Abstract
Background
Patients identified at surgical exploration with unresectable pancreatic ductal adenocarcinoma (PDAC) receive palliative, non-curative therapy. We hypothesized that accurate radiographic re-staging, multimodality treatment, and advanced surgical technique may offer patients deemed unresectable at previous exploration the possibility for curative salvage pancreatectomy.
Study Design
Review of prospectively maintained PDAC database identified all patients (1990-2010) evaluated after being deemed unresectable at first exploration elsewhere. Referring hospitals were categorized per National Cancer Data Base criteria (Academic, Community, International). Patients were re-staged using objective imaging (CT) criteria and classified based upon anatomic resectability. Clinicopathologic factors and cancer-related outcomes were assessed.
Results
We evaluated 88 patients who underwent previously unsuccessful resection attempts at Academic (n=50), Community (n=25) and International (n=13) centers. Radiographic re-staging confirmed that 7(8%) patient tumors were locally advanced and unresectable, but 81(92%) were resectable (n=61) or borderline resectable (n=20). Using a surgery first (9%) or preoperative chemoradiation (91%) approach, successful re-operative pancreatectomy was performed in 66(81%) patients with 94% receiving R0 resections. Vascular resection/reconstruction was required in 30(46%) patients and 50(76%) required complex revision of previously created biliary/gastrointestinal bypass. The major complication rate was 20% and 3(4.5%) patients died perioperatively. The median overall survival was 29.6 months for successfully resected patients vs. 10.6 and 5.1 months (p < 0.0001) for those patients with locally advanced unresectable disease at initial referral or who developed metastatic disease prior to resection, respectively.
Conclusions
In this very selected cohort of “high risk” patients, the majority of patients had anatomically resectable tumors upon re-staging. Accurate radiographic re-staging, a multimodality treatment strategy, and advanced surgical techniques may provide an opportunity for cure in a significant proportion of select patients previously deemed unresectable at exploration.
INTRODUCTION
The assessment of operative resectability is critical in patients newly diagnosed with pancreatic ductal adenocarcinoma (PDAC), as surgical resection is the only chance for cure. For a patient to receive the survival benefits of an operation, complete removal of the primary tumor and regional lymph nodes with a negative microscopic margin is recommended, if not mandatory. Multiple studies have confirmed that positive resection margins are associated with poor long-term outcome and can negate the potential benefit of resection.1,2 High quality cross-sectional imaging with multi-detector, dual phase computerized tomography (CT) can accurately predict local tumor resectability in the overwhelming majority of patients with PDAC.3,4 When properly performed and interpreted, such radiologic staging will inform the surgeon of the local extent of disease and anatomic regions at risk of positive resection margins at surgery. This can prevent an initial exploration attempt on patients where negative margins are unlikely as well as predict the need for more advanced techniques such as vascular resection in order to achieve margin negativity.
Our pancreas team relies heavily on preoperative CT imaging coupled with a complete primary medical evaluation in all new patients with apparently localized PDAC in an effort to optimize the selection of patients most likely to benefit from surgical therapy for possible cure. Patients are then risk-stratified based upon three criteria: Anatomy - tumor involvement of local vascular structures; Biology - factors associated with metastatic disease such as suspicious radiologic findings or markedly elevated CA19-9 levels; and Condition - fitness for therapy such as performance status, nutritional state, and comorbidities, many of which may be markers of more advanced occult disease. Based upon these factors, patients are clinically classified as unresectable, resectable, or borderline resectable and therapy for the individual patient is then tailored accordingly. This stratification strategy outlined above is designed to offer the possibility of a margin-negative, potentially curative resection to the greatest number of patients with localized PDAC. Patients with borderline anatomical, biological, or conditional features are at an increased risk of harboring occult metastatic disease, so preoperative (neoadjuvant) treatment is a logical strategy designed to identify and treat those high-risk patients while sparing them a potentially non-therapeutic laparotomy and failed resection attempt.5 Our group has reported several prospective trials demonstrating that this approach translates into relatively favorable cancer-related survival outcomes for patients with apparently localized PDAC.6-9
High-risk patients who complete preoperative therapy without evidence of metastasis or tumor progression are self-defined as having a “locally dominant” cancer phenotype and are therefore acceptable candidates for surgery. This selection process is critical because achieving the goals of surgery, particularly in anatomically borderline tumors, often requires complex vascular resection of involved adjacent structures. It has been demonstrated that the use of these vascular techniques, if performed safely, does not diminish survival and increases the number of potentially curative resections that can be performed.10,11 The surgeon’s appraisal of resectability through optimal radiographic staging, complete medical evaluation, and assessment of the need for advanced surgical techniques is therefore critical to the initial risk stratification in patients with PDAC, with the use and method of preoperative therapy determined by the results of this strategy. By combining these elements, patients stand the greatest chance of maximizing any survival benefit of surgical resection while minimizing the risk of ineffective surgery.
In this manuscript, we have applied this assessment and treatment strategy to a unique high-risk population: patients who have undergone previous surgical exploration with the intent to resect a PDAC that was subsequently deemed unresectable by intraoperative surgical assessment. This current report summarizes our experience in this high-risk cohort of patients with potentially curative disease, who would otherwise have gone onto palliative, non-curative therapy and resultant compromised survival.
