Abstract
Purpose
Stereotactic body radiotherapy (SBRT) can produce excellent local control of several types of solid tumor; however, toxicity to nearby critical structures is a concern. We found previously that in SBRT for lung cancer, the chest wall (CW) volume receiving 20, 30, or 40 Gy (V20, V30, or V40) was linked with the development of neuropathy. Here we sought to determine whether the dosimetric advantages of protons could produce lower CW doses than traditional photon-based SBRT.
Methods
We searched an institutional database to identify patients treated with photon SBRT for lung cancer with tumors within <2.5 cm of the CW. We found 260 cases; of these chronic grade ≥2 CW pain was identified in 23 patients. We then selected 10 representative patients from this group and generated proton SBRT treatment plans, using the identical dose of 50 Gy in 4 fractions, and assessed potential differences in CW dose between the two plans.
Results
The proton SBRT plans reduced the CW doses at all dose levels measured. The median CW V was 364.0 cm320 for photons and 160.0 cm3 for protons (P<0.0001); V30 was 144.6 cm3 for photons vs. 77.0 cm3 for protons (P=0.0012); V was 93.9 cm335 for photons vs. 57.9 cm3 for protons (P=0.005); V40 was 66.5 cm3 for photons vs. 45.4 cm3 for protons (P=0.0112); and mean lung dose was 5.9 Gy for photons vs. 3.8 Gy for protons (P=0.0001). Coverage of the planning target volume was comparable between the two sets of plans (96.4% for photons and 97% for protons).
Conclusions
From a dosimetric standpoint, proton SBRT can achieve the same coverage of the PTV while significantly reducing the dose to the CW and lung relative to photon SBRT and therefore may be beneficial for the treatment of lesions close to critical structures.
Keywords: Stereotactic body radiation therapy, protons, normal tissue toxicity, lung cancer
INTRODUCTION
Lung cancer is the leading cause of cancer-related death worldwide, with approximately 1.4 million deaths per year (1). In the United States, an estimated 221,000 individuals will be diagnosed with lung cancer and 157,000 will die from it in 2011 (2). With the advent of improved imaging techniques such as computed tomography (CT), more cases are presenting at early stages, with tumors detected during screening or found incidentally during imaging for unrelated medical issues. Stereotactic body radiation therapy (SBRT) provides an additional treatment option for some patients with early-stage tumors. Several studies have demonstrated local tumor control rates of up to 100% for early-stage lung tumors (3, 4). One study conducted in Japan showed a long-term local control rate of 92% when the biologically effective dose (BED) exceeds 100 Gy (5).
SBRT involves the use of large fraction sizes, which may contribute to high rates of toxicity to proximal critical structures, especially the mediastinum and chest wall (3, 6). Patients with high doses to the chest wall often develop acute and chronic chest wall pain, rib fractures, skin reactions, and skin contracture. Delivery of photon radiation using three-beam techniques can significantly increase the radiation dose to the chest wall and skin, thereby increasing toxicity (7). A recent review of patients treated with SBRT at MD Anderson Cancer Center (all to 50 Gy in 4 fractions) showed that the development of chronic chest wall pain after treatment correlated with the chest wall volume receiving 20, 30, 35, or 40 Gy (V20, V30, V35V40) (8).
In this study, we hypothesized that the dosimetric benefits of protons could be useful for reducing the chest wall dose while achieving comparable tumor coverage and ultimately local control of lung tumors. To test this hypothesis, we selected 10 patients who had developed grade ≥2 chest wall pain after photon-based SBRT, re-planned these cases using passive scatter proton techniques, and compared the two sets of plans for dose to the chest wall and lung.
