as the absolute value of the walked response minus the physical target location (this provides a measure of the overall magnitude of errors, ignoring their direction). Participants’ errors were averaged across repetitions before analysis. Separate repeated measures ANOVAs were conducted on the variable, constant, and absolute errors, with ‘Group’ as the between-subjects variable and ‘Target Distance’ (3 and 6 m) included as the within-subject variable. We report the exact p-values and mean squared errors (MSE) to give an indication of the effect size.
The performance of the participants on the neuropsychological tests was compared in separate analyses of variance (ANOVAs).
3. Results
There was no differences in age between groups (F[2, 12] = 2.47; MSE = 296.93, p > 0.05). Likewise, we found no differences among the groups on any of the nine neuropsychological tests (separate ANOVAs, p > 0.05). Please see Table 2 for the individual test scores by group.
Table 2.
Demographic information and neuropsychological test scores for all participant groupsa
| Healthy controlb | BPPV | UVH | |
|---|---|---|---|
| Sex (M/F) | 2/3 | 3/1 | 2/4 |
| Age | 41.4 (35–53) | 59 (47–66) | 56.7 (38–80) |
| HVLT-R | |||
| Recall (max = 36) | 24.3 (22–26) | 27.3 (21–33) | 26.3 (20–31) |
| Retained (%) | 107 (100–111.1) | 85 (66.7–100) | 93.1 (28.6–112.5) |
| Discrimination (max = 1) | 0.95 (0.92–1) | 0.94 (0.92–1) | 0.96 (0.83–1) |
| BVMT-R | |||
| Recall (max = 36) | 23.7 (15–32) | 28.8 (25–33) | 29.3 (16–32) |
| Learning (max = 12) | 3.7 (3–5) | 5.5 (2–8) | 3.8 (2–5) |
| Retained (%) | 122.3 (100–167) | 95.5 (90–100) | 90.5 (43–100) |
| Recognition (max = 6) | 6 | 6c | 5.7 (5–6) |
| DHId (max = 100) | N/A | 46 (32–62) | 46.8 e (28–56) |
| Physical (max = 28) | N/A | 9 (4–14) | 19.2 (14–24) |
| Emotional (max = 36) | N/A | 16 (12–20) | 10 (0–16) |
| Functional (max = 36) | N/A | 21 (14–32) | 17.6 (14–22) |
| VADLe, f (max = 280) | N/A | 101 (19–249) | 91.2d (62–119) |
| Functional (max = 10) | N/A | 4.5 (1–9) | 2.5 (1–4) |
| Ambulatory (max = 10) | N/A | 4.5 (2–9) | 2.9 (1–4) |
| Instrumental (max = 10) | N/A | 5 (2–10) | 3.3 (2–4) |
| Digit span (max = 30) | 18.7 (15–23) | 21 (13–30) | 18.5 (16–23) |
| Spatial span (max = 32) | 18.7 (18–20) | 16.8 (14–20) | 17 (9–20) |
| JLOT (max = 30) | 26.3 (24–28) | 26.8 (23–28) | 26.8 (21–30) |
| VOT (max = 30) | 24.7 (23.5–26.5) | 26.4 (22–29) | 26.9 (20–29) |
| SARAd (max = 24) | 0 | 0.3 (0–1) | 0.83 (0–3) |
Except where indicated, mean raw scores are presented. Values in parentheses denote the range;
n = 3 for neuropsychological battery;
n = 1;
total score;
n = 5;
median total and/or subscale scores are presented to account for omissions, extreme and/or not applicable rating.
3.1. Verbal Estimates of Target Distance
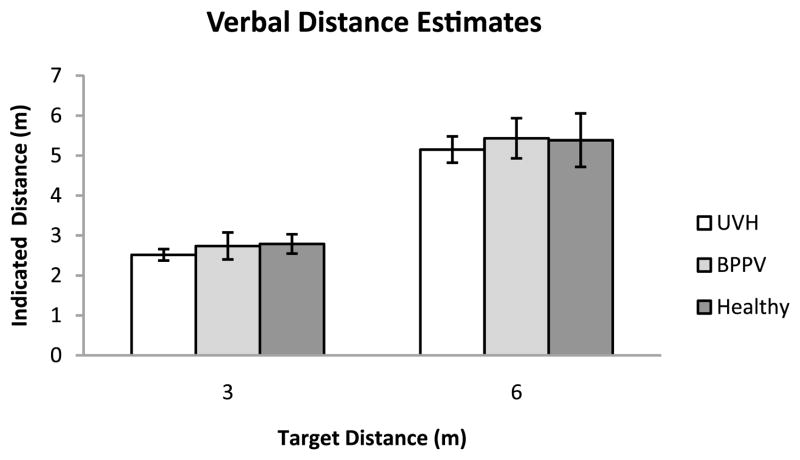
The mean indicated verbal estimates are plotted in Fig. 2.
Figure 2.
Mean indicated distance for the three groups of participants in verbal distance estimation trials. Error bars denote ± one standard error of the mean.
3.1.1. Variable Error
An ANOVA on the variable errors showed there was no reliable group difference in consistency in the verbal distance estimation task (F[2, 12] = 17.98; p = 0.887). There was a statistically significant effect of target distance on consistency (F[1, 12] = 10.59; MSE = 0.655; p = 0.007). However, we found no difference in interaction between group and verbal distance estimation (F[2, 12] = 0.65; p = 0.539). The overall mean variable errors (averaged across distances) for Healthy Control, BPPV, and UVH groups were 0.32, 0.24 and 0.29 m, respectively.
