Abstract
Background
The aging kidney has a decreased ability to repair following injury. We have shown a loss in expression of α-catenin in the aging rat kidney and hypothesize that decreased α-catenin expression in tubular epithelial cells results in diminished repair capacity.
Methods
In an effort to elucidate alterations due to the loss of α-catenin, we generated NRK-52E cell lines with stable knockdown of α(E)-catenin.
Results
α(E)-catenin knockdown resulted in decreased wound repair due to alterations in cell migration. Analysis of gene expression in the α(E)-catenin knockdown cells demonstrated almost a complete loss of bone morphogenetic protein-7 (BMP-7) expression that was associated with decreased phospho-Smad1/5/8 staining. However, addition of exogenous BMP-7 increased phosph-Smad1/5/8, suggesting that the BMP-7 pathway remained intact in C2 cells. Given the potential role of BMP-7 in repair, we investigated its role in wound repair. Inhibition of BMP-7 decreased repair in non-targeted control cells; conversely, exogenous BMP-7 restored repair in α(E)-catenin knockdown cells to control levels.
Conclusions
Taken together, the data suggests that the loss of α(E)-catenin expression and subsequent down-regulation of BMP-7, is a mechanism underlying the altered migration of tubular epithelial cells that contributes to the inability of the aging kidney to repair following injury.
Keywords: Aging, α-catenin, BMP-7, Migration, Repair
Introduction
In the past twenty-five years, a number of studies have associated age with a higher risk for acute kidney injury (AKI). Pascual et al. (1) demonstrated that the incidence of acute renal failure (ARF) is 3.5 times higher in patients over 70 than those under 70; patients older than 80 years old were 5.0 times more likely to develop AKI (2). Age above 65 years has also been shown to be an independent risk factor for AKI in a multinational, multicenter study (3). Elderly patients (≥65 years) had 10 times the incidence rate of AKI compared with those less than 65 years of age (4). Xue et al. (5) have established age as a risk factor for AKI; the incidence of AKI was 1.9% in patients younger than 65 and rose to 2.9% in those older than 85.
Given that the clinical evidence suggests that AKI is associated with delayed, or decreased, repair in the elderly, recent work has examined the impact of aging on repair. Complete recovery from ARF was reduced in the elderly patients (4). Arora et al. demonstrated that recovery, determined by normalized serum creatinine, from ARF was 3-times as long in elderly (mean 67.1 years) compared to young (32.3 years); 32 days versus 11.4 days, respectively (6). In meta-analysis of 17 studies it was found that a higher percentage of surviving elderly (>65 years) patients did not recover renal function as compared to younger patients (7).
The cadherin gene superfamily encodes for transmembrane proteins that regulate calcium-dependent cell-cell adhesion (8). A functional cadherin adhesion complex requires interaction with cytoplasmic proteins, the catenins. α-Catenin does not directly bind to cadherins; rather, it interacts with the cytoplasmic domain of cadherins via β- or γ-catenin. p120-catenin binds to the cadherin cytoplasmic domain, and shares sequence homology with β- and γ-catenin, but does not bind α-catenin. There are several forms of α-catenin; α(E)catenin is epithelial; α(N)catenin is neuronal; α(T) catenin is expressed in heart and testes, while α-catulin is a widely-expressed catenin-like molecule (9). It was long thought that α-catenin directly linked the cadherin/catenin complex to the cytoskeleton (10); as such, α-catenin was shown to interact with a variety of actin-binding proteins, including α-actinin, vinculin, and actin (11-13). This paradigm was challenged by results demonstrating that α-catenin does not simultaneously interact with cadherins and the F-actin monomers, but regulates actin dynamics (14,15). However, cadherin adhesion clearly influences actin cytoskeleton organization and actin cytoskeleton integrity is necessary for cadherin-mediated adhesion (16). Importantly, cadherin-independent functions of α-catenin are emerging (14,17-20). In both C elegans (18) and mouse keratinocytes (21), the phenotype of α-catenin loss differs from that observed following cadherin loss. In cancers where both E-cadherin and α-catenin expression is lost, prognosis is worse than loss of the individual protein; this synergistic effect would not be predicted by disruption of cell adhesion alone (22,23).
While many studies have focused on tubular epithelial cell proliferation in repair (24,25), migration is a key component of renal repair following injury (26). Previous studies in our laboratory have shown a loss of α-catenin expression in the proximal tubular epithelium in male Fischer 344 rats (27). Given the role of α(E)-catenin in actin dynamics (14,15), and the importance of actin in directed cell migration (28), we hypothesized that loss of α(E)-catenin would decrease wound repair. In the current study, we generated NRK-52E cells with a stable knockdown of α(E)-catenin and investigated the wound repair phenotype.
Materials and Methods
Animals
Male Fisher 344 (F344) rats (4 and 24 mon) were obtained from the NIA colony. On the day of the experiment, rats were anesthetized with a ketamine (80-120 mg/kg)/xylazine (5-10 mg/kg) intraperitoneal (IP) injection. Kidneys were collected and 1 mm sections were snap frozen in liquid nitrogen. All experimental procedures and animal care were approved by the University of Missouri Animal Care and Use Committee in accordance with the NIH guidelines.
Cell culture
Cells were grown in Dulbecco's modified eagle medium nutrient mixture F-12 (DMEM/F12; 1:1) containing L-Glutamine and Hepes (Gibco Cat #2013-08) supplemented with 10% fetal bovine serum (FBS; Hyclone)/50 U/ml penicillin, 50 mg/ml streptomycin (Gibco) and incubated at 37°C in 5% CO2. Cells were harvested with TrypLE Express (Gibco cat #12604-013) and pelleted at 300×g 5min at room temperature (RT).