METHODS
Patient Characteristics
Cases for this institutional-review-board–approved study were selected from an institutional database of patients enrolled in an approved trial of thoracic SBRT with photons for primary lung cancer or metastatic disease between August 2004 and February 2009 at MD Anderson. All patients had undergone treatment simulation with 4D CT and were treated with cone-beam CT. Of 360 such patients identified, 265 had had peripheral (as opposed to centrally located) tumors within 2.5 cm of the chest wall; of these patients, a subset of 23 patients who had developed grade ≥2 chest wall pain (scored according to the National Cancer Institute Common Terminology Criteria for Adverse Events V3.0) during the first year after SBRT. We selected the 10 patients out of the 23 that were above the threshold of the V30 and V35 limits for chestwall pain from the MDACC paper and the other CW paper. We did this to see if we could reduce these patients CW dose since they had the highest risk.
Stereotactic Body Radiation Therapy
All patients had been treated at a single institution in similar fashion. All simulations were done with 4D CT systems (Discovery ST, GE Medical Systems, Milwaukee, WI; or Brilliance 64, Philips Healthcare, Andover, MA) while the patient was in the treatment position, i.e., supine while immobilized and supported by a customized vacuum bag (BlueBag, Elekta, Stockholm, Sweden). Sets of 10 respiration phase datasets obtained during simulations, with maximum intensity projections and averaged CT values, were transferred to a Pinnacle V8.0 treatment planning system (Philips Healthcare). For each patient, a structure was created that enveloped the motion of the tumor during respiration (the internal gross tumor volume [iGTV]), which was then expanded uniformly by 8 mm and modified as needed by the treating physician to create the clinical target volume (CTV). The CTV was isotropically expanded by 3 mm to create the planning target volume (PTV). Our planning goal was to treat 95% of the PTV (delineated by the prescribed isodose line) to 50 Gy, to be delivered in 4 fractions. All treatments used 6-MV X-rays delivered in 6-9 treatment fields with heterogeneity corrections. Dynamic wedges were used but intensity-modulated radiation therapy was not. In-room CT images were acquired daily during the treatment with a 21 iX cone-beam CT scanner (Varian Associates, Palo Alto, CA). In-house software was used to perform a 3D-3D match that allowed set-up based on soft tissue targets. Patient shifts were made using the digital couch readouts. All patients had had all 4 treatments delivered in 1 week, generally on subsequent days. Each imaging and treatment session took 25-45 minutes.
Evaluation of Chest Wall and Lung Dose
Dose calculations, with heterogeneity corrections, were made in all cases with the Pinnacle V8.0 treatment planning system. The chest wall was not manually contoured in the original treatment plans; rather, the outer edge of each patient’s skin/chest wall was automatically outlined, and that volume was subtracted from the total lung contour. The remaining volume was defined as the chest wall volume. We then calculated the absolute volumes of the chest wall receiving 20, 30, 35, or 40 Gy (V20, V30, V35, or V40) for both the photon- and proton-based SBRT plans. Data were also collected on mean lung dose and lung V10.
Proton SBRT Plans
For this study, we generated proton SBRT treatment plans by using the same treatment volumes and dose fractionation (50 Gy in 4 fractions) for 10 patients who had had grade ≥2 chest wall pain within 1 year after photon SBRT. The dosimetrists who created the plans were blinded to the original chest wall dose-volume histogram (DVH) data; they were given the original PTV and instructed to create the best proton plan they could that had the same or better (higher percentage) PTV coverage. The treated plans use the Moyer approximation to determine range uncertainties of stopping power to Hounsfield unit conversion to ensure beam-directional coverage of the target proximally and distally (9). For inter- and intra-fractional motion and possible compensator misalignment with target and beam, the treated plans use the Urie approximation for smear radius (10). Proton plans used 4-6 beams and the standard uncertainty margins used by the Proton Therapy Center at Houston.
Statistical Analyses
Data were analyzed with Stata/SE 10.0 software. The equality of means for continuous variables was assessed with t tests. Logistic regression was used to predict the probability of occurrence of a dichotomous outcome by continuous or categorical independent variables. A P value of 0.05 or less was considered to indicate statistical significance. All statistical tests were based on a two-sided significance level.