3.1.2. Constant Error
An ANOVA on the constant errors showed no effect of Group (F[2, 12] = 0.23; p = 0.800), and no effect of Target Distance (F[1, 12] = 1.95; p = 0.188). There was no statistically significant Target Distance × Group interaction (F[2, 12] = 0.01; p = 0.989). The overall mean constant errors for the Healthy Control, BPPV, and UVH group were −0.41, −0.42 and −0.67 m, respectively, with corresponding between-subject standard errors 0.32, 0.36 and 0.30 m.
3.1.3. Absolute Error
An ANOVA on the absolute errors showed no effect of Group (F[2, 12] = 0.12; p = 0.887). There was a significant effect of Target Distance on absolute error (F[1, 12] = 12.67; MSE = 2.623; p = 0.004). Results showed that this main effect was attributable to a larger amount of absolute error in the 6 m target distance than in the 3 m target distance, averaging 1.09 and 0.49 m, respectively. There was no statistically significant Target Distance × Group interaction (F[2, 12] = 0.14; p = 0.277). The overall absolute mean error for the Healthy Control, BPPV and UVH group were 0.83, 0.82 and 0.73 m, respectively, with corresponding between-subject standard errors 0.17, 0.19 and 0.15 m.
3.2. Target-Directed Walking Estimates
The mean walked responses are plotted in Fig. 3.
Figure 3.
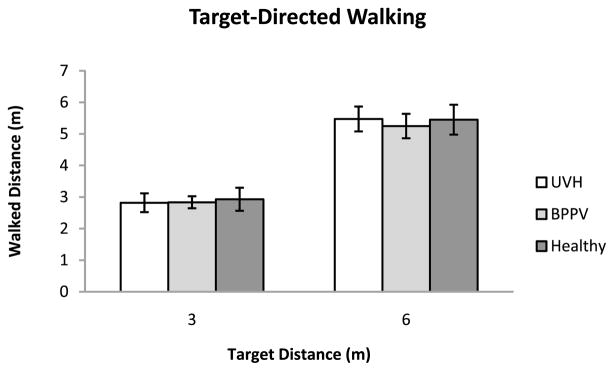
Mean walked distances in the target-directed walking trials for the three groups of participants. Error bars denote ± one standard error of the mean.
3.2.1. Response Time
An ANOVA showed no effect of Group (F[2, 11] = 3.38; p = 0.072) on response time (s) to walk to target distances of 3 or 6 m (Table 3).
Table 3.
Mean response time as a function of stimulus distance in the target-directed walking task
| Distance | Participant group
|
||
|---|---|---|---|
| Healthy Control a | BPPV b | UVH c | |
| 3 m | 3.5 (0.38) | 3.5 (0.61) | 4.7 (0.66) |
| 6 m | 6.0 (0.46) | 7.0 (1.2) | 8.0c (1.6) |
Notes: Response times are expressed in seconds; values in parentheses denote the between-subjects standard error of the mean.
n = 5;
n = 4;
n = 5.
3.2.2. Variable Error
The mean variable error for each stimulus distance in the target-directed walking task is plotted in Fig. 4(a). An ANOVA on the variable errors showed no effect of Group on mean variable error in the target-directed walking task (F[2, 12] = 1.47; p = 0.268). There was no main effect of target distance (F[1, 12] = 1.69; p = 0.218). There was no statistically significant Target Distance × Group interaction (F[2, 12] = 0.05; p = 0.956). The overall mean variable error for the Healthy Control, BPPV and UVH group were 0.28, 0.37 and 0.48 m, respectively, with corresponding between-subject standard errors 0.09, 0.10 and 0.08 m.
Figure 4.
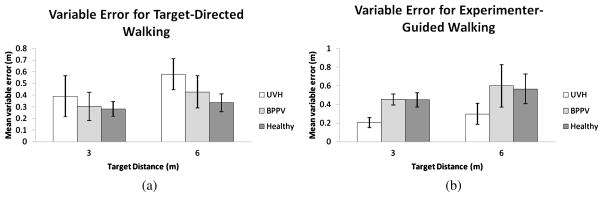
Mean within-subject standard deviations in each stimulus condition, calculated across participants, in (a) target-directed walking and (b) experimenter-guided walking. Error bars denote ± one standard error of the mean.
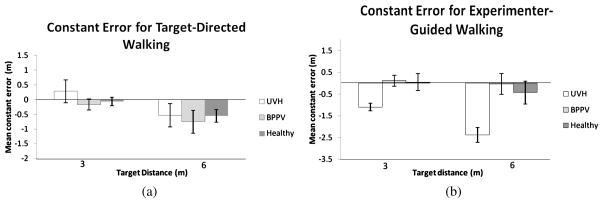
3.2.3. Constant Error
An ANOVA on the constant errors showed no effect of Group (F[1, 12] = 0.09; p = 0.916). There was a main effect of target distance (F[1, 12] = 9.60; MSE = 1.564; p = 0.009), with constant errors increasing in the larger stimulus distance. Participants tended to undershoot when attempting to walk to the 6 m target (Fig. 5(a)). There was no statistically significant Target Distance × Group interaction (F[2, 12] = 0.21; p = 0.813). The overall mean constant error for the Healthy Control, BPPV and UVH groups were −0.32, −0.46 and 0.36 m, respectively, with corresponding between-subject standard errors 0.23, 0.25 and 0.21 m.
Figure 5.
