Abstract
Hemes are porphyrins that play a critical role in diverse biological processes. Heme synthesis culminates in the mitochondrial matrix, but the eight-step biosynthetic pathway is spatially shared between the mitochondria and cytoplasm. A recent paper describes the nature of the transporter which translocates the heme precursor coproporphyrinogen III into the mitochondria for heme synthesis. The identification of ABCB6 and future studies aimed at precisely delineating the mechanism and the physiological nature of its ligand(s) will further enhance our current understanding of the intracellular movement of tetrapyrroles in eukaryotes.
Barring few exceptions, heme, a metalloporphyrin, is synthesized via a multi-step biosynthetic pathway with well-defined intermediates that are highly conserved throughout evolution. Depending upon the organelle and cell type, heme pathway intermediates are utilized for the synthesis of other tetrapyrrole compounds including bilins, chlorophylls, and corrins (1-3). Despite our extensive knowledge of heme biosynthesis per se, the intracellular trafficking of heme and porphyrins are not well understood (Fig. 1). In a recent issue of Nature, Krishnamurthy et al. report on the identification of ABCB6, a mitochondrial outer membrane transporter, which was shown by different experimental approaches to be able to transport porphyrins (4). ABCB6 is proposed to translocate coproporphyrinogen III (CPgenIII), a heme precursor, from the cytoplasm to the mitochondria, for synthesis of heme. Their findings therefore represent a significant advancement in our understanding of intraorganellar porphyrin transport in mammalian cells.
Fig. 1.
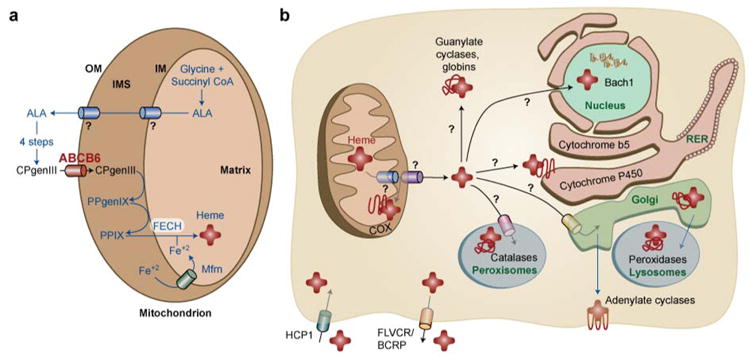
Schematic model of mammalian heme homeostasis. Presumptive heme pathways that are currently unknown are marked with a ? .
(A) In eukaryotic cells, heme is synthesized via a multi-step pathway that is spatially separated between the cytosol and mitochondria. The export of delta-aminolevulinic acid (ALA) from the mitochondria is unknown. ALA is converted to CPgenIII via four enzymatic reactions and CPgenIII is imported into the mitochondria by the outer membrane (OM) transporter, presumably ABCB6 or OGC. CPgenIII is converted to protoporphyrinogen (PPgenIX) and PPIX on the intermembrane space (IMS) side of the inner membrane (IM). The final step of heme synthesis occurs by the enzymatic chelation of ferrous iron (Fe+2) into PPIX catalyzed by ferrochelatase (FECH) located on the matrix side of the IM. Mitoferrin (Mfrn) / Mrs 3/4 is predicted to import Fe+2 into the mitochondria.
(B) The nascent heme moiety is somehow transported through two mitochondrial membranes and incorporated into a multitude of hemoproteins, presumably by hemochaperones, in different cellular compartments. Recent studies have identified HCP-1 as the heme importer, primarily in intestinal cells, while FLVCR and BCRP is predicted to export heme in erythrocytes.
Porphyrins are heterocyclic organic rings made from four pyrrole subunits linked via methine bridges. The name porphyrin is derived from the Greek word “prophura” for purple because the extensive conjugation of these tetrapyrroles gives them their violet-red hues. Heme is an iron-containing porphyrin and serves as a prosthetic group for many biological processes including oxidative metabolism, xenobiotic detoxification, the synthesis and sensing of diatomic gases, cellular differentiation, gene regulation at the level of transcription, protein translation and targeting, and protein stability.