METHODS
Patients and Data Collection
The Institutional Review Board at The University of Texas MD Anderson Cancer Center approved this study. We identified all patients who were evaluated at our institution from 1990-2010 for non-metastatic PDAC (n = 1363). These patients were then reviewed to identify those who underwent previous exploration for PDAC but were deemed unresectable at outside facilities. We specifically excluded patients who underwent surgical exploration for diagnostic purposes or other indications without the specific intent to resect a known malignancy as such patients may not have had the extensive preoperative staging necessary to determine resectability prior to their first exploration. The remaining patients represented our study group. For all patients the original outside operative reports were reviewed to determine the findings and reasons reported for unsuccessful resection. All demographic, clinicopathologic, and outcomes data were retrospectively retrieved from our prospectively collected clinical database maintained within the Department of Surgical Oncology.12 In an effort to characterize specific center expertise and experience in a standardized and minimally arbitrary fashion, the referring hospitals were categorized as “Academic”, “Community” or “International” centers in accordance with National Cancer Data Base criteria.13 Although academic designation does not specifically imply expertise in pancreatic resection techniques, upon review of the specific individual centers within the academic group, the overwhelming majority of these centers are considered by reputation and by previous published experiences as major pancreatic surgery centers.
Patient Assessment, Therapy, and Follow Up
A pathologic diagnosis of PDAC was confirmed in all patients. Radiographic re-staging was performed using a multidetector, contrast-enhanced, multi-phase pancreas protocol CT scan in all patients at initial referral. For this study, previously defined imaging criteria were used to anatomically characterize each patient’s tumor as unresectable (locally advanced), resectable, or borderline resectable.5,14 Resectable tumors were anatomically defined by the following: (1) absence of extrapancreatic disease; (2) no evidence of tumor extension to the superior mesenteric artery (SMA), celiac axis, or hepatic artery as defined by the presence of a tissue plane between the tumor and these arteries; and (3) a patent superior mesenteric-portal vein (SMPV) confluence with or without venous involvement. Borderline resectable tumors were anatomically defined by those that demonstrated tumor abutment of the SMA or celiac axis (180° or less of the circumference of the vessel); tumor abutment or encasement of a short segment of the hepatic artery; or short-segment occlusion of the superior mesenteric vein, portal vein, or SMPV confluence that was amenable to vascular resection and reconstruction. An evaluation by a multidisciplinary pancreatic tumor study group including surgical oncologists, radiation oncologists, medical oncologists, and body imaging radiologists was routine. At this review the results of the radiographic re-staging anatomic tumor classification was combined with biologic and conditional data to clinically risk stratify patients. Using this evaluation an off-protocol treatment plan was developed for each patient. Patients who received preoperative therapy were re-staged and assessed for tumor progression, distant metastasis, and fitness for surgery prior to a re-attempt at tumor resection.
Pancreatic resection was performed in a standard fashion as previously described and the oncologic technical principles have remained relatively unchanged during this series.15 Tangential or segmental resection of the superior mesenteric vein, portal vein, or SMPV confluence was performed when the operating surgeon could not separate the pancreatic head or the uncinate process from these vessels without leaving residual tumor on the vessel or risking uncontrolled venotomy. The types of venous reconstruction performed were categorized as V1-V5 as previously described.11 Simple partial vein resection with primary venorraphy was not considered venous reconstruction. When involvement of the common hepatic artery or SMA was identified, segmental resection was performed with primary anastomosis or interposition grafting. The indications for arterial reconstruction were based on preoperative imaging and only performed in a few highly selected patients in an effort to provide a microscopically margin negative resection.
Standardized pathologic evaluation of the surgical specimen was performed as previously described.16 Specifically, the technique for assessment of SMA margin status was the same regardless of whether or not vascular resection was performed. The SMA margin, posterior to the groove of the SMPV confluence defined as the soft tissue margin directly adjacent to the proximal 3 to 4 cm of the SMA, was inked and submitted in its entirety for microscopic examination on permanent sections by sectioning the specimen perpendicular to the inked margin. A margin was designated “R1” if any tumor cells were present at the inked SMA margin and we do not consider a <1mm margin as positive.
Major postoperative outcomes were recorded and surgical complications graded according to Clavien-Dindo criteria.17 Pancreatic leaks were identified according to ISGPF criteria and all grade leaks were considered. Recurrence was defined by radiographic development of new suspicious low-density masses with or without biopsy confirmation. For the purpose of statistical survival comparison, patients were grouped according to resection status after review and disposition as: unresectable at referral, metastasis prior to resection, and successfully resected.
Statistical Considerations
Categorical data were compared by X2 analysis or Fisher’s exact tests. Student’s T-test and ANOVA was used to assess the differences in continuous variables. Overall (OS) and recurrence-free (RFS) survival curves were constructed using the Kaplan-Meier product limit method, and the log-rank test was used to evaluate the statistical significance of differences. Overall survival was defined as the time from successful re-resection or the time from being deemed unresectable (either at initial radiographic re-staging or at the time metastatic disease was identified prior to re-resection attempt) to the date of death or to the time of last follow-up at which point the data were censored. Recurrence-free survival was defined as the time from successful re-resection to the date of clinically documented recurrence or death or last follow-up at which point the data were censored. Statistical analysis was performed using software from MedCalc (V. 11.6) and GraphPad Prism (V. 5.0d). A two-sided significance level of 0.05 was used for all statistical analyses.