Targeted and non-targeted clones for α(E)-catenin were generated by lentivirus-mediated shRNA by Sigma-Aldrich (St. Louis, MO). Single-cell clones of non-targeted and α(E)-catenin-targeted cells were generated by growing the parental cell line to confluency, harvesting them with TrypLE Expess and pelleting the cells at 1,000×g for 5 min at RT. Cells were then counted and a series of serial dilutions were seeded into a 96-well plate in DMEM/F12 containing 10% FBS and 5 μg/ml puromycin. On day 6 post-plating, wells were visualized on the microscope and any wells containing more than 1 colony of cells were discarded. Fresh media was put into each well every 3-5 days. Single cell colonies were grown to confluence and passaged to larger plates. Non-targeted vector control cells (NT3) and α(E)-catenin knockdown cells – clonal line 2 (C2) and 4 (C4) cells were grown in the presence of 5 μg/ml puromycin (Sigma-Aldrich) for lentivirus maintenance. The cell lines (NT3, C2 and C4) were used within 20 passages of establishing a clonal cell line.
Real-time PCR
RNA was isolated using the RNeasy mini kit (Qiagen, Valencia, CA) or EZ Tissue/Cell Total RNA Miniprep Kit (EZ BioResearch, St. Louis, MO) with on-column DNase digestion from 5×106-1×107 cells. RNA concentration and quality was determined by spectrophotometry with Nanodrop 2000c (Thermo Scientific) and confirmed by agarose gel electrophoresis. cDNA was generated from 2μg RNA using the High Capacity cDNA Synthesis Kit (Life Technologies, Carlsbad, CA) following the kit protocol. Quantitative PCR was performed in duplicate with 50ng cDNA/reaction using Taqman assays (Applied Biosystems) using SsoFast™ Probes Supermix with ROX (Bio-Rad) and the CFX96 Touch system (Bio-Rad) with the following cycling conditions: 95°C for 20 sec., then 40 times at 95°C for 1 sec and 60°C for 20 sec.
Commerically available TaqMan primer sets were used to asssess α(E)-catenin (Rn01406769_mH), BMP-7 (Rn01528889_m1), BMPR1B (RN01500616_m1), Kcp (Rn01500616_m1), Noggin (Rn01467399_s1) and Sostdc1 (Rn00596672_m1). Relative quantitation was performed using the Pfaffl method (29) normalized to cancer susceptibility candidate gene 3 (Casc3; Rn00595941_m1). The Ct values ranged from 24.08-24.55 (p=0.99) for Casc3 (Cancer susceptibility candidate gene 3 protein) across cell type or passage number. Glyceraldehyde 3-phosphate dehydrogenase (Gapdh), however, had Ct values ranging from 19.9-21.3. Therefore, Casc3 was selected as the reference gene for all gene expression studies and the efficiency of all assays were >95%.
Western Blot
Subconfluent cells were washed twice with ice-cold PBS (Phosphate Buffered Saline, Gibco, Life Technologies) and lysed with 10mM Tris-1% SDS buffer with Halt™ Protease/Phosphatase inhibitor. Cells were scraped and incubated for 15 min at 4°C on a rocker. Cells were further disrupted by passing through a 20 gauge needle and spun at 12,000xg for 15 min at 4°C. Tissue lysates were isolated using a 10mM Tris-1% SDS buffer supplemented with Halt™ Protease Inhibitor Cocktail (Thermo Fisher-Pierce, Rockford, IL). Protein concentration was determined by the BCA method using a commercially available kit (Thermo Fischer-Pierce).
The following antibodies were used: anti-α-catenin (monoclonal, clone 5) and anti-β-actin (monoclonal, clone AC-74 Sigma-Aldrich). Goat-anti-mouse HRP conjugate (Jackson ImmunoResearch Laboratories, West Grove, PA) was used at 1:20,000 dilutions. Blots were developed using West Femto (Thermo Fisher-Pierce) and imaged using the ChemiDoc imaging system and quantitation performed using the ImageLab 3.0 software (Bio-Rad, Hercules, CA).
Immunofluoresence
Cells were grown on 2-well glass chamber slides (Nuc Lab-Tek II; Thermo Scientific). Cells were washed with serum-free medium, fixed in 2% paraformaldehyde for 10 min, permeabilized with 1% TritonX for 30 min, and blocked with Background Sniper (Biocare Medical, Concord, CA) for 1-2 h. Primary anti-phospho SMAD1/5/8 antibody (CellSignaling, Beverly, MA catalog #9511) was isolated from BSA carrier using Protein A/G magnetic beads (Pierce, catalog # 88802), labeled with Alexa Fluor ® 488 5-TFP at pH 9.0 for 1h at room temperature and isolated from free label using Protein A/G magnetic beads. Cells were incubated in directly-labeled antibody overnight on rocker at 4°C. Cells were subsequently washed 3× in PBS-T, shaken dry, and counterstained with Fluoroshield with DAPI (Sigma, catalog # 6057). Immediately prior to imaging 1-2 drops of SlowFade Gold antifade reagent (Invitrogen, catalog # S36937) were applied to the cells to further prevent bleaching. Cells were imaged on an Olympus IX51 microscope (Olympus, Center Valley, PA) under a 60× oil immersion lens with a UC50 digital camera using cellSense software (Olympus, Center Valley, PA) at same exposure times. Original images (tiff or jpg files for merged images) were assembled into multi-panel pictures and further enhancements were performed on all images simultaneously.
