Abstract
The Balance Study is a randomized controlled trial designed to reduce cardiovascular disease (CVD) risk in 200 American Indian (AI) participants with metabolic syndrome who reside in southwestern Oklahoma. Major risk factors targeted include weight, diet, and physical activity. Participants are assigned randomly to one of two groups, a guided or a self-managed group. The guided group attends intervention meetings that comprise education and experience with the following components: diet, exercise, AI culture, and attention to emotional wellbeing. The self-managed group receives printed CVD prevention materials that are generally available. The duration of the intervention is 24 months. Several outcome variables will be compared between the two groups to assess the effectiveness of the intervention program.
Keywords: CVD prevention, American Indians, Holistic intervention
Introduction
Cardiovascular disease (CVD) was once rare among the American Indian (AI) population, but it is now the leading cause of death and major disability in this population (Casper et al., 2005; Lee et al., 1998). Moreover, the recent decline in age-adjusted CVD death rates experienced by the general U.S. population is not being observed in the AI population (Casper et al., 2005; Indian Health Service [IHS] Program Statistics Team, 1999). This is largely the result of the increased prevalence of obesity; physical inactivity (Denny, Holtzman, & Cobb 2003); type 2 diabetes (Hu et al., 2004; Knowler et al., 2002; Kriska et al., 2003; Lee et al., 2002); excessive intake of total and saturated fats; low levels of high-density lipoprotein cholesterol (HDL-C); increased levels of low-density lipoprotein cholesterol, total triglycerides (TG), and total cholesterol; hypertension or elevated systolic blood pressure (SBP); poor glycemic control; and albuminuria (Howard et al., 1999; Vasan et al., 2001; Wang et al., 2006; Xu et al., 2005) in AI populations.
Several lifestyle intervention trials targeting multiple risk factors for type 2 diabetes and CVD in the general U.S. population (Appel et al., 2003; Beresford et al., 2006; Howard et al., 2006; Miller et al., 2002; The Diabetes Prevention Program Research Group, 1999) have shown that intensive lifestyle intervention through diet and physical activity can effectively reduce weight and the risk of type 2 diabetes and CVD. However, similar data for the AI population are scant. Large studies for this population targeting diabetes and CVD, such as Look AHEAD (Action for Health in Diabetes) (Hoskin et al., 2005) and the Kahnawake School Diabetes Prevention Project (Macaulay et al., 1997), may be successful since several smaller studies have achieved various levels of success in reducing risk factors for diabetes and CVD (Armstrong, 2000; Gilliland, Azen, Perez, & Carter 2002; Hodge, Hodge, Pasqua, Marquez, & Geishirt-Cantrell 2002). However, it is not clear if these prevention programs are effective in all AI tribes. The traditional AI concept of health includes four intricately woven elements of life: the mental, physical, emotional, and spiritual; and any imbalance of the four health elements results in poor health. In addition, whereas the traditional AI lifestyle promoted healthy diets and physical activity, it has been increasingly replaced by a lifestyle of unhealthy high-fat and high-sugar diets and physical inactivity over the last several decades, which has contributed significantly to the onset of various diseases in this population. Guided by the abovementioned conceptual framework, we have designed and implemented a prevention program comprising four components—cultural/spiritual, emotional/motivational, nutritional, and physical—to reduce CVD risk in AI adults with metabolic syndrome, a condition known to increase the risk of developing CVD. This article describes the design and the methods used in the Balance Study.
Methods
Objectives and Hypotheses
The primary objective of this two-arm randomized controlled trial was to determine the impact of the intervention program on weight (as measured by body mass index [BMI]), physical activity, consumption of saturated fat and sodium, the status of metabolic syndrome, and incident CVD (including those listed in exclusion criteria 2[a] of Table 1). The primary hypothesis was that the intervention program would reduce BMI more than would the comparison intervention; and the secondary hypothesis was that the intervention program would decrease fat intake, increase physical activity, reduce metabolic syndrome, and lower incidence of CVD more than would the comparison intervention.
Table 1.
Eligibility criteria for the Balance Study
|
Target Population
The target population consisted of AI men and women, mostly members of the Apache, Caddo, Comanche, Delaware, Ft. Sill Apache, Kiowa, and Wichita tribes. Notably, these tribes have lived in close association in southwestern Oklahoma for more than 150 years and have intermarried and worked closely together. Thus, their cultural norms are now quite similar. Almost all individual tribal customs are primarily restricted to certain songs, dances, and celebrations. As a result, attention to tribally specific practices was not necessary and did not pose a problem throughout the study.