RESULTS
Initial Surgical Resection Attempt
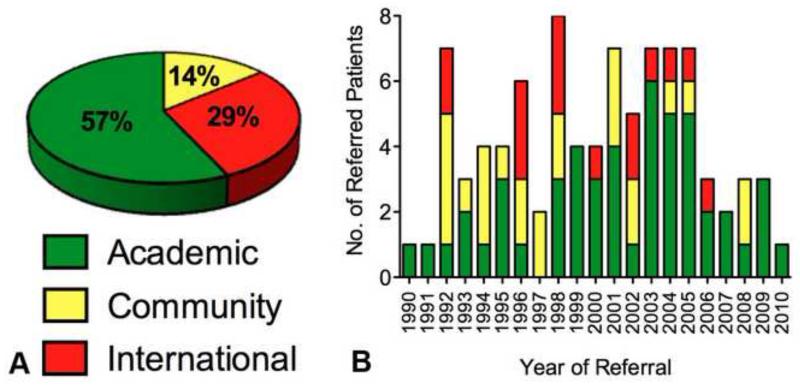
Between 1990 and 2010, a total of 88 patients were evaluated after a prior unsuccessful attempt at resection of PDAC elsewhere. (Table 1) The attempted resections were performed at Academic (n=50), Community (n=25) and International (n=13) centers located in 22 different states in the US and 10 different countries. We identified no specific annual trend in the type of hospital or the number of patients referred during the study period. (Figure 1) In all cases patients were previously deemed unresectable due to perceived unresectable involvement of adjacent vasculature by the operating surgeon. Palliative surgical bypass (biliary and/or GI) was performed in 65 (74%) of these patients at the time of their first resection attempt.
Table 1.
Patient Demographics of Study Population
| Variable | All patients, n = 88 |
Unresectable at referral, n = 7 |
Metastasis before resection, n = 15 |
Successfully resected, n = 66 |
p Value |
|---|---|---|---|---|---|
|
| |||||
| Sex, n (%) | |||||
| Female | 43 (49) | 2 (29) | 10 (67) | 31 (47) | NS |
| Male | 45(51) | 5 (71) | 5 (33) | 35 (53) | |
|
| |||||
| Race, n (%) | |||||
| Caucasian | 74 (85) | 7 (100) | 12 (80) | 55 (83) | |
| African-American | 2 (2) | 0 (0) | 1 (7) | 1 (15) | NS |
| Hispanic | 9 (10) | 0 (0) | 2 (13) | 7 (11) | |
| Asian | 2 (2) | 0 (0) | 0 (0) | 2 (3) | |
| Other | 1 (1) | 0 (0) | 0 (0) | 1 (1.5) | |
|
| |||||
| Age at referral, y | NS | ||||
| Mean | 58.1 | 54.9 | 56.3 | 58.8 | |
| SD | 11.6 | 17.2 | 9.7 | 11.4 | |
|
| |||||
| Time interval from first resection attempt to referral/re-staging, d |
|||||
| Mean | 44.3 | 31.1 | 58.4 | 42.5 | NS |
| SD | 28.7 | 14.1 | 26.9 | 29.3 | |
|
| |||||
| Previous center, n (%) | |||||
| Academic | 50 (57) | 3 (43) | 6 (40) | 41 (62) | |
| Community | 25 (28) | 4 (57) | 4 (27) | 17 (26) | NS |
| International | 13 (15) | 0 (0) | 5 (33) | 8 (12) | |
|
| |||||
| Previous biliary/GI bypass, n (%) |
NS | ||||
| No | 23 (26) | 3 (43) | 4 (27) | 16 (24) | |
| Yes | 65 (73) | 4 (57) | 11 (73) | 50 (76) | |
|
| |||||
| Neoadjuvant therapy before second resection attempt, n (%) |
NS | ||||
| No | 2 (13) | 5 ( 8) | |||
| Yes | 13 (87) | 61 (92) | |||
|
| |||||
| Initial CA19-9 | |||||
| Mean | 299 | 824 | 120.5 | 303.2 | NS |
| SD | 773.8 | 617 | 97 | 817 | |
|
| |||||
| Preop CA19-9 | |||||
| Mean | 106.6 | - | 96.3 | 113.2 | NS |
| SD | 191.8 | 82.3 | 211 | ||
|
| |||||
| Fold-change in CA19-9 | |||||
| Mean | 0.69 | - | 0.97 | 0.68 | NS |
| SD | 0.63 | 0.74 | 0.65 | ||
|
| |||||
| Time interval from first to second resection attempt, mo |
|||||
| Mean | 5.6 | 5.8 | NS | ||
| SD | 2.7 | 4.1 | |||
Figure 1.
(A) Proportion of study patients who underwent previous resection attempts categorized according to NCDB criteria. (B) Number of referred patients per year by type of referring institution showing no trend over time.