Mean constant (signed) error in each stimulus condition, calculated across participants, in (a) target-directed walking and (b) experimenter-guided walking. Error bars denote ± one standard error of the mean.
3.2.4. Absolute Error
An ANOVA on the absolute errors showed no effect of Group (F[2, 12] = 1.49; p = 0.264). There was a significant effect of Target Distance on overall error (F[1, 12] = 23.01; MSE = 1.176; p = 0.001). Results showed a larger amount of overall error in the 6 m target distance than in the 3 m target distance, averaging 0.78 and 0.38 m, respectively. There was no statistically significant Target Distance × Group interaction (F[2, 12] = 1.75; p = 0.215). The overall mean absolute error for the Healthy Control, BPPV, and UVH groups were 0.45, 0.65 0.65 m, respectively, with corresponding between-subject errors 0.10, 0.11 and 0.09 m.
3.3. Experimenter-Guided Walking Estimates
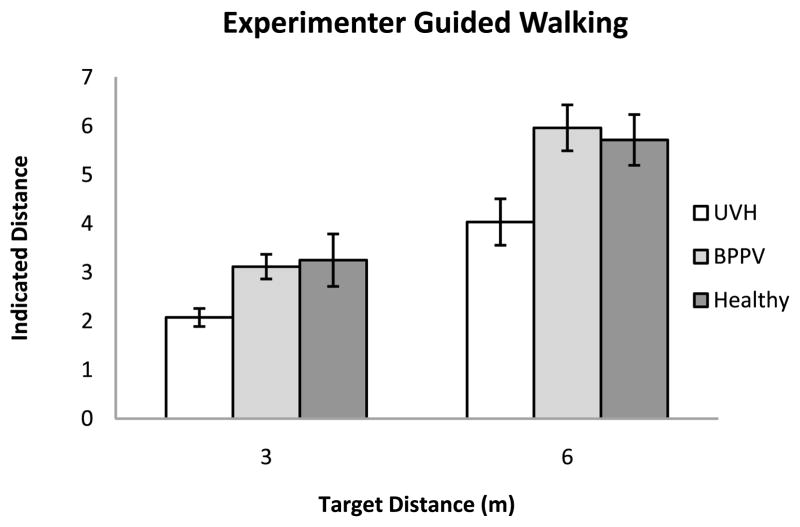
The mean indicated walked distances are plotted in Fig. 6.
Figure 6.
Mean indicated distances in the experimenter-guided walking trials for the three groups of participants. Error bars denote ± one standard error of the mean.
3.3.1. Variable Error
The mean variable error for each stimulus distance in the experimenter-guided walking task is plotted in Fig. 4(b). An ANOVA on the variable errors showed no effect of Group on mean variable error in the experimenter-guided walking task (F[2, 12] = 3.24; p = 0.075). There was no main effect of stimulus distance on response consistency (F[1, 12] = 2.24; p = 0.160). There was no statistically significant Distance × Group interaction (F[2, 12] = 0.113; p = 0.894). The overall mean variable error for the Healthy Control, BPPV and UVH groups were 0.54, 0.54 and 0.25 m, respectively, with corresponding between-subject standard errors 0.10, 0.11 and 0.09 m.
3.3.2. Constant Error
An ANOVA on the mean constant errors showed a significant effect of Group (F[2, 12] = 9.45; MSE = 10.997; p = 0.003). Pairwise planned contrasts (alpha = 0.05) were conducted to directly compare combinations of the groups’ overall error. Results suggest that the effect is attributable to the tendency of the UVH group to underestimate the stimulus distance relative to the other two groups There was an effect of Distance (F[1, 12] = 14.89; MSE = 3.161; p = 0.002), with greater error for the 6 m stimulus distance. These effects were qualified by a significant interaction between Target Distance × Group (F[2, 12] = 3.99; MSE = 0.848; p = 0.047). Pairwise planned contrasts (alpha = 0.05) were conducted in order to directly compare combinations of each group and each of the stimulus distances so that we could further isolate the source of this interaction. Results showed that this interaction was driven by the UVH group significantly undershooting target distances compared to the two control groups, with this tendency becoming more pronounced in experimenter-guided walking to the 6 m stimulus distance. The BPPV and Healthy control groups did not differ (see Fig. 5(b)). The overall mean constant error for the Healthy Control, BPPV and UVH group were −0.02, −0.04 and −1.74 m, respectively, with corresponding between-subject standard errors 0.34, 0.38 and 0.31 m.
3.3.3. Absolute Error
The results of analyses on the absolute error for experimenter-guided walking mirrored those of the constant error. An ANOVA on the absolute errors showed an effect of Group on the overall errors in the experimenter-guided walking task (F[2, 12] = 10.38; MSE = 3.598; p = 0.002). Pairwise planned contrasts (alpha = 0.05) of the groups’ overall absolute errors showed that this main effect was primarily due to differences between the UVH group and the control groups. There was also a significant effect of Distance on overall error (F[1, 12] = 22.402; MSE = 3.716; p = 0.001). Results showed that this main effect was attributable to a larger amount of overall error in the 6 m stimulus distance than in the 3 m stimulus distance, averaging 1.4 and 0.73 m, respectively. There was a statistically significant Distance × Group interaction (F[2, 12] = 3.94; MSE = 0.645; p = 0.048), with larger overall errors in the UVH group when walking to the 6 m stimulus distance than in the 3 m stimulus distance, averaging 2.38 m vs. 1.01 m, respectively. The overall mean variable errors for Healthy Control, BPPV and UVH groups were 0.32, 0.24 and 0.29 m, respectively.