Wound Healing Assay
Cells were seeded in 6-well plates at 2×104/cm2 and grown to 80-90% confluence (approximately 24 hr). A cross-scratch was made using a 200 μl pipetman tip. Media was removed and the wells washed 2× with serum-free media. Cells were then incubated with or without serum plus 5 μg/ml puromycin. For the mitomycin C assay, 5 μg/ml was added to serum-free-media plus 5 μg/ml puromycin. Healing was visualized at 4× magnification over 24 hours; at the end of the experiment cells were stained with crystal violet. To quantify healing, the center area of the scratch was measured using the closed polygon tool (CellSense) and per-cent closure was determined. In certain experiments, cells were exposed to 30 nM of a chemical BMP-7 type I receptor inhibitor was used, 6-(4-(2-(piperidin-1-yl)ethoxy)phenyl)-3-(pyridine-4-yl)pyrazolo[1,5-a]pyrimidine hydrochloride (DM-3189 LDN193189; Biovision Milpitas, CA) (30). In other experiments, exogenous recombinant human BMP-7 (100 ng/ml; Prospec Bio Rehovot, Israel) was added to cultures (31).
Statistics
Results are expressed as mean ± SD. A one-way or two-way (wound healing) analysis of variance (ANOVA) was performed followed by Student's t-test using the statistical software GraphPad Prism 6 (GraphPad Software, La Jolla, CA). The differences were considered statistically significant when p<0.05.
Results
In an effort to understand the impact of loss of α-catenin on tubular epithelial cells, stable knockdowns of α(E)-catenin were generated in NRK-52E cells. Several shRNA constructs targeting α(E)-catenin were designed and cell lines were generated that demonstrated varying levels of α(E)-catenin knockdown at the gene and protein level (data not shown). We generated clonal lines from vector control and α(E)-catenin knockdown cell lines by single cell cloning; the NT3 (vector control) and C2 and C4 (targeted α(E)-catenin knockdown) cells. The C2 and C4 cells had significant knockdown of α(E)-catenin at the gene and protein level (Figure 1A, and 1B, respectively). α-Catenin expression at the plasma membrane was seen in NT3 cells, with almost a complete loss of staining in the C2 cell line (Figure 1C). The knockdown of α(E) catenin was stable for at least 20 passages (data not shown).
Figure 1. Characterization of Stable Knockdown of α(E)-Catenin in NRK-52E Cells.
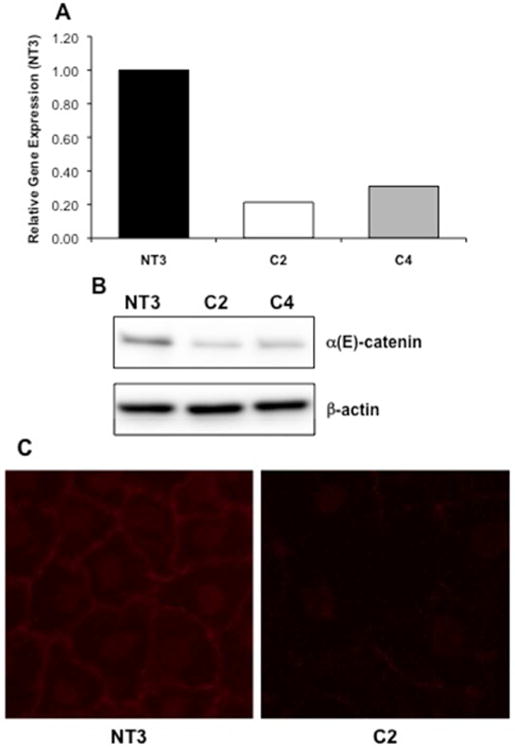
A: Loss of α(E)-catenin gene expression in single cell clones generated from stable shRNA-mediated knockdown in NRK-52E cells (C2 and C4 cells) is seen as compared to the vector control cell line (NT3). B: A corresponding loss of protein expression is seen via western blot analysis. C: Immunofluorescence demonstrates typical cell membrane staining of α-catenin in the NT3 cells; staining is reduced in the C2 cells.
Wound healing was significantly inhibited in C2 cells in both serum and serum-free conditions (Figure 2A & 2B). Interestingly, in C2 cells in serum or serum-free conditions, the wound was never completely healed, while domes of cells formed on the remaining monolayer in 10% serum. The fact that differences were seen in 10% serum suggested that the deficits in C2 cells might be due to disrupted migration. The hypothesis was supported by the finding that C2 cell proliferation was not decreased in either 10% serum or serum-free media at 12 and 24 hr. When cells were incubated with mitomycin C to halt cell proliferation in the wound healing assay, a significant difference between NT3 and C2 cells was still observed (Figure 2D). Interestingly, mitomycin C inhibited wound healing in both cell types, but C2 cells were only able to repair 60% of the wound as compared to NT3 cells in either control or mitomycin C conditions. These data indicate that alterations in migration underlie the decreased ability of C2 cells to repair.
Figure 2. Loss of α(E)-Catenin Reduces Wound Healing.
