Abstract
Purpose
We previously found that up to 75% of treatment failures after chemoradiation for unresectable esophageal cancer appear within the gross tumor volume (GTV) and that intensity-modulated [photon] radiation therapy (IMRT) may allow dose escalation to the tumor without increasing normal-tissue toxicity. Proton therapy may allow further dose escalation with even lower normal-tissue toxicity. Here we compared dosimetric parameters for photon IMRT with intensity-modulated proton therapy (IMPT) for unresectable, locally advanced distal esophageal cancer.
Methods and Materials
Four plans were created for each of 10 patients: IMPT delivered via anteroposterior/posteroanterior (AP/PA) beams, left posterior oblique/right posterior oblique (LPO/RPO) beams, or AP/LPO/RPO beams; and IMRT delivered with a concomitant boost to the GTV. Doses were 65.8 Gy to the GTV and 50.4 Gy to the planning target volume (PTV) in 28 fractions.
Results
Relative to IMRT, the IMPT (AP/PA) plan led to considerable reductions in mean lung dose (3.18 Gy vs 8.27 Gy, p<0.0001) and in lung V5, V10, and V20 (p≤0.0006) but did not reduce cardiac dose; the IMPT LPO/RPO plan also reduced the mean lung dose (4.9 Gy vs 8.2 Gy, p<0.001) as well as reducing heart doses (mean cardiac dose and cardiac V10, V20, and V30, p≤0.02) and liver doses (mean hepatic dose 5 Gy vs 14.9 Gy, p<0.0001). The IMPT AP/LPO/RPO plan led to considerable reductions in dose to the lung (p≤0.005), heart (p≤0.003), and liver (p≤0.04).
Conclusions
Compared with IMRT, IMPT for distal esophageal cancer can lower the dose to the heart, lung, and liver. The AP/LPO/RPO beam arrangement was optimal for sparing all three organs. The dosimetric benefits of protons will need to tailored to each patient based on their specific cardiac and pulmonary risks. IMPT for esophageal cancer will soon be investigate further in a prospective trial at our institution.
Keywords: esophagus, dose escalation, IMPT, dosimetry
INTRODUCTION
Trimodality therapy (surgery, chemotherapy, and radiotherapy) for esophageal cancer has improved patient outcomes, with radiotherapy believed to contribute to improvements in local control and survival (1, 2). Although radiation planning, tumor imaging, and radiation delivery have advanced rapidly over the past several decades, the radiation dose used to treat esophageal cancer has remained relatively unchanged. This may be a reflection of the difficulty in standardizing advance radiation technique among multiple institutions, making it difficult for our large cooperative groups to run the studies needed to answer these questions. In a previous review of failure patterns among patients with unresectable esophageal cancer treated with chemoradiation therapy to 50.4 Gy, we found that 75% of those failures occurred within the gross tumor volume (GTV) (3). This finding suggests that current trimodality therapies are beneficial in some cases, but local disease control, specifically within the GTV, remains a problem. The benefits of dose escalation in terms of both local control and survival have been demonstrated for tumors in other anatomic sites and could perhaps be applicable to esophageal cancer as well (4-6). However there is no guarantee that enhanced local control will translate into improved survival as the majority of our patient’s still die of metastatic disease, and the overall benefit of improved local control may not be realized until systemic therapies improve.
Prior studies have been performed to evaluate the effectiveness of dose escalation for esophageal tumors. The Intergroup (INT) 0123 / Radiation Therapy Oncology Group (RTOG) 94-05 study evaluated the feasibility of escalating the dose to 65.8 Gy; results showed no benefit in terms of local control or survival and relatively high toxicity (7). However, that study involved the use of two-dimensional (2D) conformal radiotherapy with a sequential boost and the use of large margins for both the primary and high-dose volumes. Perhaps the outcome of that study would have been different if more modern techniques had been applied. In the time since the INT 0123/RTOG 94-05 trial was conducted, new radiation planning and delivery techniques such as intensity-modulated radiation therapy (IMRT) have proven beneficial in tissue sparing (8). The dosimetric advantages of charged particles and 3D treatment planning have also offered further improvements is normal tissue sparing (9).