RESULTS
Patient and Tumor Characteristics
Characteristics of the 10 patients selected are shown in Table 1. The median distance between tumor and chest wall was 1.26 cm (range 0-2.0 cm), and the median age of the patients (4 men, 6 women) was 73 years (range 50-83 years). More tumors (n=6) were located in the anterior thorax than in the posterior thorax (n=4). One patient had had acute grade 2 chest wall pain; 9 patients had chronic grade 2 chest wall pain and 1 had chronic grade 4, with average time to onset 11 months (range 0-19 months). Four patients required narcotics for pain management within 3-18 months after treatment. Median follow-up time for all patients was 13.5 months (range 3.9-19.2 months).
Table 1.
Planning parameters for photon-based SBRT
| Tumor location |
Dist. to CW (cm) |
Beams | Target | Avg range (cm) |
Margins | Smear (cm) |
Normalization (%) |
CW ccs (V30) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | Prox. | Distal | Lat. | |||||||
| RUL | 1.5 | 30 | 180 | 222 | 264 | 306 | 348 | CTV | 7.8 | 0.4 | 0.6 | 1 | 1 | 97.5 | 58.7 |
| RLL | 0.1 | 35 | 190 | 231 | 272 | 313 | 354 | CTV | 12.1 | 0.6 | 7 | 1 | 1 | 96.2 | 69.4 |
| RLL | 0.7 | 120 | 160 | 200 | 240 | 280 | 320 | CTV | 9.6 | 0.4 | 0.6 | 1 | 1 | 96.7 | 83.2 |
| RML | 0.8 | 15 | 195 | 240 | 285 | 330 | CTV | 10.2 | 0.5 | 0.6 | 1 | 1 | 97.36 | 80.8 | |
| LLL | 0.8 | 200 | 90 | 145 | 60 | CTV | 9.4 | 0.45 | 0.65 | 1 | 1 | 97.8 | 60.7 | ||
| LUL | 0.1 | 320 | 0 | 60 | 100 | CTV | 11.4 | 0.4 | 0.6 | 1.2 | 1 | 96.4 | 111.2 | ||
| LLL | 0 | 48 | 93 | 138 | 183 | 228 | CTV | 8.2 | 0.4 | 0.6 | 1 | 1 | 96.7 | 79.3 | |
| LUL | 0.4 | 340 | 90 | 140 | 30/330 | CTV | 10.5 | 0.2 | 1 | 1 | 1 | 94 | 74.5 | ||
| RLL | 0.4 | 150 | 200 | 270 | 300 | CTV | 13 | 0.5 | 0.7 | 0.8 | 0.6 | 95 | 100 | ||
| LUL | 0.4 | 335 | 5 | 35 | 125 | CTV | 11 | 0 | 0.6 | 1 | 0.7 | 94 | 50 | ||
Avg = average; Dist. = distance; Lat. = lateral; LLL = left lower lobe; LUL = left upper lobe; Prox. = proximal; RLL = right lower lobe; RML = right middle lobe; RUL = right upper lobe (of lung).