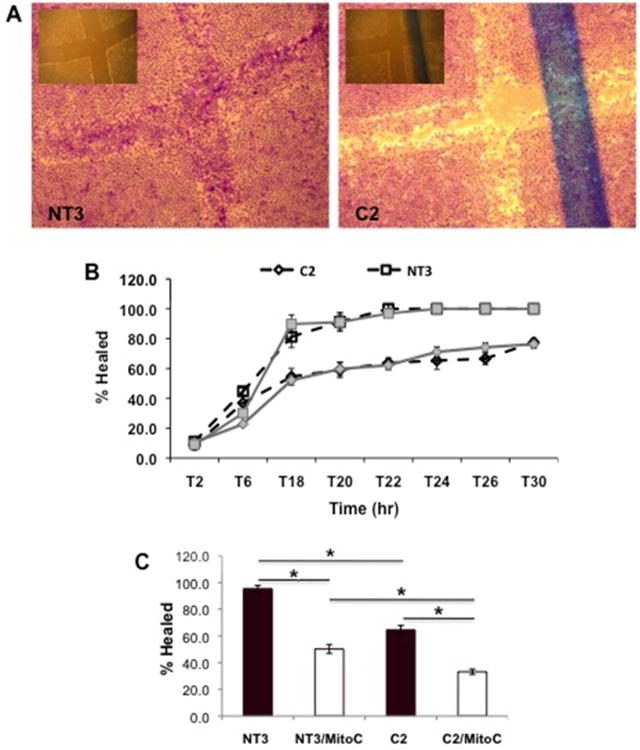
A: Representative images of NT3 and C2 cells 24 hr following injury in serum-free conditions; the inset is the T0 timepoint. B: The time course of repair in NT3 and C2 cells is shown, * indicates a statistically significant difference between C2 and NT3, or C2 serum-free (SF) and NT3 SF. For the wound healing, each data point represents 5 measurements over 3 independent experiments; each data point represents the mean±SD. C: Wound healing at 24 hr in the presence of mitomycin C, which inhibits cell proliferation; each data point represents the mean+SD from 2 independent experiments (n=3 per experiment); * indicates a significant difference from respective control.
A complete loss of BMP-7 gene expression was seen in C2 cells as compared to the NT3 controls using RNA-sequencing (data not shown). Quantitative PCR confirmed that that BMP-7 expression was significantly reduced in both C2 and C4 cells (Figure 3A). A correlation between the level of α(E)-catenin knockdown and expression of BMP-7 is seen, i.e. there is less α(E)-catenin and BMP-7 expression in C2 cells compared to C4. We also examined other components of the BMP7 pathway in C2 cells. The expression of a BMP-7 receptor, BMPR1b (32), and KCP, a BMP-7 agonist (33) were unchanged in C2 cells (Figure 3B). The expression of an endogenous BMP-7 antagonist, noggin (34), was not significantly increased; however, the expression of sostdc (USAG-1), another antagonist (35), was significantly increased in C2 cells (Figure 3B). Smad1, 5 and 8 are downstream mediators of BMP-7 signaling (36); and there is a reduction in nuclear staining of phospho-Smad1/5/8 in C2 cells (Figure 3C). Addition of exogenous BMP-7 to C2 cells significantly increased phospho-Smad1/5/8, suggesting that the downstream pathway for BMP-7 was intact in these cells, despite loss of BMP-7 expression (Figure 3C). The gene expression of BMP-7 in kidney lysates from 24 mon old male Fischer 344 rats, which exhibit decreased α(E)-catenin expression in the proximal tubules (27), is highly variable (Figure 3D). Two of the six animals expressed BMP-7 at, or above, levels in young (4 mon) controls, two had between 10-20% loss of BMP-7 expression, and two had between 20-35% loss of expression. When averaged, the difference between BMP-7 expression in young and aged kidney was not statistically significant, although there is a trend toward a decrease.
Figure 3. Loss of α(E)-Catenin Decreases BMP-7 Expression.
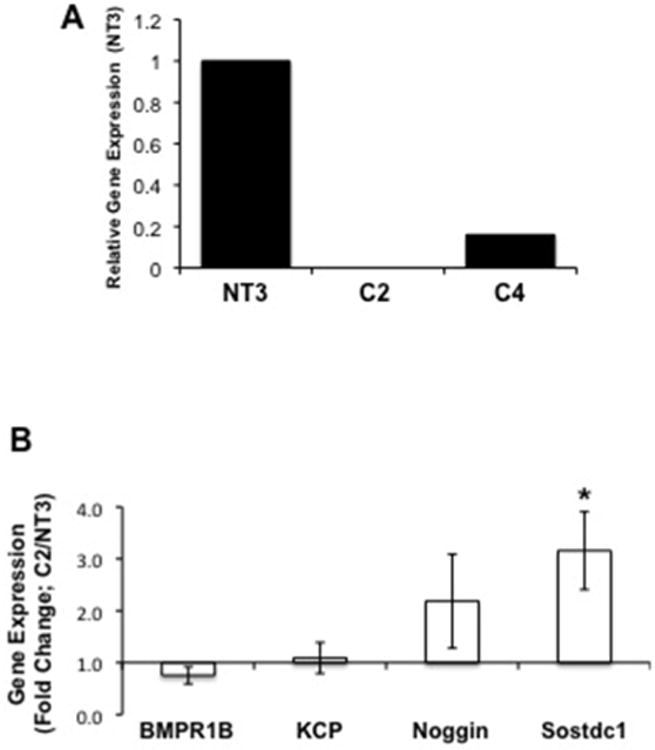
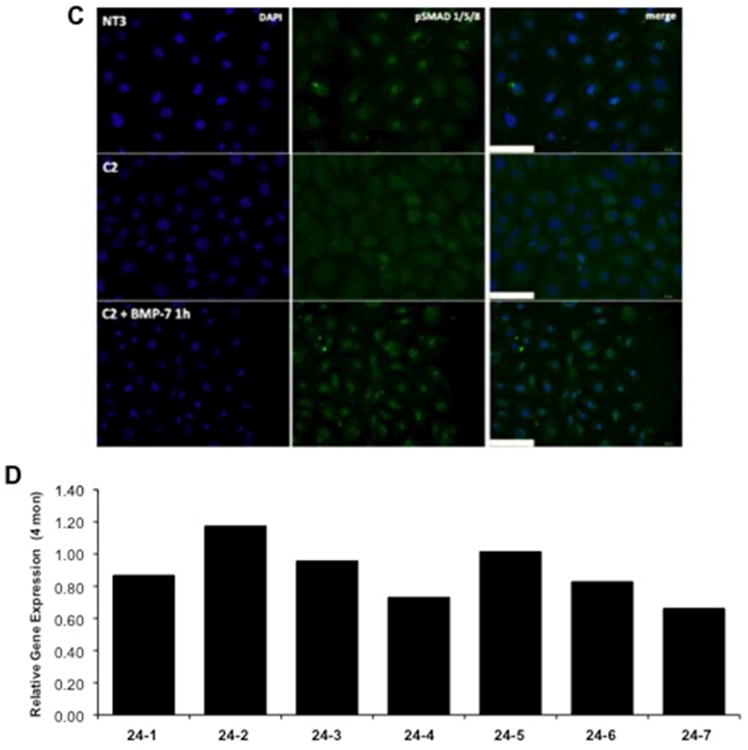
A: BMP-7 gene expression is reduced in C2 and C4 cells. Data is representative of three independent experiments. B: Gene expression of a BMPR1B, KCP, noggin and Sostdoc1 in NT3 and C2 cells was examined using quantitative PCR. each data point represents the mean+SD from 2 experiments, n=4 per experiment; * indicates a significant difference from respective control. C: Nuclear phosphoSmad 1/5/8 staining is decreased in C2 cells as compared to NT3 controls; addition of exogenous BMP-7 to C2 cells increases the staining. Data is representative of two independent experiments. D: Gene expression data in 24-mon old kidney samples demonstrates a trend of decreased BMP-7 expression; each bar represents the data from 1 of 7 individual animals compared to the mean of 8 4-month old samples.