Previously we showed that IMRT given with a simultaneous integrated boost (SIB) for esophageal cancer could achieve a 28% increase (p=0.0001) in the GTV dose while significantly reducing the mean total dose to the lung by 23% (p=0.007) and the mean cardiac dose by 30% (p=0.001) (Welsh et al., submitted). Here we explored whether intensity-modulated proton therapy (IMPT) could be used to escalate the dose to the GTV while further reducing the volume of exposed normal tissues. To do so, we compared dose-volume parameters from an IMRT-SIB plan with those of three different IMPT plans designed with different beam arrangements.
PATIENTS AND METHODS
We retrospectively identified 10 patients with biopsy-proven carcinoma of the distal esophagus treated at The University of Texas MD Anderson Cancer Center whose staging evaluations included positron emission tomography (PET) /CT, and endoscopic ultrasonography. This analysis of these patients’ treatment plans was approved by the appropriate institutional review board of MD Anderson Cancer Center.
For treatment simulation and planning purposes, all patients had undergone 4D CT scanning to account for respiratory motion. The CT images were acquired first while the patient was free-breathing, with 4D images acquired immediately thereafter. During the 4D CT image acquisition, patient respiration was monitored with an external respiratory gating system (Real-Time Position Management Respiratory Gating System; Varian Medical Systems, Palo Alto, CA). Each 4D CT image set consisted of 10 CT data sets representing 10 equally divided breathing phases in a complete respiratory cycle. The 4D CT images provided quantitative time-dependent 3D information about internal organ motion, allowing quantitative description of internal organ motion for both treatment targets and normal organs.
The GTV was delineated by the attending physician using all available resources, including fused PET/CT data, endoscopic reports, and diagnostic CT imaging. Based on the 4D CT data set an internal gross tumor volume (IGTV) was constructed which accounts for tumor motion. The IGTV was expanded to form the clinical target volume (CTV) by extending coverage 3 cm superiorly, 3 cm inferiorly, 1 cm laterally, and 3 cm into the mucosa of the stomach, depending on the attending physician’s preference. The planning target volume (PTV) was the CTV plus a uniform by 0.5-cm expansion margin. Organs at risk were outlined. Calculations of the total lung volume and mean lung dose excluded portions of the lung that were included in the IGTV. The heart was contoured from the apex to the base of the right pulmonary artery. For each of the 10 patients, we then developed four plans: an IMRT plan that included an SIB and three unique IMPT plans, all of which were to deliver 65.8 Gy to the GTV and 50.4 Gy to the PTV, with all radiation given in 28 fractions. We then evaluated the dose-volume histogram (DVH) parameters for each plan to estimate the dose to critical structures, specifically the lung, heart, liver, and spinal cord.
The IMRT-SIB plans were generated by using a step-and-shoot technique and a Pinnacle planning system (Phillips Medical Systems, Andover, MA). Beam arrangements were optimized for each patient with the goal of reducing both cardiac and pulmonary dose. The prescribed dose was 65.8 Gy (28 fractions at 2.3 Gy per fraction) to the GTV, and the CTV and PTV received the standard IMRT dose of 50.4 Gy (28 fractions of 1.8 Gy per fraction). These IMRT-SIB plans where generated by using 6x beams and using the same five beams at 80, 110, 160, 210, and 240 degrees. For this planning study we did not use conventional proton planning as it does not allow for a simultaneous integrated boost, conventional proton planning could only accomplish this with a cone down boost technique.