Eligibility and Ineligibility Criteria
Eligibility criteria included being AI (as documented by having a Certificate of Degree of Indian Blood, or CDIB, card), aged 30–75 years, and at high risk of developing CVD. High risk was defined as (1) having a BMI of at least 25 and (2) having metabolic syndrome as defined by the National Heart, Lung, and Blood Institute (NHLBI; Grundy, Brewer, Cleemen, Smith, & Lenfant 2004). Inclusion and exclusion criteria are listed in Table 1. All participants provided written informed consent. Approvals for this study were obtained from the seven major tribes and the institutional review boards of the University of Oklahoma Health Sciences Center and of the Oklahoma City Area IHS. The study protocol was also approved by an independent Protocol Review Committee appointed by the NHLBI.
Sample Size Determination
The sample size was determined using data from the primary and secondary outcome measures collected from the Strong Heart Study Phase IV participants of similar ages as estimates for the self-managed group. The intervention program was designed to approach the goal of an average difference of 18.3 lb between the guided group and the self-managed group. A two-sided test at α = .05 and 80 % statistical power would result in a sample size of 90 in each group, which allowed us to detect a minimum difference of 17.2 lb (Fleiss, 1986). A sample size of 90 in each group would provide 80 % statistical power with α = .05 to detect a minimum difference of 661 kcal per day and 1,715 steps per day. These figures were smaller than the differences of 664 kcal per day and 2,000 steps per day required by the study. Thus, the sample size of 90 participants in each group was adequate. Considering 10 % possible withdrawals and losses to follow-up based on experience with the Strong Heart Study, we decided to recruit 100 participants per group for a total sample size of 200.
Recruitment
Recruitment (from January 2008 to September 2009) utilized presentations by study personnel at community meetings, health care facilities, and tribal meetings. An introductory letter was mailed to more than 500 potentially eligible individuals, inviting them to a screening session. Other recruitment methods included telephone follow-up; public announcements in newspapers, on local TV, and on radio programs; and study posters displayed at various public facilities.
Study Design, Screening, and Randomization
Figure 1 is a CONSORT flow diagram for the Balance Study. Potential participants (n = 585) were requested to appear after fasting overnight. Written informed consent forms were obtained before any study procedures were performed. Height and weight were then measured and BMI was calculated. From potential participants who satisfied the inclusion criteria for age and BMI, a small amount of blood was obtained by finger stick for an immediate Cholestech assessment of fasting TG, HDL-C, and glucose levels. If any of these values was in the exclusionary range, persons were ruled ineligible and did not undergo further screening. However, for those who satisfied the eligibility requirements for BMI, TG, HDL-C and glucose levels, the screening procedure continued with measurements of waist circumference and blood pressure, as well as a personal interview including medical history to complete the screening.
Fig. 1.
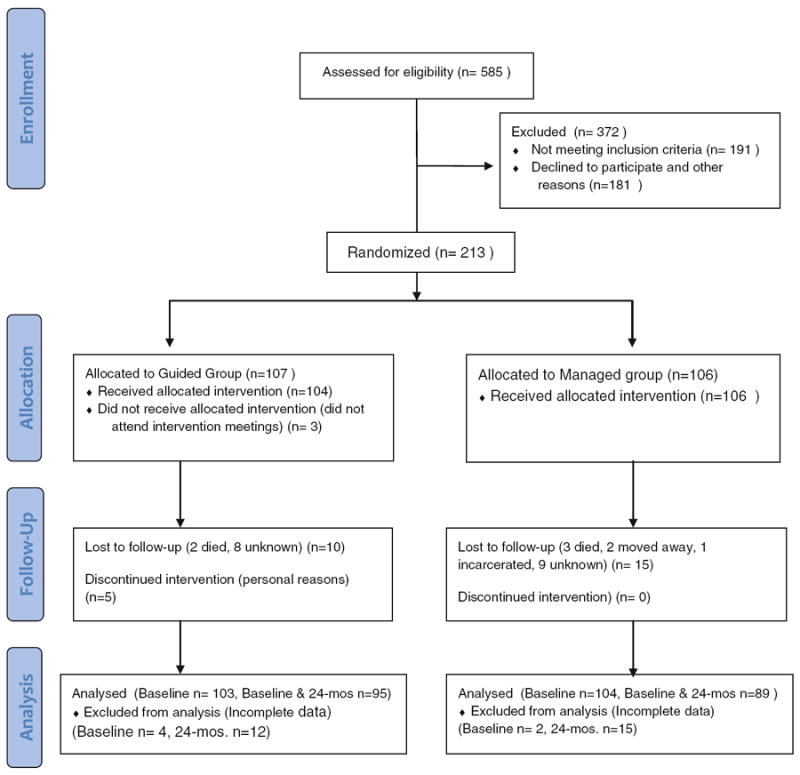
CONSORT flow diagram—Balance Study
For those who completely satisfied all of the eligibility criteria, an additional blood sample and a urine sample were taken for laboratory assessment of the measures of hemoblobin A1c, fasting glucose, TG, HDL-C, LDL-C, SBP, diastolic blood pressure (DBP), urinary sodium, urinary potassium, and urine albumin and creatinine. Additional questionnaires on health practice, quality of life, self-efficacy (general, nutrition and exercise), physical activity and food frequency were administered (Table 2). Similar procedures were repeated every 6 months thereafter for a total follow-up period of 24 months. At the 12-month and 24-month visits, a shorter form, omitting some unnecessary repeated questions for medical history and health practice were to be used. All blood and urine samples were used only for the tests indicated in Table 2, and the remaining biological specimens will be destroyed after completion of the research project.