Photon Versus Proton SBRT Plans
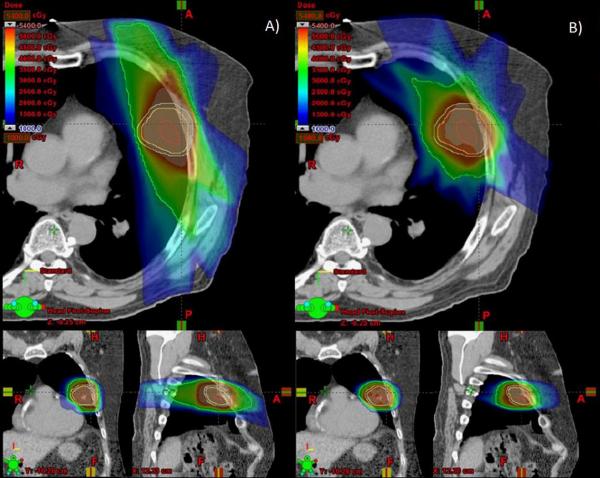
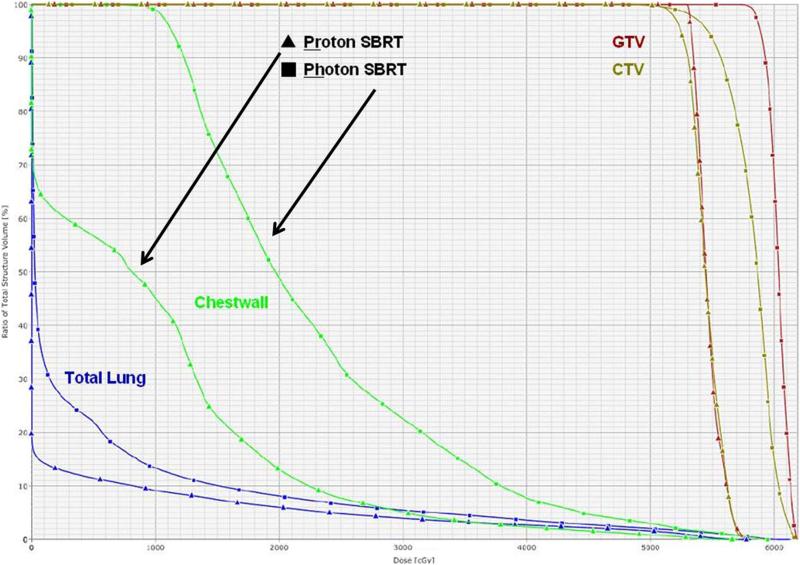
The proton SBRT plans produced comparable coverage of the PTV but lower doses to the chest wall at most dose levels measured (Table 2). Median chest wall V20 for the original photon plans was 364.0 cm3 (range 240.0-511.5 cm3) versus 160.0 cm3 (range 5.0-253.0 cm3) for the proton plans (P<0.0001). All chest wall dosimetric parameter we measured such as V20, V30, V35 and V40 were reduced for example the V30 was 144.6 cm3 (range 82.3-238.5 cm3) for photons vs. 77.0 cm3 (range 30.3-111.4 cm3) for protons (P=0.0012). These differences are illustrated in Figures 1 and 2. In Figure 1, the green isodose wash indicating the exposure to 30 Gy is visibly smaller in the proton plan than in the photon plan. Figure 2 is a DVH comparison between photon and proton SBRT.
Table 2.
Patient characteristics and toxicity of selected 10 patients
| Characteristic | Value |
|---|---|
| Age, median (y) | 73 (range, 50 to 83) |
| Sex | |
| Male | 4 |
| Female | 6 |
| Tumor location | |
| Anterior | 6 |
| Posterior | 4 |
| Tumor distance from chest wall (median) (cm) | 0.00 (range, 0 to 1.26) |
| Acute pain grade | |
| 1 | 0 |
| 2 | 1 |
| 3 | 0 |
| 4 | 0 |
| Chronic pain grade | |
| 1 | 0 |
| 2 | 9 |
| 3 | 0 |
| 4 | 1 |
| Rib fracture | 1 |
| Time to onset of pain (mo) | 11 (range, 0 to 19) |
| Skin toxicity grade | |
| 0 | 4 |
| 1 | 6 |
| 2 | 0 |
| 3 | 0 |
| Narcotics treatment | |
| Before treatment | 1 |
| Within 2 mo after treatment | 1 |
| Within 3 to 18 mo after treatment | 4 |
Figure 1.
Axial, coronal, and sagittal images of photon-based stereotactic body radiation therapy (SBRT) plans (left) and proton-based SBRT plans (right), both delivering 50 Gy in 4 fractions. A, colorwash of the photon SBRT plan shows the volume of the chest wall receiving 30 Gy in green. B, colorwash of the same CT data set used to plan for proton SBRT shows that significantly less chest wall receives 30 Gy.
Figure 2.