Evaluation and Staging
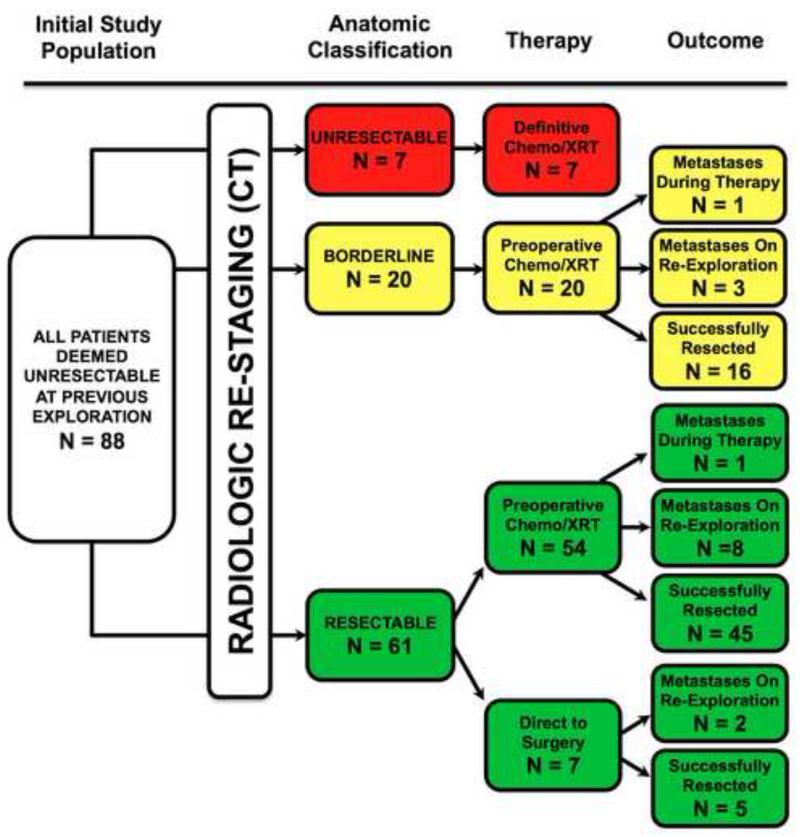
The median (mean) duration from initial resection attempt elsewhere to subsequent consultation and re-staging at our institution was 35 (44) days (range 13-148). Radiographic re-staging identified 7 (8%) patient tumors as anatomically locally advanced, and therefore unresectable. The remaining 81 (92%) patient tumors were radiographically categorized as either anatomically resectable (61 patients) or anatomically borderline resectable (20 patients). (Figure 2)
Figure 2.
Radiographic anatomical re-staging classification with subsequent therapy disposition and outcomes for entire study cohort.
Therapy
Chemoradiation was administered preoperatively in 74 (91%) patients while 7 (9%) patients went directly to surgery following re-staging. (Figure 2) All patients were considered for preoperative therapy however those 7 (9%) patients went directly to surgery primarily due to patient preference, socioeconomic factors, and surgical risk acceptability. Preoperative chemoradiation prior to the second resection attempt was administered to all patients with anatomically borderline resectable tumors. All patients who underwent surgery after preoperative chemoradiation exhibited no evidence of radiographic or biochemical cancer progression. Although no local progression of the primary tumor occurred during preoperative therapy, metastatic disease was radiographically detected during preoperative therapy in two (3%) patients. Metastatic disease was identified at reoperation in 11 (15%) of the 74 patients that received preoperative therapy and in 2 (29%) of the 7 patients that went directly to surgery; this was not significantly different between groups.
Complete resection with curative intent was achieved in 66 (75%) of the original 88 patients. Statistical comparison between the 7 unresectable patients, the 15 patients not resected due to development of metastatic disease, and the 66 who were successfully resected identified no difference in patient demographics, type of institution where first attempt occurred, palliative surgical procedures performed, the time interval between first surgery and re-evaluation, or the CA19-9 at re-evaluation (for those in whom values were available). Statistical comparison of the 15 patients not resected due to development of metastatic disease and those 66 who were successfully resected identified no difference in the use of preoperative therapy or changes between initial and preoperative in CA19-9 values after preoperative therapy. Although those that were successfully resected experienced a greater reduction in CA19-9 levels after preoperative therapy, this was not statistically significant. (Table 1)
Surgical Methods and Outcomes
The surgical and pathologic outcomes of all resected patients (n=66) in this current series are described in Table 2 and Table 3 and are compared to our previously published series of anatomically borderline (Type A) patients (n=32).5 Pancreaticoduodenectomy was performed in 64 (97%) patients and total pancreatectomy in 2 (3%). Although all patients in this cohort had presumed vasculature involvement per outside operative reports, vascular resection and reconstruction was only required in 30 (46%) of patients. Venous resection was necessary in 25 (83%), arterial resection in 3 (10%), and combined resection in 2 (7%) patients. The types of venous reconstruction were as follows: V1 in 9 (33%); V2 in 3 (11%); V3 in 2 (8%); V4 in 6 (22%); and V5 in 7 (26%).11 Arterial reconstruction of the hepatic artery was necessary in four patients and the SMA in one patient. Following tumor removal, complex revision of previously created palliative biliary/gastrointestinal bypass was required in 61 (94%) patients to provide biliary drainage and gastric emptying. These were carried out using previously described techniques.18
Table 2.
Surgical Outcomes
| Variable | Current series, n = 66 | Previous borderline-A series, n=32 | ||
|---|---|---|---|---|
|
| ||||
| n | % | n | % | |
|
| ||||
| Procedure | ||||
| Pancreaticoduodenectomy | 64 | 97 | 28 | 88 |
| Total Pancreatectomy | 2 | 3 | 4 | 12 |
|
| ||||
| Vascular reconstruction | ||||
| No | 36 | 54 | 18 | 59 |
| Yes | 30 | 46 | 14 | 41 |
|
| ||||
| Type of vascular reconstruction | ||||
| Venous | 25 | 83 | 12 | 88 |
| Arterial | 3 | 10 | 2 | 12 |
| Both | 2 | 7 | 0 | 0 |
|
| ||||
| Class of venous reconstruction | ||||
| V1 | 9 | 33 | N/A | |
| V2 | 3 | 11 | ||
| V3 | 2 | 8 | ||
| V4 | 6 | 22 | ||
| V5 | 7 | 26 | ||
|
| ||||
| Type of arterial reconstruction | ||||
| Hepatic artery | 4 | 80 | N/A | |
| SMA | 1 | 20 | ||
|
| ||||
| Revision of previous biliary/GI bypass | ||||
| No | 16 | 24 | N/A | |
| Yes | 50 | 76 | ||
|
| ||||
| OR Time, min | ||||
| Mean | 543 | 444 | ||
| SD | 136 | N/A | ||
|
| ||||
| Estimated blood loss, mL | ||||
| Mean | 1,350 | 977 | ||
| SD | 953 | N/A | ||
|
| ||||
| Hospital stay, d | ||||
| Mean | 12.5 | 11 | ||
| SD | 6.9 | N/A | ||
|
| ||||
| Major complications (Clavien/Dindo) | ||||
| No | 53 | 80 | 26 | 80 |
| Yes | 13 | 20 | 6 | 20 |
|
| ||||
| Perioperative mortality | ||||
| No | 63 | 95.5 | 32 | 100 |
| Yes | 3 | 4.5 | 0 | 0 |
Table 3.