4. Discussion
The main result of this study is that, relative to normal controls and patients with BPPV, only patients with UVH show significant deficits in linear path integration on an experimenter-guided walking task. Without vision, UVH patients tended to underestimate the extent of their self-motion when guided along a linear path to an unknown target distance. By contrast, UVH patients performed quite accurately when walking without vision to a previously seen target location. When no locomotion was required, UVH patients were able to accurately indicate the same target distances; thus, the underestimation in experimenter-guided walking was not simply due to poor verbal calibration.
These findings are in agreement with the emerging body of data that suggests non-visual walking deficits in peripheral vestibular defective patients are due to impairments in path integration (Borel et al., 2004; Cohen, 2000; Cohen and Kimball, 2002; Péruch et al., 1999, 2005). Moreover, we expand on previous findings in that our results suggest that this impairment stems from misperceptions in self-motion, which is attributable to the impaired vestibular afference in UVH (see Schubert and Minor, 2004, for a review of the neurophysiology underlying vestibular hypofunction). Comparable performance on the neuropsychological tasks indicated that cognitive deficits do not account for the systematic underestimation in self-motion. Also, the mean duration to complete the target-directed walking task was similar across groups suggesting these differences were not due to differences in gait velocity, contrary to recent evidence (Cohen and Sangi-Haghpeykar, 2011). Our data complement previous findings that peripheral vestibular dysfunction induces an asymmetrical perception of self-orientation in external space during whole-body rotations (i.e., turn errors) (Borel et al., 2008; Glasauer et al., 2002; Péruch et al., 2005). Taken together, these results highlight the important role of the vestibular system in path integration.
Impairment in path integration requires teasing apart error arising from processes underlying self-motion sensing and updating from other related component processes (e.g., visuospatial processing, working memory). Multimodal (or cross-modal) tasks are useful for separating the various subcomponents of path integration. In contrast to the simple walking task employed by Cohen (2000), in both of our walking tasks, the stimulus distance and response are presented in different modalities. In the target-directed walking task the stimulus distance is specified by direct visual perception of the target location, and distance is indicated using a walked response. In the experimenter-guided walking task, the stimulus distance is supplied via dynamic idiothetic information generated via locomotion (i.e., vestibular, proprioceptive, kinesthetic inputs) and the response is given verbally. Unlike unimodal tasks, which may conceal systematic perceptual errors, multimodal tasks are more sensitive to systematic errors in path integration (Philbeck et al., 2001, 2004; Sun et al., 2004b).
Our tasks required participants to (1) localize the initial target location (specifically, the target-directed walking and verbal estimation tasks), (2) create and encode the spatial image (Loomis et al., 2007) in working memory (target-directed and verbal estimation tasks) and (3) sense and integrate internally-generated self-motion signals (i.e., vestibular, proprioceptive, kinesthetic and efference copy) over time to yield an estimate of linear displacement (i.e., walked distance) relative to their starting point (target-directed and experimenter-guided walking tasks). Contributions from each of these sub-processes can be identified by comparing performance using a variety of tasks that involve common subsets of each of the components. The verbal distance estimation task draws upon the first two sub-processes and does not require path integration. The fairly accurate verbal estimation of target distances by the UVH group (without locomotion) suggests that our finding of their underestimation of distance walked was not merely due to imprecise localization of target location caused by deficits in distance perception, and/or spatial memory, as described above in factors (1) and (2). UVH patients’ accurate performance on the target-directed walking task also suggests that there were no deficits in maintaining the target location in memory for short durations. Together, these results suggest the participants with UVH were able to localize the initial target location, and encode and maintain the spatial image in working memory (Loomis et al., 2007). This suggests that the underestimation of walked distance found in the experimenter-guided walking task stems from a decreased ability to (3) sense and integrate internally-generated self-motion signals.
If the misperception of self-motion during non-visual navigation underlies the systematic errors found in our experimenter-guided walking task, then we would expect similar errors to be manifest in the target-directed walking task. However, patients performed quite accurately on the target-directed walking task. One possible explanation is related to the difference between active and passive locomotion. It has been shown that perception of self-motion is more accurate during active locomotion than passive for both angular and linear displacements (Féry et al., 2004; Jurgens et al., 1999; Philbeck et al., 2001; Sun et al., 2004a). This is reportedly due to differential processing for active versus passive motion signals at the secondary vestibular neurons (Cullen and Roy, 2004). Therefore, it is plausible that the two tasks employed in this study involved two different streams of vestibular processing. However, we feel our experimenter-guided walking task is not a passive task in the sense that although our participants did not have prior knowledge of the upcoming trajectory, our participants still had to generate a motor output to walk (Philbeck et al., 2001); this is in contrast to a passive wheelchair transport, where only feedback information about the movement is available (see Allen et al., 2004). So, the distinction between active and passive locomotion by itself is not likely to provide a complete explanation of the differences in performance on the two walking tasks.