Dose-volume histograms comparing the exposure of the total lung, chest wall, gross tumor volume (GTV) and clinical target volume (CTV) for proton-based and photon-based stereotactic body radiation therapy (SBRT) plans. The proton SBRT plan results in considerably less of the chest wall receiving 10, 20, or 30 Gy.
DISCUSSION
As SBRT is increasingly being used to treat both early-stage lung primary tumors and metastatic pulmonary disease, establishment of guidelines regarding maximum doses to critical structures becomes increasingly important to avoid or minimize subsequent toxicity. In the past, the emphasis was mostly on minimizing acute toxicities, as the overall survival time for many patients after treatment was relatively short. However, the increasing ability to detect early-stage disease in relatively healthy patients mandates that radiation planning delivery techniques incorporate means of minimizing both early and late toxic effects. Our findings suggest that proton-based SBRT can deliver the same dose to the PTV while significantly reducing the dose to the chest wall and the lung, making it an attractive option that might be expected to minimize both acute and late toxicity.
Several SBRT treatment planning modalities have been shown to reduce dose to surrounding structures and potentially reduce chest wall toxicity (8), including use of 5-to 10-beam arrangements to distribute the dose energy more equally to eliminate hot spots, use of beam angle optimizations utilizing the lateral edge of the field to improve dose fall-off, and the addition of field-in-field techniques. Use of 4D CT to assess tumor motion can also be useful for evaluating chest wall invasion; for tumors close to the chest wall that are fixed, the typical CTV expansion into the chest wall that would be appropriate for a mobile tumor without extension into the chest wall one could potentially be tighten the CTV. Pulmonary gating can also help to reduce tumor motion and to minimize the volume of chest wall needing treatment. Fraction size and overall treatment duration are also important predictors of toxicity. Fowler et al. (11) showed that fraction size has a large influence on toxicity to the skin and nerves, which have a lower alpha/beta ratio than other organs. Therefore, prolonging treatment by using higher doses delivered in smaller fractions, such as in 70 Gy in 10 fractions, may be beneficial in avoiding toxicity when treating larger tumors or tumors next to critical structures; however, this needs to be weighed carefully against the potential for reducing local control (12). Although modifications such as these can improve the chest wall dose in photon-based SBRT, use of proton-based SBRT has the potential to reduce chest wall dose even further, potentially reducing toxicity even further or avoiding the use of lower dose regimens for treating tumors close to critical structures.
The unique dosimetric aspects of charged particles may be one avenue by which toxicity to critical structures can be reduced while maintaining tumor control. Chang et al. (13) found that use of protons (to a dose of 87.5 Gy(RBE)) led to significant reductions in mean total lung doses compared with conventionally fractionated photon radiotherapy to 66 Gy for the treatment of stage I and III NSCLC. Doses to surrounding critical structures were also significantly improved with protons, findings that have been supported in other studies as well (14, 15).
In addition to reducing chest wall doses, we also found that proton SBRT led to lower mean lung doses. Although the volume of exposed lung is not often a dose-limiting variable for SBRT, it becomes increasing relevant when treating patients with limited lung function, prior thoracic surgery or prior radiation, or multiple lesions. Clinical studies have indicated that minimizing the irradiated lung volume significantly reduces the risk of potentially life-threatening complications (16). The mean lung dose in particular is a strong predictor of lung injury and thus should be considered carefully in treatment planning (17). Several groups have compared photon-versus proton-based SBRT plans with an eye toward improving lung doses as well as toxicity profiles. Hoppe et al. (7) found that dosimetrically, proton-based SBRT led to better V5, V10, V20, V40 and mean lung doses than photon-based SBRT as well as significant reductions in dose to the heart, esophagus and bronchus. Register et al. (18) also found lower mean total lung dose and total lung volume from protons compared with photons, as well as smaller mean maximum doses to the aorta, brachial plexus, heart, pulmonary vessels and spinal cord. Similar findings from other studies support the potential of proton therapy for reducing the overall lung dose and overall toxicity while still delivering sufficiently high doses of radiation to control gross disease (19, 20).