Pathologic Outcomes
| Variable | Current series, n = 66 | Previous borderline-A series, n=32 |
|---|---|---|
|
| ||
| Tumor size, cm | ||
| Mean | 2.9 | 2.5 |
| SD | 1.6 | N/A |
|
| ||
| Positive lymph nodes, n (%) | ||
| No | 36 (55) | 20 (62) |
| Yes | 30 (45) | 12 (38) |
|
| ||
| No. of lymph nodes harvested | ||
| Mean | 20 | 21 |
| SD | 9.7 | N/A |
|
| ||
| SMA Margin positivity, n (%) | ||
| No | 62 (94) | 7) (9 31 |
| Yes | 4 (6) | (3) |
|
| ||
| Adjuvant therapy, n (%) | ||
| No | 54 (82) | 26 (80) |
| Yes | 12 (18) | 6 (20) |
An R0 resection was achieved in 62 (94%) patients. Final pathologic findings included a mean tumor size of 2.9 centimeters with 30 (45%) patients harboring metastatic regional lymph nodes with a mean yield of 20 lymph nodes. Mean operating time was 543 minutes with a mean estimated blood loss of 1390 milliliters. There were 13 (20%) patients that developed major complications including 6 (9%) with pancreatic leak. Three patients died in the perioperative period (30-day), two from multi-organ failure and one patient from a postoperative hemorrhage, yielding a mortality rate of 4.5%. Mean hospital stay for all resected patients was 12.5 days. Only 12 (18%) patients underwent adjuvant therapy after resection.
Cancer Survival
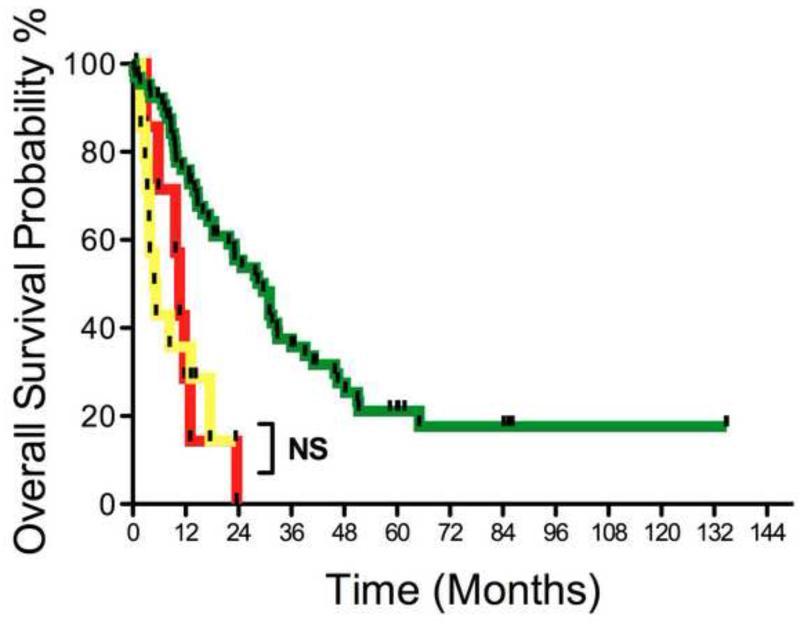
Survival data of all patients is shown in Table 4 and Figure 3. The median overall survival (OS) for all 88 patients in this study was 18.4 months. The OS for those deemed locally advanced and unresectable at initial radiographic re-staging was 10.6 months and the OS for those that did not undergo re-resection due to development of metastatic disease was 5.1 months; these two groups were not significantly different. The OS for those 66 patients that successfully underwent pancreatectomy was 29.6 months; this was significantly different from the other groups (P<0.0001). During the median follow-up of 29 months, 43 (65%) patients developed recurrence with the site of first recurrence local in 21%, regional in 27%, and distant in 52%. The recurrence-free survival (RFS) for those that were successfully resected was 15.9 months. There were no long-term survivors in those identified with locally advanced unresectable disease at initial re-staging or in those patients that developed metastatic disease prior to re-resection attempt. In contrast, there were 10 (15%) actual 5-year survivors among those successfully resected (three of whom were initially radiographically re-staged as anatomically borderline resectable).
Table 4.
Overall and Recurrence-Free Survival
| Variable | All Patients, n = 88 |
Unresectable at referral, n = 7 |
Metastasis before resection, n = 15 |
Succesfully resected, n = 66 |
p Value |
|---|---|---|---|---|---|
| Median overall survival, mo |
18.4 | 10.6 | 5.1 | 29.6 | <0.0001 |
| 8.4 | 29.6 | <0.0001 | |||
| 10.6 | 5.1 | - | NS | ||
| Recurrence, n (%) | |||||
| No | - | - | - | 23 (35) | - |
| Yes | 43 (65) | ||||
| Median recurrence-free survival, mo |
- | - | - | 15.9 | - |
| 5-Year survivors, n (%) | 10/88 (11) | 0 (0) | 0 (0) | 10/66 (15) | <0.0001 |
Figure 3.