The same PTVs and CTVs used to generate the IMRT plans were also used for the IMPT plans, which were optimized by single-field optimization by a treatment planning system (Eclipse, version 8.1 , Varian Medical Systems, Palo Alto, CA). The single-field optimization option allows each field to be independently optimized to deliver the prescribed dose(s) to the target volume(s). This technique is, therefore, less sensitive to proton range uncertainties than multiple field optimization (all spots from all fields are optimized simultaneously) (10, 11). The scanning nozzle delivers the IMPT treatment both “spot-by-spot” and “layer-by-layer” (12, 13). The treatment planning system determined the energy, spot position, and number of monitor units for each spot. The spot spacing for each field was determined as the fraction of the full width at half maximum (FWHM) of the spot in air at the isocenter for the highest energy used for the field, SS = 0.65 × FWHMair. Once the spot spacing was determined for the field, it was not changed even if lower energies were used for the proximal layers. The treatment planning system used did not consider constraints on monitor units (e.g., between 0.005 MU and 0.04 MU for each spot) during optimization; constraints were applied only during postprocessing, thereby converting the raw optimized spots to deliverable spots that had monitor units within the range defined by the minimum and maximum values (14). An analytical proton pencil-beam convolution dose algorithm was used to calculate the dose distribution. The in-air fluence for individual spots was described by a single 2D Gaussian function. Three sets of IMPT plans were designed for each of the 10 patients: one used an anterior/posterior (AP) beam arrangement, another a right posterior oblique beam (RPO) and a left posterior oblique beam (LPO) arrangement, and a three-field AP/LPO/RPO arrangement. The plan optimization process included efforts to minimize the doses to the total lung, heart, liver, and spinal cord.
Data analysis was performed with Stata/MP 11.0 statistical software. The equality of means for continuous variables was assessed by using t tests. A p value of 0.05 or less was considered to indicate statistical significance.
RESULTS
IMRT versus IMPT (AP/PA)
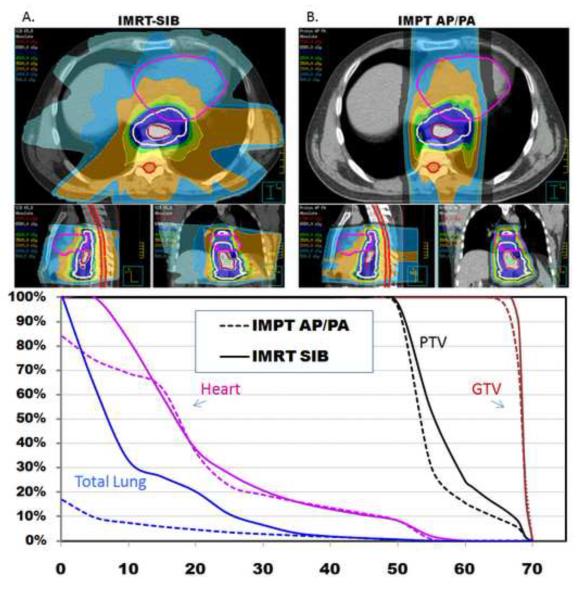
First we compared the dose-volume parameters from an IMRT-SIB plan with those from a two-beam IMPT plan in an AP/PA arrangement, which we hypothesized would maximize pulmonary sparing (Fig. 1). Separate plans were generated and optimized for all 10 patients in which the GTV was to be treated to 65.8 Gy and the PTV to 50.4 Gy (Table 1). Relative to the IMRT plan, the IMPT (AP/PA) would have delivered roughly the same mean cardiac dose (19.9 vs 21.2 Gy) and cardiac V20, V30, V40, and V50 (Table 1), although the cardiac V10 was slightly lower with the IMPT AP./PA plan (66% vs 79%, p<0.0342). However, relative to the IMRT plan, the IMPT plan reduced the mean lung dose by 61% (3.18 vs 8.27 Gy, p<0.0001), the lung V5 by 68% (12% vs 38%, p<0.0001), the lung V10 by 57% (10% vs 23%, p=0.0001), and the lung V20 by 50% (7% vs 14%, p=0.0006). Similarly, the mean hepatic dose was 4.9 Gy vs 14.9 Gy (p<0.0001), and the V30 was 7% vs 13% (p=0.0006). Both the maximum dose to 1cm3 of the spinal cord and the maximum point dose were both markedly lower in the AP/PA IMPT plan than in the IMRT plan (p=0.004 and p=0.01) (Fig. 1).
Figure 1.
A) Axial, sagittal, and coronal views of an IMRT-SIB plan in which the PTV is treated to 50.4 Gy while the GTV is boosted to 65.8 Gy. B) Axial, sagittal, and coronal views of an IMPT (AP/PA) plan in which the PTV is treated to 50.4 Gy while the GTV is boosted to 65.8 Gy. The AP/PA beam arrangement is optimal for pulmonary sparing compared to the IMRT plan.
