Introduction
Acinetobacter baumannii are aerobic Gram-negative bacilli that are increasingly becoming one of the major causes of sporadic and epidemic nosocomial infections [1, 2]. Hospital isolates of A. baumannii are commonly found to be resistant to extended spectrum cephalosporins and carbapenems [3–6]. Also, increasing numbers of reports show that A. baumannii quickly becomes endemic after it is introduced into an institution [7, 8]. The molecular epidemiology that drives the propagation of these newly introduced and multidrug resistant (MDR) strains is poorly understood.
In June 2006, we observed a significant increase in the number of A. baumannii isolates that were MDR (here defined as being resistant to all cephalosporins, penicillins, carbapenems, aminoglycosides and fluoroquinolones and susceptible only to colistin and/or tigecycline). This MDR A. baumannii phenotype was mostly found in the surgical intensive care unit (SICU). After this outbreak the clinical microbiology laboratory continued to collect MDR A. baumannii strains throughout the hospital and performed a genotypic analysis to understand the molecular epidemiology and transmission dynamics of this organism in our institution. Our forty month observation period represents one of the few longitudinal studies that describe the endemic and epidemic behavior of A. baumannii in an acute health care facility [7, 9, 10].
Methods
Study Population and Design
Temple University Hospital is a 600-bed tertiary care facility that serves a local population in North Philadelphia. An increased rate of MDR A. baumannii infections was noted in the SICU beginning in June 2006. Using standard methods for susceptibility testing, these MDR A. baumannii strains were defined as being resistant to all cephalosporins, penicillins (including those with beta-lactamase inhibitors), carbapenems, aminoglycosides and fluoroquinolones and susceptible only to colistin and/or tigecycline. Thereafter, continuous surveillance of MDR A. baumannii infection/colonization rates was performed by the infection control department and rates of MDR A. baumannii acquisition were calculated monthly. Representative samples of MDR A. baumannii isolate were preserved in trypticase Soy Broth with 20% glycerol (Becton Dickinson and Co., Sparks, MD) at −70° C for genotypic analysis.
Microbiological Analysis
A. baumannii isolates were processed in the clinical microbiology laboratory using the Phoenix Automated Microbiology System (Becton Dickinson and Co., Sparks, MD) for identification and susceptibility testing. Isolates of MDR A. baumannii were collected and stored at −70°C until they were submitted for analysis of genetic relatedness. Genomic DNA was extracted using MioBio Ultraclean DNA isolation kit per manufacturer recommendations. Using the DiversiLab A. baumannii kit (bioMerieux, Marcy l’Etoile, France) we preformed repetitive extragenic palindromic polymerase chain reaction (rep-PCR) analysis using the Agilent 2100 Bioanalyzer and the data were analyzed using DiversiLab software v3.3 to produce dendrograms. We chose a cutoff of >95% similarity on dendrogram to denote genetic relatedness.
Results
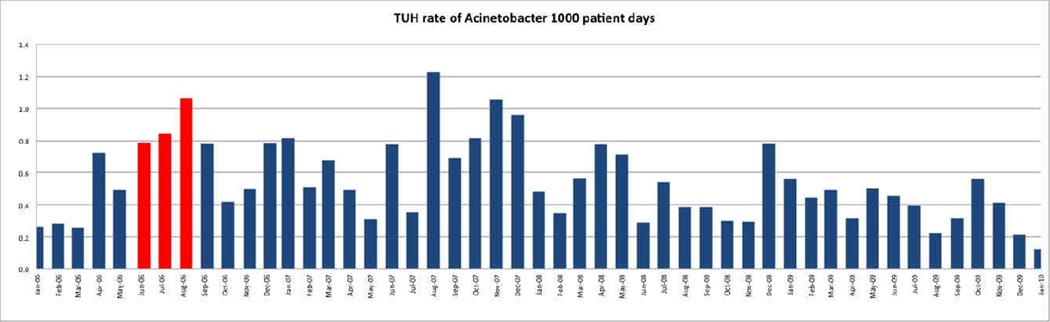
The incidence curve for MDR A. baumannii acquisition between January 2006 and January 2010 is shown in Figure 1. Multiple samples from one patient were counted as a single episode. The average incidence of unique new MDR A. baumannii isolates at Temple University Hospital during the period of January through May 2006 was 0.36 cases (range 0.2 – 0.7) per 1000 patient days. During the same period the average incidence rate of MDR A. baumannii in the SICU was 2.9 cases (range of 0 – 6.6) per 1000 patient days. In June through August 2008 the incidence increased to 0.87 in our hospital and 11.1 in the SICU. After implementation of enhanced infection control measures including reinforcement of hand hygiene, environmental cleaning and educational sessions, a decline in the incidence of MDR A. baumannii was seen between September and November 2006 (hospital incidence rate 0.53; SICU rate 6.4). This decline was transient and followed a cyclical pattern that was not identical from year to year.