Kaplan-Meier overall survival curves for all patients by eventual outcome. Red line, unresectable at referral (n=7), median overall survival 10.5 mo; yellow line, metastasis before resection (n=15), median overall survival 5.1 mo; and greend line, successfully resected (n=66), median overall survival 29.6 mo; p<0.0001.
DISCUSSION
This report demonstrates that accurate radiographic re-staging with risk stratification, selective preoperative therapy delivered in a multidisciplinary approach, and the use of advanced surgical techniques can provide a second chance for cure in highly selected patients that have been deemed surgically unresectable after first operative exploration. Upon radiographic re-staging the majority (69%) of these patients were identified with anatomically resectable tumors. Out of an initial cohort of 88 patients, 66 (75%) were able to receive a potentially curative resection with a median overall survival of 29.6 months, which corresponds favorably with published cancer-related outcomes after successful pancreatectomy.1,19 Importantly, 15% of the re-resected patients survived 5-years, a rate not yet reported after palliative therapy alone. The excellent long-term outcomes in this series of high-risk patients are most likely a result of ideal patient and tumor biological selection utilizing a standardized institutional patient risk stratification approach.
The initial proof of concept for this approach was demonstrated in a small series of different patients reported previously by our group 18. Other authors have reported similar small series results.20-23 This current study differs from these initial reports in that all the patients in this current cohort underwent a previously planned resection of their known pancreatic cancer but were deemed unresectable by the surgeon due to presumed vascular involvement as assessed intraoperatively. This manuscript explores the results of our assessment and treatment approach to these unique high-risk patients and underscores that accurate radiographic staging with surgical interpretation is the critical first step for clarification of the local extent of tumor that will ultimately determine any required and necessary surgical procedures. In our approach, a patient’s anatomic tumor classification is then combined with biologic and comorbidity data to risk stratify patients and tailor therapy through a multimodality approach.
This current report provides a snapshot of the general approach to delivering therapy for apparently localized pancreatic cancer over the last two decades. Over half of the patients in this cohort were initially explored at major academic surgical centers. If we assume that all of these tumors were considered at least initially anatomically resectable preoperatively, as all the patients went onto surgical exploration with intent for cure, our opinions as to the potential explanations for the failure to resect at initial operation are: inadequate pre-operative image quality, inadequate radiologic interpretation, discrepancy between intraoperative findings and pre-operative imaging with lack of trust of radiographic findings, lack of technical capability or willingness to perform vascular resection to achieve potential negative margin resections, and finally a sense of surgical nihilism based on historical overall poor outcomes and early recurrence in the majority of patients resected for potential cure.
If one assumes that technically experienced surgeons performed the initial exploration, this suggests that appropriate imaging and interpretive skills were the missing component. Failure to execute this critical component would lead to misclassification of locally advanced tumors as surgically resectable as well as fail to identify those patients with borderline anatomy where preoperative therapy may have been of benefit. Unfortunately the majority of the pre-referral scans were not available for review, thus we were only able to re-review our institutional re-staging scans at initial patient referral. Upon re-staging, 8% of tumors were anatomically unresectable (locally advanced) and another 25% were anatomically borderline resectable, thus failure to recognize these features pre-operatively and the resultant unexpected identification of a more advanced tumor at initial exploration may have accounted for some of these unsuccessful resection attempts. We and others have demonstrated that high resolution, multi-phase, helical pancreatic protocol CT with thin-cuts is highly predictive for tumors that are free of local vasculature and those in which vascular resection might be necessary.24-26 Close communication between the surgeon and radiologist is a critical component. Our group utilizes and advocates a structured radiologic report containing fields in which critical clinical information (such as vessel patency, abutment, and relative degree of involvement of all important vascular structures) can be populated by the radiologist and reviewed by the surgeon preoperatively to guide subsequent decision-making and surgical planning.
The related component to this clinical scenario is the potential lack of expertise of the surgeons performing the initial procedure. Although the necessity of vascular resection cannot always be predicted with certainty in any individual patient, published literature from our group and others provides useful data. In this current series of high-risk patients vascular resection and reconstruction was necessary to obtain negative margins in nearly 50% of patients. This is not unexpected given that up to 40% of patients required vascular resection in previous reports.5,7,9,19 Thus many of the failures to resect at first attempt may have been due to the inability or unwillingness to perform venous resection in order to obtain negative margins despite 75% of patients of those successfully resected having clearly anatomically resectable (non-borderline tumors) at re-staging. Patients with tumors that appear to involve surrounding vascular structures at initial radiographic staging will likely require vascular resection. Knowing this, an inexperienced surgeon could anticipate this need preoperatively and plan appropriately by asking another surgeon with necessary skill set to assist during the case or by referring the patient to a surgeon or center with the necessary expertise. Radiographic evidence of a cancer that may require vascular resection does not warrant an “attempt” at resection without the technical capacity to do so. Our institutional anatomic criteria for anatomically resectable pancreatic cancer is more broad and inclusive compared to NCCN (National Comprehensive Cancer Network) guidelines as tumors at our institution that involve a patent SMV/PV confluence are considered in the same anatomically resectable category as those tumors that do not involve venous structures, thus our venous resection rate for anatomically resectable tumors may be higher than other institutions who utilize NCCN criteria for resectability. That being said, in our opinion as a significant proportion of resectable patients may require venous resection in an effort to provide a margin negative resection, surgeons who hope to render a potentially curative operation should be prepared and have the technical capacity to perform more advanced vascular techniques. Taken together, the data in this manuscript suggest that the combination of inaccurate imaging and/or interpretation and the lack of necessary skill set in the operating room may be a common scenario leading to failed attempt at resection.