Another non-exclusive hypothesis for the apparent discrepancy between the two tasks involves the response modes and the use of contextual information. Prior to walking in the target-directed task, participants were shown an actual target location. In contrast, for the experimenter-guided walking task, no information regarding the upcoming trajectory (i.e., walking distance) was given. Previous research has shown that vision of target locations and salient landmarks while viewing the environment provides contextual information for self-motion updating tasks (Arthur et al., 2007, 2009; Philbeck et al., 2001). It has been speculated that specifically the anticipation of actively walking to a predetermined location forms the basis of the spatiotemporal framework for incoming sensory signals during goal-directed linear locomotion (Philbeck et al., 2001). We did not control for the potential use of visual cues. In fact, our test environment afforded participants with a highly structured context (well-lit hallway, pictures on the walls). Previous work has validated that previewed landmarks along linear and angular trajectories do facilitate path integration, even when the observer cannot directly sense the objects as they pass (Arthur et al., 2007, 2009; Israël et al., 1996; Philbeck and O’Leary, 2005; Philbeck et al., 2001). However, the contextual information was available to participants in both the target-directed and experimenter-guided tasks.
What, then, can account for the differences in UVH patients’ performance on the two walking tasks? One likely explanation is the response phases in the two blind-walking tasks involved different modalities (verbal vs. walking). One major distinction between verbal and walking responses is the frame of reference underlying the response modes. Verbal responses are egocentric-referenced in that there is no requirement to respond relative to an external location (e.g., target) in the environment. In contrast, walking responses are generally considered exocentric-referenced in that they are explicitly directed to remembered locations external to the body. The difference in the frame of references underlying responses in our two blind-walking tasks could have contributed to the self-motion estimation errors found in the UVH patients. Previous work by Borel et al. (2004) suggest that unilateral vestibular deficiencies can lead to high-level adaptive mechanisms, such as switching between exocentric and egocentric frames of reference depending on the availability of salient visual cues in the external environment. Moreover, recent findings in healthy participants show that the benefit of spatial memory is particularly likely in spatial updating tasks in which one’s self-location estimate is referenced to external space (Arthur et al., submitted). In particular, UVH patients may have relied heavily on non-sensory inputs, such as memory of the surrounding environment, as a strategy to compensate for the lack of peripheral vestibular afference in the target-directed walking non-visual task. In this task one’s self-location estimate must be referenced to external space (i.e., walking to a memorized target location), so spatial memory may be beneficial for updating. In contrast, in the experimenter-guided task, they may have abandoned such a strategy and relied on idiothethic cues, presumably because information about the external environment is no longer strictly required for the task. Instead, participants are required to estimate walked stimulus distance using verbal responses relative to a frame of reference centered on their own body. Therefore, in this task, participants may be less inclined to maintain and use the remembered spatial framework to keep track of their orientation during the body rotation. In fact, our finding of increased variability within the UVH group during the target-directed walking task compared to the experimenter-guided walking task may be related to the varied use of such cognitive strategies (see Fig. 4). Thus, our interpretation is that accurate performance in the target-directed walking task may not necessarily imply accurate self-motion updating. Participants’ underestimation of the extent of self-motion in the experimenter-guided walking task may be due to deficits in the sensing and integrating of self-motion signals. Importantly, the accurate performance during the target-directed walking task in patients with UVH suggests that their deficit does not affect their ability to compensate during active locomotion and walk to a memorized target.
The use of adaptive mechanisms and strategies in unilateral vestibular patients has been noted by other researchers (Borel et al., 2004, 2008; Péruch et al., 1999). Péruch et al. (1999) tested eight patients with UVH (Ménière’s disease) before and after surgical treatment (vestibular neurotomy) on a non-visual walking task that required participants to navigate to memorized target locations. Interestingly, the experimenters found that patients performed better than the healthy controls, in terms of turn error and latency, before surgery. Additional studies showed that compared to controls, unilateral vestibular defective patients performed well on simple active blind-walking tasks (path reproduction and path reversal), yet exhibit linear and angular impairments on more complex navigation task (shortcutting) (Péruch et al., 2005). The authors concluded that the proprioceptive cues compensated for vestibular loss during the simple active blind-walking task. This raises the issue that some tasks, such as the target-directed walking task, may be less sensitive to deficits in path integration. Together with our findings, these data suggest that the performance of patients with UVH is task-dependent. Accurate performance on certain goal-directed tasks may in fact be due to compensatory mechanisms. This process of compensation for abnormal vestibular input can be one of sensory substitution (e.g., proprioception, kinesthesia, efference copy) coupled with non-sensory (cognitive) strategies, such as the use of spatial memory, that can be used to substitute for abnormal vestibular afferent signals (Borel et al., 2008). Further exploration on tasks that limit sensory input to vestibular information with a greater variety of stimulus distances would yield important insights into this issue.
Notably, our healthy control group was strikingly accurate during the experimenter-guided walking task, which contrasts with previous work (Peruch et al., 2005; Philbeck et al., 2004). Using similar blind-walking tasks, previous studies have shown an underestimation of linear displacement in neurologically-intact and UVH participants (Peruch et al., 2005; Philbeck et al., 2004). It is unclear from our study exactly what type of cues and/or cognitive strategies participants in the control group may have used to compensate for the lack of real-time visual input. Further investigation is required to fully illuminate this issue.
To our knowledge, this is the first study to use an extensive neuropsychological battery to assess cognitive functions in conjunction with path integration for patients with vestibular hypofunction. We found no evidence that patients with UVH are at a cognitive disadvantage; neither did their cognitive functioning explain difficulty in estimating walked distance in the dark. However, the extent to which our preliminary results can be informative on the contribution of vestibular afferent signals to self-motion sensing in normal and patient populations is limited. Admittedly, more extensive testing is required to confirm our current findings. Therefore, readers should be cautious about the extent to which these results from the two patient populations tested can be applied to the patient population at large. In the future, studies investigating the link between vestibular dysfunction and path integration deficits will need to focus on more standardized neuropsychological tests and non-visual walking tasks in a larger patient population with adequate control groups. Future studies should also test a broader range of stimulus and response distances to increase the sensitivity of detecting path integration deficits. Furthermore, a passive wheelchair conveyance task (e.g., Allen et al., 2004) with continuous blind pointing (e.g., Siegle et al., 2009) to investigate linear path integration in UVH would corroborate these data.