Figure 1.
Incidence of MDR Acinetobacter spp. Between January 2006 through January 2010. (Red: SICU outbreak period)
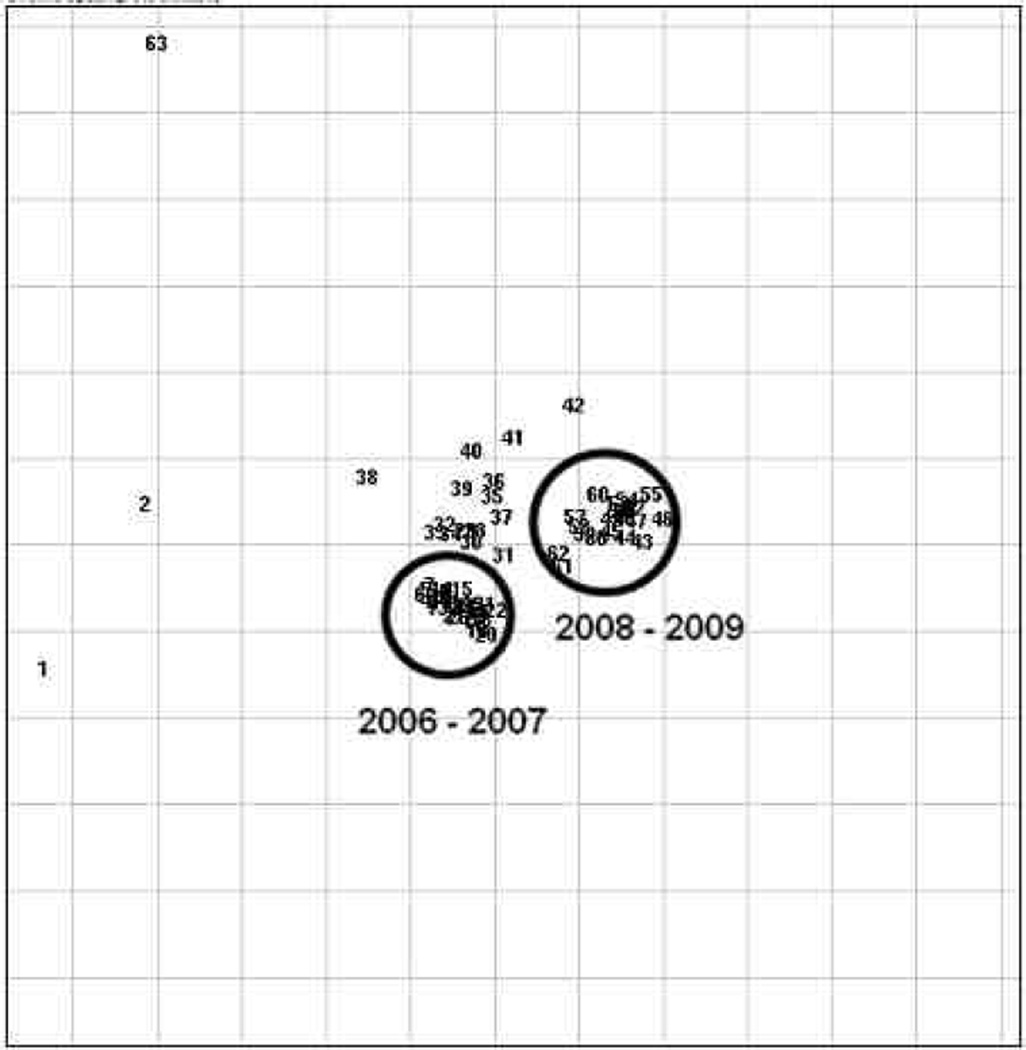
Fifty-six strains were collected and saved during this study period. These strains were used to delineate the longitudinal epidemiology MDR A. baumannii in our institution. Genotyping of these strains through rep-PCR was performed. Thirty strains were from 2006, 2 strains from 2007, 16 from 2008 and 8 were from 2009. Results showed that the 2006–2007 clinical isolates were a single dominant clone during this time period. Twenty-four more strains collected between 2008 and 2009 showed a different predominant strain. Of interest, the predominant strains differed between 2006–2007 and 2008– 2009 (Figure 2).