Heme synthesis culminates in the mitochondrial matrix, but the eight sequential enzymatic steps are spatially separated between the cytoplasm and the mitochondria. CPgenIII is a product of the fifth enzyme in the heme pathway, uroporphyrinogen III decarboxylase, and enters the mitochondria to undergo three additional enzymatic reactions to generate heme (Fig 1A) (5). The pathway culminates when ferrous iron is catalytically inserted into the protoporphyrin IX (PPIX) ring by ferrochelatase, a mitochondrial inner membrane-associated enzyme (6,7). Importantly, iron and porphyrins are toxic to cells; iron generates hydroxyl radicals from Fenton-based reactions, and PPIX catalyzes light-dependent generation of oxygen radicals. Moreover, the end-product of the synthetic pathway is heme, a cytotoxic macrocycle with peroxidase activity. Consequently, cellular heme synthesis is coupled with iron availability and apo-protein synthesis to prevent uncoordinated accumulation of iron, porphyrin intermediates and heme (8,9).
The two unanswered questions pertinent to heme synthesis in eukaryotic cells are: a) how does delta-aminolevulinic acid, the first intermediate of the pathway exit from the mitochondrial matrix into the cytoplasm, and b) how does CPgenIII translocates from the cytoplasm into the intermembrane mitochondrial space. The results from Krishnamurthy et al. provides evidence that ABCB6 may be responsible for CPgenIII transport (4). However, the recent work by Kabe et al. suggest that the 2-oxoglutarate carrier (OGC) may also perform the same function (10). Thus, additional work is required to firmly resolve the identity of the transporter, and the biological nature of its ligand. Neither of these studies utilizes the physiologically relevant substrate CPgenIII, a reduced nonplanar porphyrin, as opposed to the commercially available oxidized planar conjugated macrocycle, coproporphyrin III (CPIII).
The findings that ABCB6 expression is coordinated with heme synthesis and cellular function provides further proof for the importance of orchestrated timing of biological processes during development. As erythrocytes mature, the primary function of these oxygen-carrying cells is hemoglobin synthesis which is underscored by a dramatic and highly coordinated upregulation of heme and globin production. Using biochemical and cell culture assays with wild-type and mutant forms of ABCB6, the authors provide evidence that Abcb6 mRNA and protein is upregulated in erythroleukemia and G1ER cells, the immature red cell precursor cell lines (4). They show that ABCB6 binds porphyrins, including heme, but binding and competition assays suggest that the substrate is likely to be CPIII. ABCB6 activity was found to be highly regulated by cellular heme levels. Inhibition of heme synthesis by succinyl acetone resulted in down regulation of Abcb6, while ectopic expression of Abcb6 resulted in increased PPIX accumulation and upregulation of mRNA for several heme biosynthesis genes. Notably, knock-down of Abcb6 by RNA-mediated interference resulted in diminished heme synthesis providing further proof that Abcb6 expression is coordinated with heme synthesis. Furthermore, genetic ablation of a single copy of Abcb6 in mouse embryonic stem cells revealed a haploid insufficiency phenotype; Abcb6+/- heterozygous cells had accumulated half the levels of PPIX compared to wild-type cells when treated with the heme precursor, delta-aminolevulinic acid. Taken together the authors provide evidence that ABCB6 plays an important role in regulating heme synthesis either by directly channeling CPgenIII to the mitochondria (Fig. 1A), or by indirectly regulating another step in the pathway.