Table 1.
Dosimetric comparison of IMRT-SIB and IMPT plans
| Treatment Plans | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Organ | IMRT |
Proton AP/PA |
Proton LPO/RPO |
Proton AP/LPO/RPO |
|||||||
| Mean | S.E. | Mean | S.E. | p value* | Mean | S.E. | p value* | Mean | S.E. | p value* | |
| GTV | |||||||||||
| Mean dose, Gy | 67.90 | 0.20 | 68.50 | 0.40 | 0.3 | 68.60 | 0.35 | 0.17 | 68.50 | 0.34 | 0.29 |
| Lung | |||||||||||
| Mean dose, Gy | 8.27 | 1.01 | 3.18 | 0.51 | <0.0001 | 4.90 | 0.69 | 0.001 | 4.30 | 0.64 | 0.0002 |
| V5, % | 38 | 5 | 12 | 2 | <0.0001 | 19 | 2 | 0.0002 | 19 | 3 | 0.0003 |
| V10, % | 23 | 3 | 10 | 2 | 0.0001 | 15 | 2 | 0.004 | 15 | 2 | 0.005 |
| V20, % | 14 | 2 | 7 | 1 | 0.0006 | 11 | 2 | 0.15 | 7 | 1 | 0.0008 |
| Heart | |||||||||||
| Mean dose, Gy | 21.20 | 1.58 | 19.90 | 1.52 | 0.31 | 11.90 | 0.98 | <0.0001 | 17.00 | 1.33 | 0.003 |
| V10, % | 79 | 4 | 66 | 5 | 0.0342 | 29 | 2 | <0.0001 | 61 | 4 | 0.0044 |
| V20, % | 44 | 4 | 46 | 5 | 0.71 | 22 | 2 | 0.0001 | 29 | 3 | 0.0003 |
| V30, % | 25 | 3 | 23 | 2 | 0.18 | 17 | 2 | 0.002 | 20 | 2 | 0.01 |
| V40, % | 15 | 2 | 15 | 2 | 0.94 | 13 | 1 | 0.07 | 14 | 2 | 0.35 |
| V50, % | 8 | 1 | 9 | 1 | 0.12 | 8 | 1 | 0.60 | 9 | 1 | 0.15 |
| Liver | |||||||||||
| Mean dose, Gy | 14.90 | 1.48 | 4.90 | 0.60 | <0.0001 | 5.00 | 0.63 | <0.0001 | 5.40 | 0.63 | <0.0001 |
| V20, % | 26 | 3 | 12 | 2 | 0.0010 | 9 | 1 | 0.0002 | 9 | 1 | 0.0001 |
| V30, % | 13 | 2 | 7 | 1 | 0.0006 | 6 | 1 | 0.0004 | 7 | 1 | 0.0005 |
| V40, % | 7 | 1 | 5 | 1 | 0.007 | 5 | 1 | 0.004 | 5 | 1 | 0.004 |
| V50, % | 4 | 1 | 3 | 1 | 0.06 | 3 | 1 | 0.07 | 3 | 1 | 0.04 |
| Spinal Cord | |||||||||||
| Maximum dose, Gy | 36.70 | 3.01 | 24.80 | 0.47 | 0.004 | 36.80 | 3.74 | 0.97 | 24.70 | 1.19 | 0.0008 |
| Maximum dose to 1 cm3, Gy | 34.30 | 2.84 | 22.80 | 0.35 | 0.01 | 34.35 | 2.11 | 0.99 | 20.73 | 0.89 | 0.002 |
vs. IMRT plan
Abbreviations: IMRT-SIB, intensity-modulated [photon] therapy with simultaneous integrated boost; IMPT, intensity-modulated proton therapy; AP/PA, anteroposterior/posteroanterior; LPO/RPO, left posterior oblique/left posterior oblique; S.E., standard error; GTV, gross tumor volume
IMRT versus IMPT (LPO/RPO)
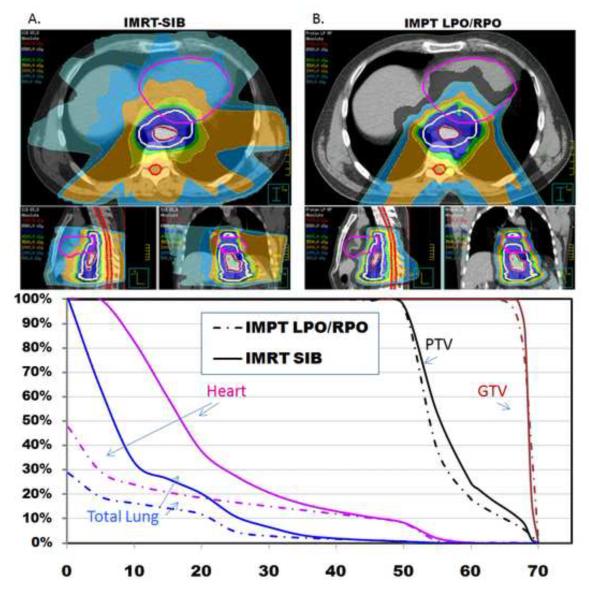