Our current study suffers from the weaknesses inherent in any retrospective study, and other factors aside from chest wall dose could have contributed to the development of chest wall pain. Additionally, photon SBRT is often performed in combination with pulmonary gating, which is not available at many proton centers; thus patients with tumors that move more than 1 cm with respiration may preferentially benefit from gated photon techniques. Indeed, we are not suggesting that protons be used for conventional SBRT for pulmonary disease when dose constraints to critical structures can be achieved with photons; the goal is to achieve the highest possible probability of local control. Additionally, proton treatments have several uncertainties, such as range, setup and tumor motion uncertainties which, which are addressed by using the Uri and Moyers approximations (9, 10), however larger treatment volume may still be needed. Addressing these uncertainties especially tumor motion, may be more of an issue in treatment plans treated with only 2-3 beams, which is one of the reasons our treatment plans used 4-6 beams. Because we achieved similar PTV coverage with photons and protons in our study, we would expect similar rates of local control—or perhaps better control if charged particles are able to enhance the probability of killing cancer stem cells as others have postulated (21). Perhaps in the future molecular profiles of tumors will allow identification of the rare lung tumors that are resistant to the BED threshold of 100 (22); for such tumors, protons might allow dose escalation without violating safe dose limits to critical structures.
In summary, although photon SBRT has proven highly effective for early-stage lung cancer, the toxicity and dose limits to critical structures associated with this technique need further exploration. The two predominant anatomic challenges for the use of thoracic SBRT are still central tumors and peripheral tumors that are close to the chest wall. For the latter, because chest wall dose correlates strongly with toxicity, efforts to reduce chest wall dose, perhaps by using charged particles, may help to reduce the morbidity associated with treatment of such tumors.
Table 3.
Dosimetric comparison of photon-based SBRT and proton-based SBRT therapy plans
| Characteristic | Photon-based SBRT plan | Proton-based SBRT plan | p Value |
|---|---|---|---|
| median (range) | median (range) | ||
| PTV | |||
| Percentage coverage | 96 (95 to 99) | 97 (95 to 99) | |
| Chest wall (cm3) | |||
| V20 | 364.0 (240.0 to 511.5) | 160.0 (5.0 to 253.0) | < 0.0001 |
| V30 | 144.6 (82.3 to 238.5) | 77.0 (30.3 to 111.4) | 0.0012 |
| V35 | 93.9 (54.8 to 167.7) | 57.9 (21.3 to 93.6) | 0.0054 |
| V40 | 66.5 (36.9 to 123.1) | 45.4 (15.1 to 78.5) | 0.0112 |
| Lung | |||
| Mean dose (Gy) | 57.5 (38.0 to 80.2) | 38.8 (29.0 to 44.9) | 0.0001 |
| V10 (cm3) | 17.5 (11.0 to 29.0) | 12.7 (9.2 to 16.0) | 0.0010 |
Acknowledgments
Supported in part by the family of M. Adnan Hamed to the MD Anderson Cancer Center Thoracic Radiation Oncology program and by National Institutes of Health grant CA16672 to MD Anderson.