The molecules and the mechanisms involved in heme transport across biological membranes to various cellular destinations are poorly understood partly because of the use of in vitro biochemical approaches, static microscopic techniques, and inappropriate genetic model systems. Based on the well-established paradigm for intracellular copper trafficking pathways (11), it is likely that specific pathways also exist for transport, trafficking, sequestration and egress of heme in cells (Fig. 1B). Although there are several parallels between copper and heme, the most noteworthy is that both are essential cofactors that participate in electron transfer reactions, but are toxic compounds when found in excess. A major difference, however, is that nutritional copper is acquired exogenously, while heme is produced endogenously by a defined and regulated pathway. Although the pathway and intermediates for heme synthesis have been well defined, the handling of heme from its point of synthesis in the mitochondria to its insertion into hemoproteins remains poorly understood (Fig. 1B). Heme is a hydrophobic molecule and is insoluble in the aqueous cellular milieu. Free heme is toxic to biological macromolecules. How then is heme transported through the mitochondrial inner membranes to specific hemoproteins that reside in the cytoplasm, peroxisomes, mitochondrial inter-membrane space, secretory pathway, and the nucleus? What are the mechanisms for incorporating heme into apo-hemoproteins? Are these mechanisms specific to specific target proteins or the milieu of a sub-cellular compartment? Humans have intracellular hemoproteins such as hemo-, myo-, neuro- and cyto- globins, and heme enzymes including cytochrome P450s, adenylate cyclases, soluble guanylate cyclases, peroxidases, catalases, and respiratory cytochromes. These enzymes are located in different cellular organelles and they perform diverse biological functions, which depend upon heme as a cofactor. Thus, in principle, specific intracellular pathways are likely to also exist for the safe, efficient, and accurate transfer of heme from the inner mitochondrial membrane to distinct hemoproteins that are present in various sub-cellular compartments (Fig. 1B).
Researchers have recently demonstrated that the breast cancer resistance protein (BCRP) and the feline leukemia virus receptor (FLVCR) are potential heme exporters in developing erythroid cells, and that the heme carrier protein (HCP-1) is the intestinal heme importer in mammals (12-14) (Fig. 1B). Although, it is unclear why heme export would be necessary in red blood cells given that there is an overwhelming requirement for heme for hemoglobin synthesis, these studies underscore the necessity for translocation of heme between membrane compartments. A conceptual setback in identifying heme trafficking pathways has been the difficulty in dissociating biosynthesis from downstream trafficking events for three main reasons: (a) organisms normally make endogenous heme via a highly regulated pathway, (b) defects in the heme synthesis pathway are usually lethal or have pleiotropic effects, and (c) exogenous heme/porphyrins are poorly utilized by organisms that normally make heme. Although hemes are found in all phyla, certain prokaryotic organisms neither make heme nor contain hemoproteins, and the protozoa, Leishmania spp. appears to lack seven of the eight enzymes of the heme biosynthetic pathway (2,15). An exception is C. elegans, a free-living nematode, which does not synthesize heme, but ingests dietary heme to fulfill its heme auxotrophy. C. elegans has the repertoire of hemoproteins that humans have (16). It therefore represents a unique genetic model system to dissect the cellular and molecular determinants of heme homeostasis because it has a clean genetic background devoid of endogenous heme. Worms will therefore permit external control over the flux of heme and intracellular trafficking pathways, an advantage not attainable in other model systems.
The practical implications of discoveries in porphyrin transport are far-reaching. Identification of mammalian heme transporters, including HCP-1, will allow the design of more bioavailable forms of iron or porphyrin-based “nutraceuticals” to deliver iron more effectively to iron-deficient populations. Identification of mechanisms for how enzymes such as the cytochrome P450s and guanylate cyclases acquire heme will provide novel insights into modulating biologic responses to pharmaceuticals, xenobiotics, and gases such as nitric oxide. Finally, characterization of how heme is transported in organisms may lead to the discovery of parallel trafficking pathways for other tetrapyrroles such as vitamin B12.