The next IMPT beam arrangement we tested was two posterior oblique beams (LPO/RPO), which we hypothesized would provide optimal cardiac sparing (Fig. 2). The IMPT (LPO/RPO) plan led to reductions of 44% in mean cardiac dose (11.9 vs 21.2 Gy, p<0.0001), and the cardiac V20 was reduced by 50% (22% vs 44%, p=0.001) and the cardiac V30 by 32% (17% vs 25%, p=0.002). The mean lung dose was reduced by 42% (4.9 vs 8.27 Gy, p<0.001). Although the lung V20 was no different in the two plans, both the lung V10 and V5 were significantly reduced in the IMPT plan, the V10 by 35% (15% vs 23%, p=0.004) and the V5 by 50% (19% vs 38%, p= 0.0002). The mean hepatic dose was decreased to 5 Gy vs 14.8 Gy (p<0.0001) and the liver V30 was decreased to 6% vs 13% (p=0.0004). Both the maximum dose to 1cm3 of the spinal cord and the maximum point dose to the spinal cord were no different in the two plans (Table 1).
Figure 2.
A) Axial, sagittal, and coronal views of an IMRT-SIB plan in which the PTV is treated to 50.4 Gy while the GTV is boosted to 65.8 Gy. B) Axial, sagittal, and coronal view of an IMPT (LPO/RPO) plan in which the PTV is treated to 50.4 Gy while the GTV is boosted to 65.8 Gy. The LPO/RPO beam arrangement is optimal for cardiac sparing compared to the IMRT plan.
IMRT versus IMPT (AP/LPO/RPO)
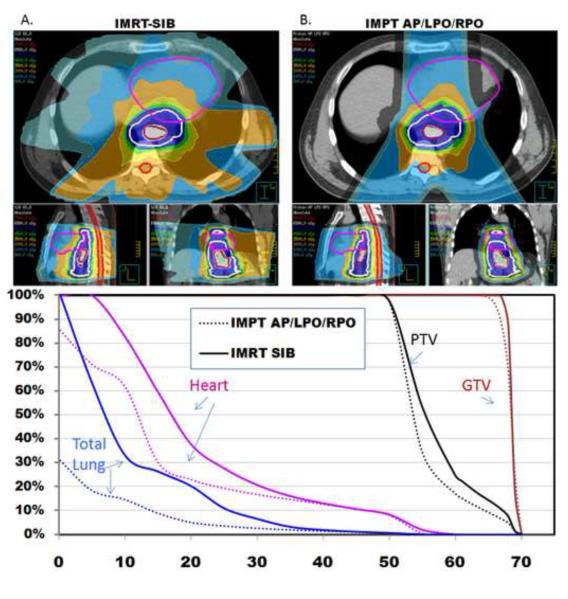
Lastly we hypothesized that a three-field IMPT plan (AP/LPO/RPO) could effectively reduce the dose to both the heart and lung relative to the two other plans owing to the multiple beam arrangement (Fig. 3). Compared with the IMRT-SIB plan, the three-field IMPT plan resulted in a lower mean cardiac dose (17 Gy vs 21 Gy, p=0.003). The heart V30 was reduced by 20% (20% vs 25%, p=0.01), the heart V20 by 35% (29% vs 44%, p=0.0003), and the heart V10 was reduced by 23% (61% vs 79%, p=0.0044). The mean lung dose was reduced by 49% (4.3 Gy vs 8.27 Gy, p=0.0002), as was the lung V20 (from 14% to 7%, p=0.0008); the lung V10 was reduced by 35% (15% vs 23%, p=0.005), and the lung V5 was reduced by 50% (19% vs 38%, p=0.0003). The mean hepatic dose was reduced from 14.9 Gy to 5.4 Gy (p<0.0001), and the hepatic V30 was 7% vs 13% (p=0.0005). Both the maximum dose to 1cm3 of the spinal cord and the maximum point dose were both markedly lower in the AP/PA IMPT plan than in the IMRT plan (p=0.0008 and p=0.002) (Table 1).