Meeting Presentations: This work was submitted for presentation at the 53rd (2011) ASTRO meeting.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, et al. Cancer Statistics. CA Cancer J Clin. 2010:caac. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Chang JY, Balter PA, Dong L, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;72:967–971. doi: 10.1016/j.ijrobp.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen NP, Garland L, Welsh J, et al. Can stereotactic fractionated radiation therapy become the standard of care for early stage non-small cell lung carcinoma. Cancer Treat Rev. 2008;34:719–727. doi: 10.1016/j.ctrv.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2:S94–100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 6.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 7.Hoppe BS, Huh S, Flampouri S, et al. Double-scattered proton-based stereotactic body radiotherapy for stage I lung cancer: a dosimetric comparison with photon-based stereotactic body radiotherapy. Radiother Oncol. 97:425–430. doi: 10.1016/j.radonc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Welsh J, Thomas J, Shah D, et al. Obesity Increases the Risk of Chest Wall Pain from Thoracic Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2010.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moyers MF, Miller DW, Bush DA, et al. Methodologies and tools for proton beam design for lung tumors. International journal of radiation oncology, biology, physics. 2001;49:1429–1438. doi: 10.1016/s0360-3016(00)01555-8. [DOI] [PubMed] [Google Scholar]
- 10.Urie M, Goitein M, Wagner M. Compensating for heterogeneities in proton radiation therapy. Physics in medicine and biology. 1984;29:553–566. doi: 10.1088/0031-9155/29/5/008. [DOI] [PubMed] [Google Scholar]
- 11.Fowler JF. The radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncol. 2005;44:265–276. doi: 10.1080/02841860410002824. [DOI] [PubMed] [Google Scholar]
- 12.Stephans KL, Djemil T, Reddy CA, et al. A comparison of two stereotactic body radiation fractionation schedules for medically inoperable stage I non-small cell lung cancer: the Cleveland Clinic experience. J Thorac Oncol. 2009;4:976–982. doi: 10.1097/JTO.0b013e3181adf509. [DOI] [PubMed] [Google Scholar]
- 13.Chang JY, Zhang X, Wang X, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in Stage I or Stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65:1087–1096. doi: 10.1016/j.ijrobp.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 14.Lee CH, Tait D, Nahum AE, et al. Comparison of proton therapy and conformal X-ray therapy in non-small cell lung cancer (NSCLC) Br J Radiol. 1999;72:1078–1084. doi: 10.1259/bjr.72.863.10700825. [DOI] [PubMed] [Google Scholar]
- 15.Glimelius B, Ask A, Bjelkengren G, et al. Number of patients potentially eligible for proton therapy. Acta Oncol. 2005;44:836–849. doi: 10.1080/02841860500361049. [DOI] [PubMed] [Google Scholar]
- 16.Lee HK, Vaporciyan AA, Cox JD, et al. Postoperative pulmonary complications after preoperative chemoradiation for esophageal carcinoma: correlation with pulmonary dose-volume histogram parameters. Int J Radiat Oncol Biol Phys. 2003;57:1317–1322. doi: 10.1016/s0360-3016(03)01373-7. [DOI] [PubMed] [Google Scholar]
- 17.Ricardi U, Filippi AR, Guarneri A, et al. Dosimetric predictors of radiation-induced lung injury in stereotactic body radiation therapy. Acta Oncol. 2009;48:571–577. doi: 10.1080/02841860802520821. [DOI] [PubMed] [Google Scholar]
- 18.Register SP, Zhang X, Mohan R, et al. Proton stereotactic body radiation therapy for clinically challenging cases of centrally and superiorly located stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 80:1015–1022. doi: 10.1016/j.ijrobp.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georg D, Hillbrand M, Stock M, et al. Can protons improve SBRT for lung lesions? Dosimetric considerations. Radiother Oncol. 2008;88:368–375. doi: 10.1016/j.radonc.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Macdonald OK, Kruse JJ, Miller JM, et al. Proton beam radiotherapy versus three-dimensional conformal stereotactic body radiotherapy in primary peripheral, early-stage non-small-cell lung carcinoma: a comparative dosimetric analysis. Int J Radiat Oncol Biol Phys. 2009;75:950–958. doi: 10.1016/j.ijrobp.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Cui X, Oonishi K, Tsujii H, et al. Effects of Carbon Ion Beam on Putative Colon Cancer Stem Cells and Its Comparison with X-rays. Cancer Research. 2011 doi: 10.1158/0008-5472.CAN-10-2926. [DOI] [PubMed] [Google Scholar]
- 22.Onishi H, Shirato HM, Nagata YM, et al. Hypofractionated Stereotactic Radiotherapy (HypoFXSRT) for Stage I Non-small Cell Lung Cancer: Updated results of 257 patients in a japanese multi-institutional study. J Thorac Oncol. 2007;2:94–100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]