Table 2.
Schedule of visits and measures at each visit
| Measure | Screening | Baseline | Months
|
|||
|---|---|---|---|---|---|---|
| 6 | 12 | 18 | 24 | |||
| Behavior modification outcomes | ||||||
| Total caloric intake | X | X | X | X | X | |
| Physical activity level | X | X | X | X | X | |
| One-week pedometer data | X | X | X | X | X | |
| Questionnaire | X | X | X | X | X | |
| Urinary sodium | X | X | X | X | X | |
| Urinary potassium | X | X | X | X | X | |
| CVD risk factor outcomes | ||||||
| Weight | X | X | X | X | X | X |
| Fasting glucose | X | X | X | X | X | X |
| Hemoglobin A1c | X | X | X | |||
| Triglycerides | X | X | X | X | X | X |
| HDL-C | X | X | X | X | X | X |
| LDL-C | X | X | X | X | X | X |
| Systolic blood pressure | X | X | X | X | X | X |
| Diastolic blood pressure | X | X | X | X | X | X |
| Urine albumin and creatinine | X | X | X | |||
| Physical measurements | ||||||
| Height and waist circumference | X | X | X | X | X | X |
| Questionnaire | ||||||
| Sociodemographics | X | |||||
| Medical history | X | X | X | |||
| Short form | Short form | |||||
| Health practice | X | X | X | |||
| Short form | Short form | |||||
| Quality of life | X | X | X | X | X | |
| Self-efficacy (General, Nutrition, and Exercise) | X | X | X | |||
| CHF risk appraisal | X | X | X | X | X | |
CVD cardiovascular disease, HDL-C high-density lipoprotein cholesterol, LDL-C low-density cholesterol, CHF congestive heart failure, Short form shorter medical history form without some un-necessary repeated questions
Participants (n = 213) were then randomized into either the guided group (n = 107) or self-managed group (106) according to the block randomization method (Chow & Liu, 2004). The guided group received a culturally appropriate intervention program by attending intervention meetings. The self-managed group received CVD prevention materials published by the American Heart Association (AHA), as well as printed materials developed by the Balance Study, without attending any intervention meetings. The study protocol was designed and implemented using a community-based participatory research approach (Wallerstein & Duran, 2006; Horowitz, Robinson, & Seifer 2009) with input from community focus groups and a Community Advisory Board comprising members from each of the seven local tribes.
Intervention Program
Intervention for the Guided Group
In the first year (intensive phase) of the intervention, the 100 participants, divided into five groups of approximately 20 persons, attended staggered biweekly group and individual meetings in the evenings. Each meeting included a short presentation on each of the four components (cultural/spiritual, emotional/motivational, nutritional, and physical), a healthy meal, and staff-led exercise. Healthy food preparations were also demonstrated frequently. The participants were asked to set personal goals in all areas to help monitor their progress. All study staff, trained to present and facilitate their respective components, were local AI individuals. At each meeting, the participants signed in, were weighed, and reported if they had followed our recommendations regarding diet and physical activity; counseling was provided if they had not. During the week in which no group intervention meeting occurred, intervention participants were visited individually by telephone or in person by study staff to provide reinforcement. During the second year of the intervention, group meetings and individual visits occurred monthly during lunch hours and a healthy lunch was served.