Given a role for BMP-7 in renal repair (37), we examined the impact of BMP-7 on wound repair. Inhibition of BMP-7 in NT3 cells resulted in a complete loss of repair (Figure 4A), demonstrating that BMP-7 is requisite and necessary for wound repair in this model. Addition of exogenous BMP-7 to C2 cells partially attenuated repair (Figure 4B). Taken together, the data suggests that the α(E)-catenin regulates BMP-7 expression and there is a requirement for BMP-7 in repair.
Figure 4. BMP-7 Regulates Repair in NRK-52E Cells.
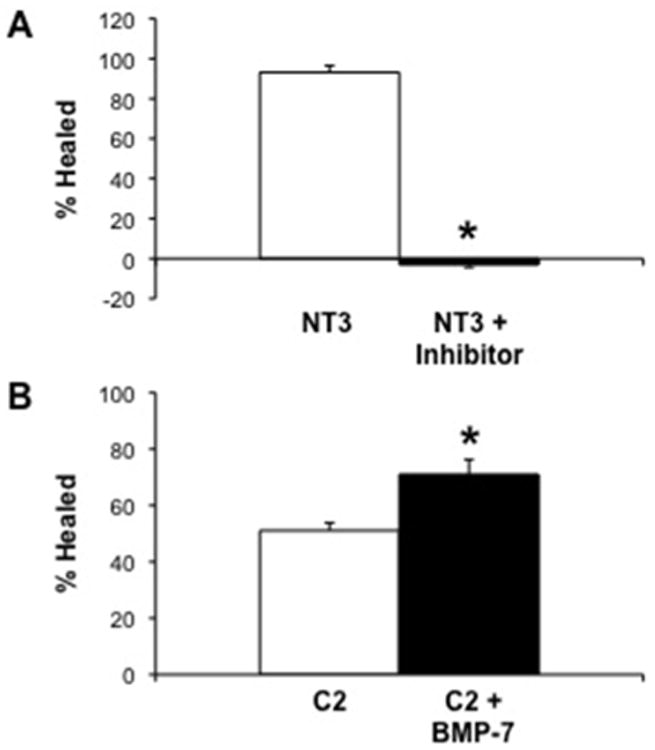
A: Addition of a chemical inhibitor to the BMP type I receptor (30 nM), completely inhibits wound repair in NT3 cells. B: Addition of exogenous recombinant BMP-7 (100 ng/ml) to C2 cells significantly increases wound repair. Each data point represents the mean±SD of four independent experiments (3 replicates/experiment), * indicates a significant difference from untreated control.
Discussion
Classically, the function of α-catenin has been thought to be limited to its role in cadherin-mediated cell adhesion; however, cadherin-independent functions of α-catenin are emerging (17-20). For example, in several tumors the loss of α-catenin is stronger prognostic factor for dedifferentiation (38) and invasion (38-40) as compared to E-cadherin loss. More specifically, increased sensitivity to growth factors, sustained activation of the ras-ERK pathway, increased NF-κB activity, and regulation of actin cytoskeleton dynamics are all regulated by α-catenin in a cadherin-independent manner (17-20). The loss of α-catenin expression leads to increased cell proliferation in tumor cells (41), the mouse epidermis (21) and central nervous system (42). This may involve aberrant activation of several pathways, including ras-ERK (21) and NF-κB (19).
Loss of α(E)-catenin was associated with decreased ability to repair in a wound healing assay, due to alterations in cell migration. Our results are generally consistent with the phenotype of α(E)-catenin knockdown reported in MDCK cells where α(E)-catenin knockdown was associated with altered cell morphology and disrupted cell-cell adhesion; however in the wound healing assay the knockdown cells repaired at the same rate as control cells (14). While the migration rate was increased following α(E)-catenin knockdown in MDCK cells, migration was not coordinated and it was suggested that migratory deficit in α(E)-catenin knockdown cells is due to decreased cadherin-mediated cell adhesion (14).