Acknowledgments
This work has not been previously published elsewhere in any medium (paper or electronic), nor has the content been simultaneously submitted for publication in any form. This work was supported in part by NIH Postdoctoral Fellowship Grant to JCA and National Institute on Deafness and Other Communication Disorders (K23-007926) Grant to MCS. The authors thank the anonymous reviewers for their insightful comments and contributions.
References
- Allen GL, Kirasic KC, Rashotte MA. Aging and path integration skill: kinesthetic and vestibular contributions to wayfinding. Percept Psychophys. 2004;66:170–179. doi: 10.3758/bf03194870. [DOI] [PubMed] [Google Scholar]
- Arthur JC, Philbeck JW, Chichka D. Spatial memory enhances the precision of angular self-motion updating. Exper Brain Res. 2007;183:557–568. doi: 10.1007/s00221-007-1075-0. [DOI] [PubMed] [Google Scholar]
- Arthur JC, Philbeck JW, Chichka D. Non-sensory inputs to angular path integration. J Vestib Res. 2009;19:111–125. doi: 10.3233/VES-2009-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avni R, Elkan T, Dror AA, Shefer S, Eilam D, Avraham KB, Mintz M. Mice with vestibular deficiency display hyperactivity, disorientation and signs of anxiety. Behav Brain Res. 2009;202:210–217. doi: 10.1016/j.bbr.2009.03.033. [DOI] [PubMed] [Google Scholar]
- Barlow JS. Inertial navigation as a basis for animal navigation. J Theoret Biol. 1964;6:76–117. doi: 10.1016/0022-5193(64)90067-0. [DOI] [PubMed] [Google Scholar]
- Benedict RHB. Brief Visuospatial MemoryTest-Revised. Psychological Assessment Resources; Odessa, FL, USA: 1997. [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Dobraski M, Shpritz B. Revision of the brief visuospatial memory test: studies of normal performance, reliability, and validity. Psycholog Assess. 1996;8:145–153. [Google Scholar]
- Benton AL, Varney NR, Hamsher KS. Visuospatial judgement. Arch Neurol. 1978;35:364–367. doi: 10.1001/archneur.1978.00500300038006. [DOI] [PubMed] [Google Scholar]
- Beritoff J. Neural Mechanisms of Higher Vertebrate Behavior. Little, Brown; Boston, MA, USA: 1965. [Google Scholar]
- Borel L, Harlay F, Lopez C, Magnan J, Chays A, Lacour M. Walking performance of vestibular-defective patients before and after unilateral vestibular neurotomy. Behav Brain Res. 2004;150:191–200. doi: 10.1016/S0166-4328(03)00257-2. [DOI] [PubMed] [Google Scholar]
- Borel L, Lopez C, Péruch P, Lacour M. Vestibular syndrome: a change in internal spatial representation. Clin Neurophysiol. 2008;38:375–389. doi: 10.1016/j.neucli.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Brandt J, Benedict RHB. Hopkins Verbal Learning Test-Revised (HVLT-R) Psychological Assessment Resources; Lutz, FL, USA: 2001. [Google Scholar]
- Cattaneo D, Regola A, Meotti M. Validity of six balance disorders scales in persons with multiple sclerosis. Disabil Rehabilit. 2006;28:789–795. doi: 10.1080/09638280500404289. [DOI] [PubMed] [Google Scholar]
- Cohen HS. Vestibular disorders and impaired integration along a linear trajectory. J Vestib Res. 2000;10:7–15. [PubMed] [Google Scholar]
- Cohen HS, Kimball KT. Development of the vestibular disorders activities of daily living scale. Arch Otolaryngol — Head and Neck Surg. 2000;126:881–887. doi: 10.1001/archotol.126.7.881. [DOI] [PubMed] [Google Scholar]
- Cohen HS, Kimball KT. Improvements in path integration after vestibular rehabilitation. J Vestib Res. 2002;12:47–51. [PubMed] [Google Scholar]
- Cohen HS, Sangi-Haghpeykar H. Walking speed and vestibular disorders in a path integration task. Gait Posture. 2011;33:211–213. doi: 10.1016/j.gaitpost.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KE, Roy JE. Signal processing in the vestibular system during active versus passive head movements. J Neurophysiol. 2004;91:1919–1933. doi: 10.1152/jn.00988.2003. [DOI] [PubMed] [Google Scholar]
- Enloe LJ, Shields RK. Evaluation of health-related quality of life in individuals with vestibular disease using disease-specific and general outcome measures. Phys Therapy. 1997;77:890–903. doi: 10.1093/ptj/77.9.890. [DOI] [PubMed] [Google Scholar]
- Etienne AS, Maurer R, Séguinot V. Path integration in mammals and its interaction with visual landmarks. J Exper Biol. 1996;199:201–209. doi: 10.1242/jeb.199.1.201. [DOI] [PubMed] [Google Scholar]
- Féry Y-A, Magnac R, Israël I. Commanding the direction of passive whole-body rotations facilitates egocentric spatial updating. Cognition. 2004;91:B1–B10. doi: 10.1016/j.cognition.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Glasauer S, Amorim MA, Viaud-Delmon I, Berthoz A. Differential effects of labyrinthine dysfunction on distance and direction during blindfolded walking of a triangular path. Exper Brain Res. 2002;145:489–497. doi: 10.1007/s00221-002-1146-1. [DOI] [PubMed] [Google Scholar]