Figure 2.
PCR Fingerprint patterns of MDR Acinetobacter collected from 2006–2007 and 2008–2009. Each number indicates one strain and the distance between the strains indicate genotypic similarity. There are two distinct clusters of strains from different periods as circled.
Discussion
In 2006, when we recorded an excess number of cases of MDR A. baumannii in our hospital, our analysis showed that longer hospital stay, more days on a ventilator, and more antibiotic exposure may have contributed to this increase in the number of cases (data not shown). These risk factors have been associated with MDR A. baumannii infections by others [11]. The incidence of MDR A. baumannii decreased after institution of a “bundle” approach of enhanced infection control measures, but this decline was transient. Tracking of the incidence of MDR A. baumannii showed a resurgence of MDR A. baumannii several times in the three and one-half year follow-up period. Other studies have documented a seasonal pattern preferring the warmer months for outbreaks. A regional study of A. baumannii incidence during a 10 year observation period confirmed a "warm weather periodicity" though another study showed no seasonality [12, 13]. We were intrigued that in our SICU the incidence of MDR A. baumannii tended to increase during the summer months, but hospital-wide trends did not follow a set pattern. There have been suggestions by others that the warm temperatures can cause a “bloom” in biofilms in hospital tap waters, resulting in an increase during warm weather months[13]. We were not able to demonstrate this through our environmental sampling measures, a major part of our infection control intervention.
To understand the transmission dynamics and genetic relatedness of A. baumannii in our hospital, we performed genotypic analysis using rep-PCR on 56 unique patient isolates collected during a period of forty months. Surprisingly, the predominant strains from 2006 were distinct from the 2008 and 2009 strains. Since the antibiotic susceptibilities of MDR strains were identical during the two time periods, this strain shift would have been undetected without the use of molecular epidemiologic methods.
The phenomenon of strain replacement and succession among MDR pathogens is not completely understood as molecular typing of successive outbreaks is not commonly done. Furthermore, secular trends in the longitudinal molecular epidemiology of A. baumannii are poorly understood [7, 9, 10]. To illustrate, Go and colleagues tracked A. baumannii clinical isolates during a twelve-month period [14]. Using restriction endonuclease analysis, they demonstrated that imipenem-resistant A. baumannii were derived from previous endemic imipenem-susceptible clones. On the contrary, Corbella and colleagues [15] demonstrated that during a one and one-half year period of observation, their increase in MDR A. baumannii was the result of an acquisition of two new clones. Our findings were supportive of the observation made by Corbella and colleagues and shows that MDR A. baumannii in our institution was comprised of two major clones from different periods. This observation has important implications for the control of MDR A. baumannii. While aggressive measures to limit environmental contamination in the hospital as well as vigorous efforts to prevent patient-to-patient spread via health care workers are always recommended, an ongoing environmental niche that favors highly resistant MDR bacteria such as A. baumannii may also exist. Evidence of declining rates of MDR A. baumannii is re-assuring. On the other hand, such a decline may reflect a cyclical variation in the incidence of an existing strain or clonal replacement with new imported strains.