A critical role for BMP-7 in kidney development has been established (43). In addition, it may be renoprotective against both acute (44) and chronic kidney injury (33). In respect to chronic kidney disease, BMP-7 is suggested to have anti-fibrotic effects, in part, by attenuating TGFβ-1 signaling (45). BMP-7 signaling is tightly regulated at several levels, including expression and modulation of the pathway by both endogenous agonists and antagonists. In both normal mouse (31) and human kidneys (46), BMP-7 is predominantly localized to the distal segments of the nephron and not expressed in proximal tubules. However, BMP-7 expression is dramatically upregulated in the proximal tubules after injury (47,48). This could explain the lack of an effect of aging on overall BMP-7 gene expression as the age-dependent loss of α-catenin is seen in the proximal tubules (27). The regulation of BMP-7 expression is not completely defined. Cell culture studies have shown that protein kinase C (PKC) regulates BMP-7 expression (49). A reporter construct using a 4.6 kb portion of the human BMP-7 promoter demonstrates that both retinoic acid and prostaglandin E(2) increase BMP-7 expression (50). Futures studies will focus on identifying the pathway linking α(E)-catenin and BMP-7 regulation.
BMP-7 mediates its effects by binding to receptors, the bone morphogenic protein receptors-IA and -IB, bone morphogenic protein receptor-II, and the activin type I receptor, which have serine-threonine kinase activity (32). These receptors activate Smad transcription factors, including Smad1/5/8 (36). BMP-7 signaling is also modulated by a number of endogenous antagonists including Sostdc1 and noggin (34,35), as well as agonists, including KCP (33). This regulation adds further cellular specificity and complexity to BMP-7 signaling. Although knockdown of α(E)-catenin decreased increased expression of sostdc1, the signaling pathway remained intact and stimulable in C2 cells as demonstrated by the finding that exogenous BMP-7 elicited Smad activation. Specific targets of BMP-7 have yet to be identified in C2 cells, future studies will focus on the mechanism(s) by which BMP-7 stimulates repair in the NRK-52E model.
A role for BMP-7 in the regulation of migration has been established. The migration of metastatic breast cancer cells (4T1E/M3) was inhibited by either a BMP-7 neutralizing antibody, or miR RNAi knockdown of BMP-7 (51). BMP-7 also regulated cell migration in human bone marrow mesenchymal stem cells (52). Interestingly, the regulation of migration by BMP-7 may involved remodeling of the actin cytoskeleton via Rho-ROCK1 (53). Given the relationship between α(E)-catenin and actin dynamics, an intriguing possibility is that the decreased expression of BMP-7 is important in the disruption of the actin cytoskeleton seen in C2 cells (data not shown).
These data demonstrate that loss of α(E)-catenin expression leads to decreased BMP-7 expression, and decreased repair. Exogenous BMP-7 is able to partially rescue this repair deficit and suggest that an α(E)-catenin-BMP-7 pathway may be important in tubular epithelial repair.
Acknowledgments
The authors contributed the following to the work: Single cell cloning, IF (EAG-B), qPCR, Wound repair (LAN), Western Blot (ARP), BMP-7 experiments (AS, EAG-B), manuscript preparation (LAN, AS, EAG-B, ARP). Research reported in this publication was supported by the National Institute of Aging of the National Institutes of Health under award number RO1AG034154. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Pascual J, Orofino L, Liano F, Marcen R, Naya MT, Orte L, Ortuno J. Incidence and prognosis of acute renal failure in older patients. J Am Geriatr Soc. 1990;38:25–30. doi: 10.1111/j.1532-5415.1990.tb01592.x. [DOI] [PubMed] [Google Scholar]
- 2.Pascual J, Liano F. Causes and prognosis of acute renal failure in the very old. Madrid Acute Renal Failure Study Group. J Am Geriatr Soc. 1998;46:721–725. doi: 10.1111/j.1532-5415.1998.tb03807.x. [DOI] [PubMed] [Google Scholar]
- 3.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 4.Baraldi A, Ballestri M, Rapana R, Lucchi L, Borella P, Leonelli M, Furci L, Lusvarghi E. Acute renal failure of medical type in an elderly population. Nephrol Dial Transplant. 1998;13(S7):25–29. doi: 10.1093/ndt/13.suppl_7.25. [DOI] [PubMed] [Google Scholar]
- 5.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992-2001. J Am Soc Nephrol. 2006;17:1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 6.Arora P, Kher V, Kohli HS, Sharma RK, Gupta A, Jha R. Acute renal failure in the elderly: Experience from a single centre in India. Nephrol Dial Transplant. 1993;8:827–830. [PubMed] [Google Scholar]
- 7.Schmitt R, Coca S, Kanbay M, Tinetti ME, Cantley LG, Parikh CR. Recovery of kidney function after acute kidney injury in the elderly: A systematic review and meta-analysis. Am J Kidney Dis. 2008;52:262–271. doi: 10.1053/j.ajkd.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Wu Q, Maniatis T. A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell. 1999;97:779–790. doi: 10.1016/s0092-8674(00)80789-8. [DOI] [PubMed] [Google Scholar]