- Grimm RJ, Hemenway WG, Lebray PR, Black FO. The perilymph fistula syndrome defined in mild head trauma. Acta Otolaryngologica (Stockh) Supplementum. 1989;464:1–40. doi: 10.3109/00016488909138632. [DOI] [PubMed] [Google Scholar]
- Guidetti G, Monzani D, Trebbi M, Rovatti V. Impaired navigation skills in patients with psychological distress and chronic peripheral vestibular hypofunction without vertigo. Acta Otorhinolaryngologica Italica. 2008;28:21–25. [PMC free article] [PubMed] [Google Scholar]
- Hanes DA, McCollum G. Cognitive–vestibular interactions: a review of patient difficulties and possible mechanisms. J Vestib Res. 2006;16:75–91. [PubMed] [Google Scholar]
- Herdman SJ, Whitney SL. Treatment of vestibular hypofunction. In: Wolf SL, editor. Vestibular rehabilitation. F.A. Davis; Philadelphia, PA, USA: 2000. pp. 387–423. [Google Scholar]
- Highstein SM. How does the vestibular part of the inner ear work? In: Baloh RW, Halmagyi GM, editors. Disorders of the Vestibular System. Oxford University Press; Oxford, UK: 1996. pp. 3–11. [Google Scholar]
- Hillier SL, Hollohan V. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst Rev. 2007;4:CD005397. doi: 10.1002/14651858.CD005397.pub2. [DOI] [PubMed] [Google Scholar]
- Hilton M, Pinder D. The Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigo. Cochrane Database Syst Rev. 2004;2:CD003162. doi: 10.1002/14651858.CD003162.pub2. [DOI] [PubMed] [Google Scholar]
- Hooper HE. The Hooper Visual Organization Test Manual. Western Psychological Services; Los Angeles, CA, USA: 1958. [Google Scholar]
- Israël I, Berthoz A. Contribution of the otoliths to the calculation of linear displacement. J Neurophysiol. 1989;62:247–263. doi: 10.1152/jn.1989.62.1.247. [DOI] [PubMed] [Google Scholar]
- Israël I, Bronstein AM, Kanayama R, Faldon M, Gresty MA. Visual and vestibular factors influencing vestibular ‘navigation’. Exper Brain Res. 1996;112:411–419. doi: 10.1007/BF00227947. [DOI] [PubMed] [Google Scholar]
- Israël I, Grasso R, Georges-François P, Tsuzuku T, Berthoz A. Spatial memory and path integration studied by self-driven passive linear displacement. I Basic properties. J Neurophysiol. 1997;77:3180–3192. doi: 10.1152/jn.1997.77.6.3180. [DOI] [PubMed] [Google Scholar]
- Jacobson GP, Hunter L, Balzer G. Balance function test correlates of the dizziness handicap inventory. J Amer Acad Audiol. 1991;2:253–260. [PubMed] [Google Scholar]
- Jacobson GP, Newman CW. The development of the dizziness handicap inventory. Arch Otolaryngol — Head and Neck Surg. 1990;116:424–427. doi: 10.1001/archotol.1990.01870040046011. [DOI] [PubMed] [Google Scholar]
- Jurgens R, Boss T, Becker W. Estimation of self-turning in the dark: comparison between active and passive rotation. Exper Brain Res. 1999;128:491–504. doi: 10.1007/s002210050872. [DOI] [PubMed] [Google Scholar]
- Kallie CS, Schrater PR, Legge GE. Variability in stepping direction explains the veering behavior of blind walkers. J Exper Psychol: Human Percept Perform. 2007;33:183–200. doi: 10.1037/0096-1523.33.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis JM, Da Silva JA, Fujita N, Fukusima SS. Visual space perception and visually directed action. J Exper Psychol: Human Percept Perform. 1992;18:906–921. doi: 10.1037//0096-1523.18.4.906. [DOI] [PubMed] [Google Scholar]
- Loomis JM, Klatzky RL, Avraamides M, Lippa Y, Golledge RG. Functional equivalence of spatial images produced by perception and spatial language. In: Mast F, Jäncke L, editors. Spatial Processing in Navigation, Imagery and Perception. Springer; New York, NY, USA: 2007. pp. 29–48. [Google Scholar]
- Loomis JM, Klatzky RL, Golledge RG, Philbeck JW. Human navigation by path integration. In: Golledge RG, editor. Wayfinding Behavior: Cognitive Mapping and Other Spatial Processes. John Hopkins Press; Baltimore, MD, USA: 1999. pp. 125–152. [Google Scholar]
- Lopez MN, Lazar MD, Oh S. Psychometric properties of the Hooper Visual Orientation Test. Assessment. 2003;10:66–70. doi: 10.1177/1073191102250183. [DOI] [PubMed] [Google Scholar]
- Mittelstaedt ML, Mittelstaedt H. Homing by path integration in a mammal. Naturwissenschaften. 1980;67:566–567. [Google Scholar]
- Mittelstaedt ML, Mittelstaedt H. Idiothetic navigation in humans: estimation of path length. Exper Brain Res. 2001;139:318–332. doi: 10.1007/s002210100735. [DOI] [PubMed] [Google Scholar]
- Péruch P, Borel L, Gaunet F, Thinus-Blanc G, Magnan J, Lacour M. Spatial performance of unilateral vestibular defective patients in nonvisual vs visual navigation. J Vestib Res. 1999;9:37–47. [PubMed] [Google Scholar]