Figure 3.
A) Axial, sagittal, and coronal views of an IMRT-SIB plan in which the PTV is treated to 50.4 Gy while the GTV is boosted to 65.8 Gy. B) Axial, sagittal, and coronal views of an IMPT (AP/LPO/RPO) plan in which the PTV is treated to 50.4 Gy while the GTV is boosted to 65.8 Gy. The AP/LPO/RPO beam arrangement is optimal for achieving both pulmonary and cardiac sparing compared to the IMRT plan.
Finally, no significant differences were found with respect to target coverage of the PTV or GTV. All plans achieved excellent target coverage, with ≥ 95% of the PTV receiving at least 100% of the prescription dose as expected based on preset planning restrictions.
DISCUSSION
The results of the current study extend our prior findings regarding local failure after definitive chemoradiotherapy for unresectable distal esophageal cancer to 50.4 Gy and the potential for an IMRT-SIB technique to escalate the dose to the GTV (Welsh et al., submitted). We found here that IMPT techniques could further reduce the dose to critical structures, especially the heart and lung, during treatment of distal esophageal cancer. All three of the beam arrangements we evaluated for an IMPT scanning beam technique spared more of the heart, lung, liver, and cord than did an IMRT plan based on the same image sets. Clinical studies have indicated that minimizing of the volume of lung receiving radiation can significantly reduce the risk of potentially life-threatening complications (15, 16). Similar conclusions have been drawn from studies of radiation-induced cardiovascular and liver toxicity (17). In this study, we found that among the three techniques tested, the AP/PA field arrangement was best for reducing the pulmonary dose, the LPO/RPO technique was best for reducing the cardiac dose, and the AP/LPO/RPO plan provided some reduction in both pulmonary and cardiac exposure. All three of the IMPT plans spared the liver comparably well. Because each IMPT beam arrangement offers a unique treatment planning advantage, an optimal plan can be synthesized with consideration of each patient’s unique anatomy and health history. For example in a patient with prior cardiac disease an LPO/RPO beam arrangement may be preferred to reduce cardiac dose. Alternately, a patient with extensive nodal disease resulting in a larger thoracic CTV may benefit from an AP/PA technique to reduce the amount of normal lung treated. The potential benefits of IMPT for esophageal cancer will soon be investigate further in a prospective trial at our institution.
The past three decades have seen a large number of technologic advances in radiation therapy, with the transition from reliance on x-rays films to the use of 3D planning based on CT scans and, most recently, on PET/CT fusion scans, plus the routine use of bronchoscopy and endoscopy to accurately map the extent of disease. Nevertheless, outcomes after treatment for esophageal cancer have not significantly improved over this period. We posited here that local control could be improved through the development of more sophisticated treatment planning techniques such as IMRT and proton therapy, both of which have been shown to offer improved tissue sparing (9, 18).