We were not able to explain on genetic grounds the strain replacement phenomenon that was observed. One speculation is that differing resistance determinants or other factors that result in the same phenotype may have effects on bacterial fitness [16]. Another potential possibility is the introduction of competing strains from other institutions in our region. The spread of MDR organisms throughout a region is not unique to A. baumannii [6, 17, 18]. Given an ongoing risk of re-introduction, aggressive intervention may lead to only a brief period of eradication [7, 19].
In addition to the predominant clones of MDR A. baumannii, there were a number of distinct genetically uniform minor clones. The significance of these minor strains is unclear. Some strains were unrelated to the major clones and they may represent unsuccessful “attempts” to become part of the endemic flora. It would be interesting to know what kept them from establishing a presence in the hospital.
A limitation of our study was that we were not able to distinguish colonization from infection in individual patients throughout the study period, although our experience suggests that more than one-half of the patients received some antibiotics directed at MDR A. baumannii. We also were not able to genotype every isolate of MDR A. baumannii during the study period and our sample was not collected prospectively to represent body sites, hospital units or patient types in direct proportion to their prevalence of MDR A. baumannii.
In conclusion, our study describes the longitudinal molecular epidemiology and population dynamics of MDR A. baumannii in our institution. These successive outbreaks were most likely part of a repeated series of “waves” of this organism entering, spreading and leaving our hospital. Furthermore, our study represents one of the few long-term studies that illustrate the endemic and epidemic activities of MDR A. baumannii in an acute health care facility. Genotyping confirmed that the ongoing persistence of MDR A. baumannii was a mix of long-term persistence and periodic strain substitution of several clones. Our experience also illustrates the difficulty of eliminating A. baumannii once it becomes endemic in an institution and suggests the importance of relying on difficult-to-sustain infection control measures, as a means of limiting acquisition of A. baumannii in critically ill patients. Enhanced infection control measures (i.e. “bundle approach”) might slow the spread of MDR A. baumannii, but even with optimal implementation of these measures it can be challenging to eliminate this organism especially when it becomes endemic. Future research looking at the longitudinal epidemiology of A. baumannii may need to look at multiple institutions including long term care facilities in a region to understand the transmission dynamics and epidemiology of this organism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Villegas MV, Hartstein AI. Acinetobacter outbreaks, 1977–2000. Infect Control Hosp Epidemiol. 2003 Apr;24(4):284–295. doi: 10.1086/502205. [DOI] [PubMed] [Google Scholar]
- 2.Bergogne-Berezin E, Towner K. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clinical Microbiology Reviews. 1996;9(2):148–165. doi: 10.1128/cmr.9.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown S, Amyes SG. The sequences of seven class D beta-lactamases isolated from carbapenem-resistant Acinetobacter baumannii from four continents. Clin Microbiol Infect. 2005 Apr;11(4):326–329. doi: 10.1111/j.1469-0691.2005.01096.x. [DOI] [PubMed] [Google Scholar]
- 4.Navon-Venezia S, Ben-Ami R, Carmeli Y. Update on Pseudomonas aeruginosa and Acinetobacter baumannii infections in the healthcare setting. Curr Opin Infect Dis. 2005 Aug;18(4):306–313. doi: 10.1097/01.qco.0000171920.44809.f0. [DOI] [PubMed] [Google Scholar]
- 5.del Mar Tomas M, Cartelle M, Pertega S, et al. Hospital outbreak caused by a carbapenem-resistant strain of Acinetobacter baumannii: patient prognosis and risk-factors for colonisation and infection. Clin Microbiol Infect. 2005 Jul;11(7):540–546. doi: 10.1111/j.1469-0691.2005.01184.x. [DOI] [PubMed] [Google Scholar]