- 9.Kobielak A, Fuchs E. α-Catenin: At the junction of intercellular adhesion and actin dynamics. Nat Rev Mol Cell Biol. 2004;5:614–625. doi: 10.1038/nrm1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsukita S, Tsukita S, Nagafuchi A, Yonemura S. Molecular linkage between cadherins and actin filaments in cell-cell adherens junction. Curr Opin Cell Biol. 1992;4:834–839. doi: 10.1016/0955-0674(92)90108-o. [DOI] [PubMed] [Google Scholar]
- 11.Knudsen KA, Soler AP, Johnson KR, Wheelock MJ. Interaction of α-actinin with the cadherin/catenin cell-cell adhesion complex via α-catenin. J Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rimm DL, Koslov ER, Kebriaci P, Cianci CD, Morrow JS. α1(E)-catenin is an actin-binding and –bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci USA. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh P, Rifkin DE, Blantz RC. Chronic kidney disease: An inherent risk factor for acute kidney injury? Clin J Am Soc Nephrol. 2010;5:1690–1695. doi: 10.2215/CJN.00830110. [DOI] [PubMed] [Google Scholar]
- 14.Benjamin JM, Kwiatkowski AV, Yang C, Korobova F, Pokutta S, Svitkina T, Weis WI, Nelson WJ. αE-catenin regulates actin dynamics independently of cadherin-mediated cell-cell adhesion. J Cell Biol. 2010;189:339–352. doi: 10.1083/jcb.200910041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mege RM, Gavard J, Lambert M. Regulation of cell-cell junctions by the cytoskeleton. Curr Opin Cell Biol. 2006;18:541–548. doi: 10.1016/j.ceb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Benjamin JM, Nelson WJ. Bench to bedside and back again: Molecular mechanisms of α-catenin function and roles in tumorigenesis. Semin Cancer Biol. 2008;18:53–64. doi: 10.1016/j.semcancer.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costa M, Raich W, Agbunag C, Leung B, Hardin J, Priess JR. A putative catenin-cadherin system mediates morphogenesis of the Caenorhabditis elegans embryo. J Cell Biol. 1998;141:297–308. doi: 10.1083/jcb.141.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobielak A, Fuchs E. Links between alpha-catenin, NF-kappaB, and squamous cell carcinoma in skin. Proc Natl Acad Sci USA. 2006;103:2322–2327. doi: 10.1073/pnas.0510422103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott JA, Yap AS. Cinderella no longer: α-catenin steps out of cadherin's shadow. J Cell Sci. 2006;119:4599–4605. doi: 10.1242/jcs.03267. [DOI] [PubMed] [Google Scholar]
- 21.Vasioukhin V, Bauer C, Degenstein K, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 2001;104:605–617. doi: 10.1016/s0092-8674(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 22.Scholten AN, Aliredjo R, Creutzberg CL, Smit VT. Combined E-cadherin, alpha-catenin, and beta-catenin expression is a favorable prognostic factor in endometrial carcinoma. Int J Gynecol Cancer. 2006;16:1379–1385. doi: 10.1111/j.1525-1438.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 23.Setoyama T, Natsugoe S, Okumura H, Matsumoto M, Uchikado Y, Yokomakura N, Ishigami S, Aikou T. Alpha-catenin is a significant prognostic factor than E-cadherin in esophageal squamous cell carcinoma. J Surg Oncol. 2007;95:148–155. doi: 10.1002/jso.20610. [DOI] [PubMed] [Google Scholar]
- 24.Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol. 2003;14(S1):S55–S61. doi: 10.1097/01.asn.0000067652.51441.21. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt R, Marlier A, Cantley LG. Zag expression during aging suppresses proliferation after kidney injury. J Am Soc Nephrol. 2008;19:2375–2583. doi: 10.1681/ASN.2008010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen D, Ni L, You L, Zhang L, Gu Y, Hao CM, Chen J. Upregulation of nestin in proximal tubules may participate in cell migration during renal repair. Am J Physiol Renal Physiol. 2012;303:F1534–F1544. doi: 10.1152/ajprenal.00083.2012. [DOI] [PubMed] [Google Scholar]
- 27.Jung KY, Dean D, Jiang J, Gaylor S, Griffith WH, Burghardt RC, Parrish AR. Loss of N-cadherin and alpha-catenin in the proximal tubules of aging male Fischer 344 rats. Mech Ageing Dev. 2004;125:445–453. doi: 10.1016/j.mad.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Gardel ML, Schneider IC, Aratyn-Schaus Y, Waterman CM. Mechanical integration of actin and adhesion dynamics in cell migration. Annu Rev Cell Dev Biol. 2010;26:315–333. doi: 10.1146/annurev.cellbio.011209.122036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen Z, Seppanen H, Kauttu T, Vainionpaa S, Ye Y, Wang S, Mustonen H, Puolakkainen P. Vasohbin-1 expression is regulated by transforming growth factor-b/bone morphogenetic protein signaling pathway between tumor-associated macrophages and pancreatic cancer cells. J Interferon Cytokine Res. 2013;33:428–433. doi: 10.1089/jir.2012.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gould SE, Day M, Jones SS, Dorai H. BMP-7 regulates chemokine, cytokine, and hemodynamic gene expression in proximal tubule cells. Kidney Int. 2002;61:51–60. doi: 10.1046/j.1523-1755.2002.00103.x. [DOI] [PubMed] [Google Scholar]
- 32.Sieber C, Kopf J, Hiepen C, Knaus P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 2009;20:343–355. doi: 10.1016/j.cytogfr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Mitu G, Hirschberg R. Bone morphogenetic protein-7 (BMP-7) in chronic kidney disease. Front Biosci. 2008;13:4726–4739. doi: 10.2741/3035. [DOI] [PubMed] [Google Scholar]