The resulting inflammation from the previous surgical resection attempt and subsequent hospitalization with potential complications experienced by these patients makes this a particularly challenging patient group to care for. Upon review of the scans obtained at referral many did have evidence of postoperative inflammatory changes as expected soon after an initial resection attempt and even some infectious complications. This is one of the inherent advantages of utilizing a multimodality approach to these select patients as these associated conditions may have the necessary time to heal rendering a potentially safer resection after preoperative treatment. In fact as our low rate of pancreatic leak (9%) in this series supports, the use of preoperative therapy has the potential to decrease subsequent surgical complications.27,28 Our treatment approach for this cohort demonstrates our bias toward preoperative therapy in such high-risk patients and as such they all should be considered clinically borderline resectable. The retrospective nature of this study and small number of patients makes it difficult, however, to determine if the preoperative therapy approach is superior to careful radiologic restaging and evaluation by surgeons with experience in advanced techniques alone. It could be argued then that preoperative therapy is just a mechanism for selection of patients and may not alter the natural history of pancreatic cancer in the individual patient. We acknowledge the possibility that systemic therapy was ineffectual for the individual patients in whom metastases are identified during or after preoperative therapy. It is precisely for this reason that we employ these treatments before surgery. We assume that microscopic burden of disease is already present, particularly in the patient with borderline anatomy, biology or condition. We use preoperative therapy to select patients with “locally dominant” disease and then treat them with aggressive surgery. If systemic therapy cannot control the development of clinically detectable metastases before surgery, there is no good reason to believe this will be the case after surgery. We believe the poor survival of those patients who developed metastases during preoperative therapy (< 6 months) supports that assumption. Furthermore, in this current series 16 of 20 (80%) anatomically borderline resectable tumors were able to be successfully resected following preoperative therapy after a previous failed resection attempt. This compares favorably to our previous report of only 38%, suggesting that upfront therapy may actually be of benefit in increasing the likelihood of resectability in this special subset of patients. Although others have raised concerns that a preoperative treatment schema may result in the loss of a surgical window of opportunity for curative resection, no patients in this current series progressed locally, which is similar to results from our previous neoadjuvant trials.9 We have found that although preoperative chemoradiation can lead to some remarkable morphologic and biochemical responses, our experience has not demonstrated that preoperative therapy can convert anatomically unresectable tumors into resectable tumors nor convert those tumors requiring vascular resection on initial imaging to those that do not at final surgery. This observation underscores the importance of adequately performed and interpreted initial radiologic studies to anatomically classify patients for subsequent therapy and disposition.
Resection in the re-operative setting does not compromise long-term outcomes as evidenced by our results, however is associated with significant technical demands. The operative time, blood loss, and mortality of this patient cohort reflects the added difficulty to achieve our stated surgical goals in these high-risk patients, but it also emphasizes the need for carefully selecting those patients most likely to receive survival benefit for the surgical risk. We cannot discount the fact that a previous surgeon’s judgment considered these patients unresectable, and thus we need to take pause prior to rushing into the re-operative setting. Furthermore we do not intend to dictate the individual practice decisions of other experienced surgical providers. Although the presence of a previously created biliary/gastrointestinal bypass in the majority of the patients in this series required reconstruction and resultant prolonged operatives times and technical complexity, if the decision to bypass is due to perceived unresectable disease identified intraoperatively at first exploration and is based on sound surgical judgment then it is appropriate, and should not altered due to the potential of complicating a possible future resection attempt. Moreover the results of this manuscript are not to suggest that all or even most patients deemed unresectable at first operation can eventually undergo resection after preoperative therapy, but rather that some highly selected patients may be salvaged by such an approach.
We acknowledge the significant selection bias in this retrospective analysis, as the majority of these referrals were in fact anatomically resectable at presentation upon radiographic review. The majority of the patients referred to our surgeons for second opinion after previous failed exploration have been seen by several providers after review of studies and were thought to qualify for consideration of salvage pancreatectomy. Certainly not all truly locally advanced patients are referred to us for consideration of re-resection attempt. However, the incidence of failed resections due to locally advanced (non-metastatic) disease identified at exploration should be considerably rare due to the marked improvements in axial imaging and predictability of resectability preoperatively, thus accounting for the lower incidence of truly locally advanced cases in this series. However, our findings suggest that a multidisciplinary approach may enable an oncologically effective pancreatectomy in the re-operative setting; however this surgery carries with it significant technical demands and increased patient risk. For this reason we recommend that re-operative pancreatectomy be performed by pancreatic surgeons with experience in vascular reconstruction techniques supported by an excellent multidisciplinary team in the form of dedicated medical oncologists, radiation oncologists, radiologists, and interventionalists with a focus on preoperative therapy. We conclude that excellent preoperative radiographic staging and interpretation is critical and surgeons should have trust and confidence in staging results and need to be technically equipped to do the operation suggested by these initial studies. In patients that are re-staged with potentially resectable tumors, the use of preoperative therapy should be highly considered in this new group of highly selected, high-risk patients. Using such a strategy, patients that were once resigned to palliative therapy may be offered a chance for cure and long-term survival.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Presented at the Western Surgical Association 119th Scientific Session, Tucson, AZ, November 2011.