- Péruch P, Borel L, Magnan J, Lacour M. Direction and distance deficits in path integration after unilateral vestibular loss depend on task complexity. Cognit Brain Res. 2005;25:862–872. doi: 10.1016/j.cogbrainres.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Philbeck JW, Behrmann M, Levy L, Potolicchio SJ, Jr, Caputy AJ. Path integration deficits during linear locomotion after human medial temporal lobectomy. J Cognit Neurosci. 2004;16:510–520. doi: 10.1162/089892904323057254. [DOI] [PubMed] [Google Scholar]
- Philbeck JW, Klatzky R, Behrmann M, Loomis J, Goodridge J. Active control of locomotion facilitates nonvisual navigation. J Exper Psychol: Human Percept Perform. 2001;27:141–153. [PubMed] [Google Scholar]
- Philbeck JW, Loomis JM. Comparison of two indicators of perceived egocentric distance under full-cue and reduced-cue conditions. J Exper Psychol: Human Percept Perform. 1997;23:72–85. doi: 10.1037//0096-1523.23.1.72. [DOI] [PubMed] [Google Scholar]
- Philbeck JW, O’Leary S. Remembered landmarks enhance the precision of path integration. Psicologica. 2005;26:7–24. [Google Scholar]
- Riccio CA, Hybd GW. Validity of Benton’s judgment of line orientation test. J Psychoeducat Assess. 1992;10:210–218. [Google Scholar]
- Russell NA, Horii A, Liu P, Smith PF, Darlington CL, Bilkey DK. Bilateral peripheral vestibular lesions produce long-term changes in spatial learning in the rat. J Vestib Res. 2003;13:9–16. [PubMed] [Google Scholar]
- Schmitz-Hübsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, Giunti P, Globas C, Infante J, Kang JS. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66:1717–1720. doi: 10.1212/01.wnl.0000219042.60538.92. [DOI] [PubMed] [Google Scholar]
- Schubert MC, Minor LB. Vestibulo-ocular physiology underlying vestibular hypo-function. Phys Therapy. 2004;84:373–385. [PubMed] [Google Scholar]
- Shapiro AM, Benedict RHB, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-Revised. Clin Neuropsychol. 1999;13:348–358. doi: 10.1076/clin.13.3.348.1749. [DOI] [PubMed] [Google Scholar]
- Siegle JH, Campos JL, Mohler BJ, Loomis JM, Bülthoff HH. Measurement of instantaneous perceived self-motion using continuous pointing. Exper Brain Res. 2009;195:429–444. doi: 10.1007/s00221-009-1805-6. [DOI] [PubMed] [Google Scholar]
- Smith PF, Darlington CL, Zheng Y. Move it or lose it: is stimulation of the vestibular system necessary for normal spatial memory? Hippocampus. 2010;20:36–43. doi: 10.1002/hipo.20588. [DOI] [PubMed] [Google Scholar]
- Smith PF, Zheng Y, Horii A, Darlington CL. Does vestibular damage cause cognitive dysfunction in humans? J Vestib Res. 2005;15:1–9. [PubMed] [Google Scholar]
- Stackman RW, Herbert AM. Rats with lesions of the vestibular system require a visual landmark for spatial navigation. Behav Brain Res. 2002;128:27–40. doi: 10.1016/s0166-4328(01)00270-4. [DOI] [PubMed] [Google Scholar]
- Sun HJ, Campos JL, Chan GS. Multisensory integration in the estimation of relative path length. Exper Brain Res. 2004a;154:246–254. doi: 10.1007/s00221-003-1652-9. [DOI] [PubMed] [Google Scholar]
- Sun H-J, Campos JL, Young M, Chan GS, Ellard CG. The contributions of static visual cues, nonvisual cues, and optic flow in distance estimation. Perception. 2004b;33:49–65. doi: 10.1068/p5145. [DOI] [PubMed] [Google Scholar]
- Thomson JA. Is continuous visual monitoring necessary in visually guided locomotion? J Exper Psychol: Human Percept Perform. 1983;9:427–443. doi: 10.1037//0096-1523.9.3.427. [DOI] [PubMed] [Google Scholar]
- Wallace DG, Hines DJ, Pellis SM, Whishaw IQ. Vestibular information is required for dead reckoning in the rat. J Neurol. 2002;22:10009–10017. doi: 10.1523/JNEUROSCI.22-22-10009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler D. Weschler Memory Scale — III Manual. Psychological Corporation; San Antonio, TX, USA: 1997. [Google Scholar]
- Woods SP, Scott JC, Conover E, Marcotte TD, Heaton RK. Test–retest reliability of component process variables within the Hopkins Verbal Learning Test-Revised. Assessment. 2005;12:96–100. doi: 10.1177/1073191104270342. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Darlington CL, Smith PF. Impairment and recovery on a food foraging task following unilateral vestibular deafferentation in rat. Hippocampus. 2006;16:368–378. doi: 10.1002/hipo.20149. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Goddard M, Darlington CL, Smith PF. Long-term deficits on a foraging task after bilateral vestibular deafferentation in rats. Hippocampus. 2009;19:480–486. doi: 10.1002/hipo.20533. [DOI] [PubMed] [Google Scholar]