- 34.McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12:1438–1452. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanagita M, Oka M, Watabe T, Iguchi H, Niida A, Takahashi S, Akiyama T, Miyazono K, Yanagisawa M, Sakurai T. USAG-1, a bone morphogenetic protein antagonist abundantly expressed in the kidney. Biochem Biophys Res Commun. 2004;316:490–500. doi: 10.1016/j.bbrc.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 36.Motazed R, Colville-Nash P, Kwan JT, Dockrell ME. BMP-7 and proximal tubule epithelial cells: Activation of multiple signaling pathways reveals a novel anti-fibrotic mechanism. Pharm Res. 2008;25:2440–2446. doi: 10.1007/s11095-008-9551-1. [DOI] [PubMed] [Google Scholar]
- 37.Zhen-Qiang F, Bing-Wei Y, Yong-Liang L, Xiang-Wei W, Shan-Hong Y, Yuan-Ning Z, Wei-Sheng J, Wei C, Ye G. Localized expression of human BMP-7 by BM-MSCs enhances renal repair in an in vivo model of ischemia-reperfusion injury. Genes Cells. 2012;17:53–64. doi: 10.1111/j.1365-2443.2011.01572.x. [DOI] [PubMed] [Google Scholar]
- 38.Kadowaki T, Shiozaki H, Inoue M, Tamura S, Oka H, Doki Y, Iihara K, Matsui S, Iwazawa T, Nagafuchi A, Tsukita S, Mori T. E-cadherin and alpha-catenin expression in human esophageal cancer. Cancer Res. 1994;54:291–296. [PubMed] [Google Scholar]
- 39.Gofuku J, Shiozaki H, Tsujinaka T, Inoue M, Tamura S, Doki Y, Matsui S, Tsukita S, Kikkawa N, Monden M. Expression of E-cadherin and alpha-catenin in patients with colorectal carcinoma. Correlation with cancer invasion and metastasis. Am J Clin Pathol. 1999;111:29–37. doi: 10.1093/ajcp/111.1.29. [DOI] [PubMed] [Google Scholar]
- 40.Matsui S, Shiozaki H, Inoue M, Tamura S, Doki Y, Kadowaki T, Iwazawa T, Shimaya K, Nagafuchi A, Tsukita S. Immunohistochemical evaluation of alpha-catenin expression in human gastric cancer. Virchows Arch. 1994;424:375–381. doi: 10.1007/BF00190559. [DOI] [PubMed] [Google Scholar]
- 41.Watabe M, Nagafuchi A, Tsukita S, Takeichi M. Induction of polarized cell-cell association and retardation of growth by activation of E-cadherin-catenin adhesion system in a dispersed carcinoma line. J Cell Biol. 1994;127:247–256. doi: 10.1083/jcb.127.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lien WH, Klezovitch O, Fernandez TE, Delrow J, Vasioukhin V. AlphaE-catenin controls cerebral cortical size by regulating the hedgehog signaling pathway. Science. 2006;311:1609–1612. doi: 10.1126/science.1121449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karsenty G, Luo G, Hofmann C, Bradley A. BMP 7 is required for nephrogenesis, eye development, and skeletal patterning. Ann NY Acad Sci. 1996;785:98–107. doi: 10.1111/j.1749-6632.1996.tb56247.x. [DOI] [PubMed] [Google Scholar]
- 44.Vukicevic S, Basic V, Rogic D, Basic N, Shih MS, Shepard A, Jin D, Dattareyamurty B, Jones W, Dorai H, Ryan S, Griffiths D, Maliakal J, Jelic M, Pastorcic M, Stavljenic A, Sampath TK. Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J Clin Invest. 1998;102:202–214. doi: 10.1172/JCI2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeisberg M, Hanai JH, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. BMP-7 counteracts TGF-beta-1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nature Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 46.Wetzel P, Haag J, Campean V, Goldschmeding R, Atalla A, Amman K, Aigner T. Bone morphogenetic protein-7 expression and activity in the human adult normal kidney is predominantly localized to the distal nephron. Kidney Int. 2006;70:717–723. doi: 10.1038/sj.ki.5001653. [DOI] [PubMed] [Google Scholar]
- 47.Villanueva S, Cespedes C, Vio CP. Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. Am J Physiol Regul Integr Comp Physiol. 2006;290:R861–R870. doi: 10.1152/ajpregu.00384.2005. [DOI] [PubMed] [Google Scholar]
- 48.Marumo T, Hishikawa K, Yoshikawa M, Fujita T. Epigenetic regulation of BMP7 in the regenerative response to ischemia. J Am Soc Nephrol. 2008;19:1311–1320. doi: 10.1681/ASN.2007091040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishibashi K, Sasaki S, Akiba T, Marumo F. Expression of bone morphogenetic protein 7 mRNA in MDCK cells. Biochem Biophys Res Commun. 1993;193:235–239. doi: 10.1006/bbrc.1993.1614. [DOI] [PubMed] [Google Scholar]
- 50.Paralkar VM, Grasser WA, Mansolf AL, Baumann AP, Owen TA, Smock SL, Martinovic S, Borovecki F, Vukicevic S, Ke HZ, Thompson DD. Regulation of BMP-7 expression by retinoic acid and prostaglandin E(2) J Cell Physiol. 2002;190:207–217. doi: 10.1002/jcp.10048. [DOI] [PubMed] [Google Scholar]
- 51.Sakai H, Furihata M, Matsuda C, Takahashi M, Miyazaki H, Konakahara T, Imamura T, Okada T. Augmented autocrine bone morphogenetic protein (BMP7) signaling increases the metastatic potential of mouse breast cancer cells. Clin Exp Metastasis. 2012;29:327–338. doi: 10.1007/s10585-012-9453-9. [DOI] [PubMed] [Google Scholar]
- 52.Mishima Y, Lotz M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J Orthop Res. 2008;26:1407–1412. doi: 10.1002/jor.20668. [DOI] [PubMed] [Google Scholar]
- 53.Konstantinidis G, Moustakas A, Stournaras C. Regulation of myosin light chain function by BMP signaling controls actin cytoskeleton remodeling. Cell Physiol Biochem. 2011;28:1031–1044. doi: 10.1159/000335790. [DOI] [PubMed] [Google Scholar]