References
- 1.Ponka P. Cell biology of heme. Am J Med Sci. 1999;318:241–56. doi: 10.1097/00000441-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Panek H, O'Brian MR. A whole genome view of prokaryotic haem biosynthesis. Microbiology. 2002;148:2273–82. doi: 10.1099/00221287-148-8-2273. [DOI] [PubMed] [Google Scholar]
- 3.Papenbrock J, Grimm B. Regulatory network of tetrapyrrole biosynthesis--studies of intracellular signalling involved in metabolic and developmental control of plastids. Planta. 2001;213:667–81. doi: 10.1007/s004250100593. [DOI] [PubMed] [Google Scholar]
- 4.Krishnamurthy PC, Du G, Fukuda Y, Sun D, Sampath J, Mercer KE, Wang J, Sosa-Pineda B, Murti KG, Schuetz JD. Identification of a mammalian mitochondrial porphyrin transporter. Nature. 2006;443:586–9. doi: 10.1038/nature05125. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira GC, Andrew TL, Karr SW, Dailey HA. Organization of the terminal two enzymes of the heme biosynthetic pathway. Orientation of protoporphyrinogen oxidase and evidence for a membrane complex. J Biol Chem. 1988;263:3835–9. [PubMed] [Google Scholar]
- 6.Dailey HA. Terminal steps of haem biosynthesis. Biochem Soc Trans. 2002;30:590–5. doi: 10.1042/bst0300590. [DOI] [PubMed] [Google Scholar]
- 7.Shaw GC, Cope JJ, Li L, Corson K, Hersey C, Ackermann GE, Gwynn B, Lambert AJ, Wingert RA, Traver D, Trede NS, Barut BA, Zhou Y, Minet E, Donovan A, Brownlie A, Balzan R, Weiss MJ, Peters LL, Kaplan J, Zon LI, Paw BH. Mitoferrin is essential for erythroid iron assimilation. Nature. 2006;440:96–100. doi: 10.1038/nature04512. [DOI] [PubMed] [Google Scholar]
- 8.Hamza I, Chauhan S, Hassett R, O'Brian MR. The bacterial irr protein is required for coordination of heme biosynthesis with iron availability. J Biol Chem. 1998;273:21669–74. doi: 10.1074/jbc.273.34.21669. [DOI] [PubMed] [Google Scholar]
- 9.Rouault T, Klausner R. Regulation of iron metabolism in eukaryotes. Curr Top Cell Regul. 1997;35:1–19. doi: 10.1016/s0070-2137(97)80001-5. [DOI] [PubMed] [Google Scholar]
- 10.Kabe Y, Ohmori M, Shinouchi K, Tsuboi Y, Hirao S, Azuma M, Watanabe H, Okura I, Handa H. Porphyrin Accumulation in Mitochondria is Mediated by 2-Oxoglutarate Carrier. J Biol Chem. 2006;281:31729–31735. doi: 10.1074/jbc.M604729200. [DOI] [PubMed] [Google Scholar]
- 11.Finney LA, O'Halloran TV. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science. 2003;300:931–6. doi: 10.1126/science.1085049. [DOI] [PubMed] [Google Scholar]
- 12.Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, Sarkadi B, Sorrentino BP, Schuetz JD. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem. 2004;279:24218–25. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- 13.Quigley JG, Yang Z, Worthington MT, Phillips JD, Sabo KM, Sabath DE, Berg CL, Sassa S, Wood BL, Abkowitz JL. Identification of a human heme exporter that is essential for erythropoiesis. Cell. 2004;118:757–66. doi: 10.1016/j.cell.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, Khan Y, Warley A, McCann FE, Hider RC, Frazer DM, Anderson GJ, Vulpe CD, Simpson RJ, McKie AT. Identification of an intestinal heme transporter. Cell. 2005;122:789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 15.Sah JF, Ito H, Kolli BK, Peterson DA, Sassa S, Chang KP. Genetic rescue of Leishmania deficiency in porphyrin biosynthesis creates mutants suitable for analysis of cellular events in uroporphyria and for photodynamic therapy. J Biol Chem. 2002;277:14902–9. doi: 10.1074/jbc.M200107200. [DOI] [PubMed] [Google Scholar]
- 16.Rao AU, Carta LK, Lesuisse E, Hamza I. Lack of heme synthesis in a free-living eukaryote. Proc Natl Acad Sci U S A. 2005;102:4270–5. doi: 10.1073/pnas.0500877102. [DOI] [PMC free article] [PubMed] [Google Scholar]