- 6.Landman D, Quale JM, Mayorga D, et al. Citywide clonal outbreak of multiresistant Acinetobacter baumannii and Pseudomonas aeruginosa in Brooklyn, NY: the preantibiotic era has returned. Arch Intern Med. 2002 Jul 8;162(13):1515–1520. doi: 10.1001/archinte.162.13.1515. [DOI] [PubMed] [Google Scholar]
- 7.Apisarnthanarak A, Pinitchai U, Thongphubeth K, Yuekyen C, Warren DK, Fraser VJ. A multifaceted intervention to reduce pandrug-resistant Acinetobacter baumannii colonization and infection in 3 intensive care units in a Thai tertiary care center: a 3-year study. Clin Infect Dis. 2008 Sep 15;47(6):760–767. doi: 10.1086/591134. [DOI] [PubMed] [Google Scholar]
- 8.D'Agata EM, Thayer V, Schaffner W. An outbreak of Acinetobacter baumannii: the importance of cross-transmission. Infect Control Hosp Epidemiol. 2000 Sep;21(9):588–591. doi: 10.1086/501808. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Bano J, Garcia L, Ramirez E, et al. Long-term control of hospital-wide, endemic multidrug-resistant Acinetobacter baumannii through a comprehensive "bundle" approach. Am J Infect Control. 2009 Nov;37(9):715–722. doi: 10.1016/j.ajic.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shelburne SA, 3rd, Singh KV, White AC, Jr, et al. Sequential outbreaks of infections by distinct Acinetobacter baumannii strains in a public teaching hospital in Houston, Texas. J Clin Microbiol. 2008 Jan;46(1):198–205. doi: 10.1128/JCM.01459-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Garmendia JL, Ortiz-Leyba C, Garnacho-Montero J, et al. Risk factors for Acinetobacter baumannii nosocomial bacteremia in critically ill patients: a cohort study. Clin Infect Dis. 2001 Oct 1;33(7):939–946. doi: 10.1086/322584. [DOI] [PubMed] [Google Scholar]
- 12.Wisplinghoff H, Edmond MB, Pfaller MA, Jones RN, Wenzel RP, Seifert H. Nosocomial bloodstream infections caused by Acinetobacter species in United States hospitals: clinical features, molecular epidemiology, and antimicrobial susceptibility. Clin Infect Dis. 2000 Sep;31(3):690–697. doi: 10.1086/314040. [DOI] [PubMed] [Google Scholar]
- 13.McDonald LC, Banerjee SN, Jarvis WR. Seasonal variation of Acinetobacter infections, 1987–1996. Nosocomial Infections Surveillance System. Clin Infect Dis. 1999 Nov;29(5):1133–1137. doi: 10.1086/313441. [DOI] [PubMed] [Google Scholar]
- 14.Go ES, Urban C, Burns J, et al. Clinical and molecular epidemiology of acinetobacter infections sensitive only to polymyxin B and sulbactam. Lancet. 1994 Nov 12;344(8933):1329–1332. doi: 10.1016/s0140-6736(94)90694-7. [DOI] [PubMed] [Google Scholar]
- 15.Corbella X, Montero A, Pujol M, et al. Emergence and rapid spread of carbapenem resistance during a large and sustained hospital outbreak of multiresistant Acinetobacter baumannii. J Clin Microbiol. 2000 Nov;38(11):4086–4095. doi: 10.1128/jcm.38.11.4086-4095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marciano DC, Karkouti OY, Palzkill T. A fitness cost associated with the antibiotic resistance enzyme SME-1 beta-lactamase. Genetics. 2007 Aug;176(4):2381–2392. doi: 10.1534/genetics.106.069443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bratu S, Landman D, Haag R, et al. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch Intern Med. 2005 Jun 27;165(12):1430–1435. doi: 10.1001/archinte.165.12.1430. [DOI] [PubMed] [Google Scholar]
- 18.Lomaestro BM, Tobin EH, Shang W, Gootz T. The spread of Klebsiella pneumoniae carbapenemase-producing, K pneumoniae to upstate New York. Clin Infect Dis. 2006 Aug 1;43(3):e26–e28. doi: 10.1086/505598. [DOI] [PubMed] [Google Scholar]
- 19.Sengstock DM, Thyagarajan R, Apalara J, Mira A, Chopra T, Kaye KS. Multidrug-resistant Acinetobacter baumannii: an emerging pathogen among older adults in community hospitals and nursing homes. Clin Infect Dis. Jun 15;50(12):1611–1616. doi: 10.1086/652759. [DOI] [PubMed] [Google Scholar]