Most patients with esophageal cancer who undergo proton therapy in the United States are treated with a passive scatter technique. Passive scattering uses a range shifter and scattering foil to widen the thin proton beams to cover the length of the tumor; the proton beam then goes through a collimator to shape the beam, and finally a compensator is used to shape the distal edge of the beam to fit the tumor shape. The passive scatter technique involves delivery of an equal number of protons throughout the field to create a uniform dose distribution to the treated area. One challenge associated with this technique thus is how to deliver different doses to different treatment areas. One solution is to use a cone-down technique, which requires the development of a new plan, a composite plan, and the new collimators and compensators. An alternative solution is to use pencil beam scanning, a proton delivery method that was recently approved in the United States and has been used at MD Anderson since 2008 for the treatment of prostate cancer. Pencil beam scanning uses magnets to precisely steer the beam and proton beams with different energies obviating the need for collimators and compensators. The scanning beam technique also allows more precise delivery than does passive scatter in that it allows the simultaneous delivery of different doses to high-risk and low-risk areas without the need for a cone-down.
Nevertheless, the precision offered with scanning beams invokes other factors that must be considered before this technology is adapted for the treatment of thoracic tumors. Perhaps of the most concern is the need to consider tumor motion, because the entire treatment CTV is not being treated at the same moment in time due to the interplay between tumor motion and the scanning beam. To account for this issue, we recommend respiratory gating when treating thoracic tumors with a scanning-beam treatment plan. However, because the motion of a tumor will vary depending on its location, accounting for motion variation will be site-specific and motion control should be evaluated on an individual basis accounting for factors such as the magnitude of motion and the electron density of the surrounding structures.
Although dose escalation has been shown to improve local control and survival in tumors at other anatomic sites (19), caution needs to be applied in escalating radiation doses for esophageal cancer. The anatomy of the esophagus dictates that any such tumor will be in close proximity to, if not encompassed within, critical structures; thus we must be careful not to trade off improvements in local control for increased morbidity. Excessive esophageal doses associated with high dose-irradiation for lung cancer can result in esophageal stricture, a potentially life-treating complication (20). The initial tumor size, and changes in tumor volume over the course of treatment, are also an important and potentially dose-limiting factor that will need to be monitored and accounted for. Furthermore, the penetration of the gastric layer by the radiation beam should be restricted, as gastric tolerance is thought to be below that of the esophagus (21, 22). Lastly, patients with distal esophageal tumors may be at particular risk for increased cardiac toxicity, and further work must be done to assess the impact of cardiac motion on treatment planning.
CONCLUSIONS
In summary, tremendous advances have been made in radiation treatment planning and delivery, yet many institutions are hesitant to escalate radiation doses for esophageal cancer because of the increased risk of normal-tissue toxicity as well as the controversial results of a randomized trial (7). The GTV represents the most common site of failure after definitive chemoradiation for unresectable esophageal cancer (3). The treatment-planning series reported here illustrates that at least from a dosimetric standpoint, use of an IMPT technique can safely allow increased doses to the GTV with decreased toxicity to critical structures. Currently we are preparing to evaluate this technique in a phase I clinical trial to determine the maximum tolerated dose to which the GTV can be safely escalated. This study will provide a foundation for larger prospective studies to evaluate the potential benefits of IMPT for esophageal cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Notification The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Tepper J, Krasna MJ, Niedzwiecki D. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–1092. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh TN, Noonan N, Hollywood D. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462–467. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 3.Settle SH, Bucci MK, Palmer MB, et al. PET/CT Fusion with Treatment Planning CT (TP CT) Shows Predominant Pattern of Locoregional Failure in Esophageal Patients Treated with Chemoradiation (CRT) is in GTV. International Journal of Radiation Oncology*Biology*Physics. 2008;72:S72–S73. [Google Scholar]