REFERENCES
- 1.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. discussion 1210-1211. [DOI] [PubMed] [Google Scholar]
- 2.Hartel M, Niedergethmann M, Farag-Soliman M, et al. Benefit of venous resection for ductal adenocarcinoma of the pancreatic head. Eur J Surg. 2002;168:707–712. doi: 10.1080/00000000000000007. [DOI] [PubMed] [Google Scholar]
- 3.Lu DS, Reber HA, Kadell BM, Sayre J, et al. Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR American journal of roentgenology. 1997;168:1439–1443. doi: 10.2214/ajr.168.6.9168704. [DOI] [PubMed] [Google Scholar]
- 4.Tamm EP, Loyer EM, Faria S, et al. Staging of pancreatic cancer with multidetector CT in the setting of preoperative chemoradiation therapy. Abdominal imaging. 2006;31:568–574. doi: 10.1007/s00261-005-0194-y. [DOI] [PubMed] [Google Scholar]
- 5.Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–846. doi: 10.1016/j.jamcollsurg.2007.12.020. discussion 846-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127:1335–1339. doi: 10.1001/archsurg.1992.01420110083017. [DOI] [PubMed] [Google Scholar]
- 7.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 8.Pisters PW, Wolff RA, Janjan NA, et al. Preoperative paclitaxel and concurrent rapid-fractionation radiation for resectable pancreatic adenocarcinoma: toxicities, histologic response rates, and event-free outcome. J Clin Oncol. 2002;20:2537–2544. doi: 10.1200/JCO.2002.11.064. [DOI] [PubMed] [Google Scholar]
- 9.Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 10.Nakagohri T, Kinoshita T, Takahashi S, et al. Survival benefits of portal vein resection for pancreatic cancer. Am J Surg. 2003;186:149–153. doi: 10.1016/s0002-9610(03)00173-9. [DOI] [PubMed] [Google Scholar]
- 11.Tseng JF, Raut CP, Lee JE, et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg. 2004;8:935–949. doi: 10.1016/j.gassur.2004.09.046. discussion 949-950. [DOI] [PubMed] [Google Scholar]
- 12.Hwang RF, Wang H, Lara A, et al. Development of an integrated biospecimen bank and multidisciplinary clinical database for pancreatic cancer. Ann Surg Oncol. 2008;15:1356–1366. doi: 10.1245/s10434-008-9833-1. [DOI] [PubMed] [Google Scholar]
- 13.Winchester DP, Stewart AK, Jones RS, et al. The National Cancer Data Base: a clinical surveillance and quality improvement tool. J Surg Oncol. 2004;85:1–3. doi: 10.1002/jso.10320. [DOI] [PubMed] [Google Scholar]
- 14.Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Yen TW, Abdalla EK, Evans DB, et al. Pancreaticoduodenectomy. In: Von Hoff DD, Evans DB, Hruban RH, editors. Pancreatic Cancer. Sudbury, MA: 2005. pp. 265–286. [Google Scholar]
- 16.Raut CP, Tseng JF, Sun CC, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyler DS, Evans DB. Reoperative pancreaticoduodenectomy. Ann Surg. 1994;219:211–221. doi: 10.1097/00000658-199402000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz MH, Wang H, Fleming JB, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836–847. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimi H. Reoperative pancreaticoduodenectomy. Ann Surg. 1995;221:121–122. [PMC free article] [PubMed] [Google Scholar]
- 21.Lavu H, Nowcid LJ, Klinge MJ, et al. Reoperative completion pancreatectomy for suspected malignant disease of the pancreas. J Surg Res. 2011;170:89–95. doi: 10.1016/j.jss.2011.04.050. [DOI] [PubMed] [Google Scholar]
- 22.Robinson EK, Lee JE, Evans DB, et al. Reoperative pancreaticoduodenectomy for periampullary carcinoma. Am J Surg. 1996;172:432–437. doi: 10.1016/S0002-9610(96)00218-8. discussion 437-438. [DOI] [PubMed] [Google Scholar]
- 23.Shukla PJ, Qureshi SS, Desouza LJ, et al. Reoperative pancreaticoduodenectomy for periampullary carcinoma. ANZ J Surg. 2005;75:520–523. doi: 10.1111/j.1445-2197.2005.03438.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee JK, Kim AY, Ha HK, et al. Prediction of vascular involvement and resectability by multidetector-row CT versus MR imaging with MR angiography in patients who underwent surgery for resection of pancreatic ductal adenocarcinoma. European journal of radiology. 2010;73:310–316. doi: 10.1016/j.ejrad.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 25.Loyer EM, David CL, Charnsangavej C, et al. Vascular involvement in pancreatic adenocarcinoma: reassessment by thin-section CT. Abdominal imaging. 1996;21:202–206. doi: 10.1007/s002619900046. [DOI] [PubMed] [Google Scholar]
- 26.Fuhrman GM, Charnsangavej C, Abbruzzese JL, et al. Thin-section contrast-enhanced computed tomography accurately predicts the resectability of malignant pancreatic neoplasms. Am J Surg. 1994;167:104–111. doi: 10.1016/0002-9610(94)90060-4. discussion 111-113. [DOI] [PubMed] [Google Scholar]
- 27.Chandler NM, Canete JJ, Stuart KE, Callery MP. Preoperative chemoradiation in resectable pancreatic cancer. Journal of hepato-biliary-pancreatic surgery. 2003;10:61–66. doi: 10.1007/s10534-002-0736-5. [DOI] [PubMed] [Google Scholar]
- 28.Satoi S, Yanagimoto H, Toyokawa H, et al. Surgical results after preoperative chemoradiation therapy for patients with pancreatic cancer. Pancreas. 2009;38:282–288. doi: 10.1097/MPA.0b013e31819438c3. [DOI] [PubMed] [Google Scholar]