- 4.Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. International Journal of Radiation Oncology*Biology*Physics. 2002;53:1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 5.Martel MK, Ten Haken RK, MB. H. Estimation of tumor control probability parameters from 3-D dose distributions of non-small cell lung cancer patients. Lung Cancer. 1999:31–37. doi: 10.1016/s0169-5002(99)00019-7. [DOI] [PubMed] [Google Scholar]
- 6.Kong F-M, Ten Haken RK, Schipper MJ, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: Long-term results of a radiation dose escalation study. International Journal of Radiation Oncology*Biology*Physics. 2005;63:324–333. doi: 10.1016/j.ijrobp.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Minsky BD, Pajak TF, Ginsberg RJ. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–1174. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 8.Marks LB, Ma J. Challenges in the clinical application of advanced technologies to reduce radiation-associated normal tissue injury. Int J Radiat Oncol Biol Phys. 2007;69:4–12. doi: 10.1016/j.ijrobp.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Zhao K-l, Guerrero TM, et al. Four-Dimensional Computed Tomography-Based Treatment Planning for Intensity-Modulated Radiation Therapy and Proton Therapy for Distal Esophageal Cancer. International Journal of Radiation Oncology*Biology*Physics. 2008;72:278–287. doi: 10.1016/j.ijrobp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lomax AJ. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 1: the potential effects of calculational uncertainties. Physics in Medicine and Biology. 2008;53:1027–1042. doi: 10.1088/0031-9155/53/4/014. [DOI] [PubMed] [Google Scholar]
- 11.Lomax AJ. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 2: the potential effects of inter-fraction and inter-field motions. Physics in Medicine and Biology. 2008;53:1043–1056. doi: 10.1088/0031-9155/53/4/015. [DOI] [PubMed] [Google Scholar]
- 12.Smith A, Gillin M, Bues M, et al. The M. D. Anderson proton therapy system. Medical Physics. 2009;36:4068–4083. doi: 10.1118/1.3187229. [DOI] [PubMed] [Google Scholar]
- 13.Gillin MT, Sahoo N, Bues M, et al. Commissioning of the discrete spot scanning proton beam delivery system at The University of Texas M. D. Anderson Cancer Center, Proton Therapy Center, Houston. Medical Physics. 2010;37:154–163. doi: 10.1118/1.3259742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu XR, Sahoo N, Zhang X, et al. Intensity modulated proton therapy treatment planning using single-field optimization: the impact of monitor unit constraints on plan quality. Medical Physics. 2010;37 doi: 10.1118/1.3314073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoon KL, Ara AV, James DC, et al. Postoperative pulmonary complications after preoperative chemoradiation for esophageal carcinoma: correlation with pulmonary dose volume histogram parameters. International journal of radiation oncology, biology, physics. 2003;57:1317–1322. doi: 10.1016/s0360-3016(03)01373-7. [DOI] [PubMed] [Google Scholar]
- 16.Ramesh G, Susan LT, Ritsuko K, et al. The relationship between local dose and loss of function for irradiated lung. International journal of radiation oncology, biology, physics. 2003;56:106–113. doi: 10.1016/s0360-3016(03)00094-4. [DOI] [PubMed] [Google Scholar]
- 17.Charlie CP, Brian DK, Laura AD, et al. Radiation-Associated Liver Injury. International journal of radiation oncology, biology, physics. 76:S94–S100. doi: 10.1016/j.ijrobp.2009.06.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandra A, Liu H, Tucker SL, et al. IMRT reduces lung irradiation in distal esophageal cancer over 3D CRT. International Journal of Radiation Oncology*Biology*Physics. 2003;57:S384–S385. [Google Scholar]
- 19.Alan Pollack MD,PHD, Gunar K, Zagars MD, George Starkschall PD. Prosate cancer radation dose resposne: Results of the M.D Anderson Phase II randomized trial Int. J. Radiation Oncology Biol. Phys. 2002;53:1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 20.Marks LB, Zeng J, Light K, et al. 116: Radiation-Induced Esophageal Stricture Following Therapy for Lung Cancer: Its Clinical Course and Analysis Comparing Stricture Length With Isodose Levels. International Journal of Radiation Oncology*Biology*Physics. 2006;66:S66–S67. [Google Scholar]
- 21.van der Geld YG, Senan S, van Sörnsen de Koste JR, et al. A Four-Dimensional CT-Based Evaluation of Techniques for Gastric Irradiation. International Journal of Radiation Oncology*Biology*Physics. 2007;69:903–909. doi: 10.1016/j.ijrobp.2007.06.062. [DOI] [PubMed] [Google Scholar]
- 22.Caudry M, Escarmant P, Maire JP, et al. Radiotherapy of gastric cancer with a three field combination: Feasibility, tolerance, and survival. International Journal of Radiation Oncology*Biology*Physics. 1987;13:1821–1827. doi: 10.1016/0360-3016(87)90347-6. [DOI] [PubMed] [Google Scholar]