Our dietary intervention generally followed the 2006 Diet and Lifestyle Recommendations for Cardiovascular Disease Risk Reduction (Lichtenstein, Appel, Brands, American Heart Association Nutrition Committee et al. 2006). The general goals for the guided group at end of the first year of the intervention were: (1) at least a 25 % reduction in saturated fat intake and as much reduction as possible in trans fat intake; (2) daily calorie intake between 1,500 and 2,000, depending on weight at baseline; (3) daily consumption of two and four servings of fruits and vegetables, respectively; and (4) sodium consumption less than 2,400 mg per day. Participants were encouraged to lose 1–3 lbs per month, and were asked to use a journal to log their weight, exercise and dietary goals, as well as their daily physical activity and dietary intake in the first year of the intervention. This journal was reviewed by the staff and used as a formative assessment tool. Participants with little or no progress were counseled, and those achieving the greatest progress were recognized. During the second year of the intervention any participants who missed any of the group meetings were visited at home by study staff, who delivered the intervention materials in person.
The following is a brief description of the four intervention components:
Cultural/spiritual. A culture curriculum, including topics such as the AI holistic lifestyle, traditional foods, cultural strength and resiliency, and changing cultural norms and practices, was developed by two AI individuals who were members of the local tribes and conversant with the general spiritual and cultural practices of the participating tribes. Because the tribes have lived in close association for a long time, thus sharing most cultural practices, attention to individual tribal practices was not necessary. Therefore, the focus was on those cultural traits now shared by the participating tribes. Furthermore, care was taken to utilize local individuals who were recognized as sensitive to any tribally specific concerns that might arise during the course of the intervention program. Prior experience with the program revealed no concerns regarding tribally specific norms or practices. Materials for the cultural/spiritual component were presented using slides, intertribal songs, and story telling.
Emotional/motivational. This component was developed by two AI psychologists and behavioral scientists. Topics included emotional/behavioral health, stress, depression, self-esteem, and taking charge of one’s life. The talking circle format was employed to allow participants to freely and safely express personal and family issues. Talking circles provided a place for social connection and for sharing personal experiences/concerns and emotions/feelings surrounding the epidemic of CVD and related conditions such as diabetes, nutrition, weight loss, and exercise.
Nutritional. This component was focused on improving eating behaviors. In addition to following the 2005 Dietary Guidelines for Americans and the MyPyramid system (http://mypyramid.gov/guidelines/index.html), many strategies for weight loss were encouraged, such as reducing portion sizes, reducing consumption of fats and sugar-sweetened beverages, eating out without overeating, and incorporating healthier ingredients in current recipes. Participants were encouraged to develop their own strategies to achieve dietary goals. A gradual improvement plan for healthy mealplanning was encouraged. Periodically, a healthy meal (including traditional meals) was prepared with participation from study participants. Other nutrition-related activities included reading food labels and trips to grocery stores to select fresh and healthy foods, with attention to healthy recipes that preserve the AI culinary tradition.
Physical fitness and exercise. Walking was the primary physical activity, which was monitored with pedometers. Depending on the baseline average number of steps per day in a week, each participant was assisted in setting goals designed to reach at least 7,000 steps per day or 35,000 steps per week in 6 months and at least 10,000 steps per day or a total of at least 50,000 steps per week at the end of the first year. The number of steps was increased gradually by 5 % per week to reach 150–175 min per week of physical activity at the end of the first year. Other strongly recommended exercises included chair exercises, AI social dances, and gardening. A community garden was developed in the first year, and local master gardeners were invited to provide advice and share their experience. Study staff also discussed the relationship between physical activity and energy expenditure and the importance of long-term regular physical activity.
Intervention for the Self-Managed Group
Participants randomized to the self-managed group received pamphlets for healthy living published by the AHA, all study curricula, and recipes developed for the guided group by mail at the same time as participants in the guided group. Participants in the self-managed group were encouraged to ask questions, and they were invited to the follow-up assessments every 6 months. Persons in the self-managed group were not allowed to be guests in the guided intervention group meetings.
Retention Strategies and Referral Procedures
Retention strategies included the following: (1) educating participants and their families on the importance of nutrition and exercise to prevent heart disease; (2) making the intervention program as interesting and convenient as possible for participants; (3) establishing a good relationship between the study staff and the participants; (4) providing participants with educational materials and incentives; and (5) publishing newsletters for participants, which reported interesting events and/or special achievements of participants.
Individuals found to have health problems during the screening and examinations, or during the intervention sessions, were referred to the local IHS facilities for follow-up with attention to the severity of the condition according to procedures in the protocol and training manual for our study staff. Copies of our physical examination and laboratory results were placed in participants’ medical records.
Quality Control
All the field staff were trained and certified for interview technique and blood pressure measurements. Blood draws were performed by a certified phlebotomist. Quality control measures included maintenance of study equipment. Personal interviews were observed periodically by the study coordinator. In addition, site visits were regularly made by the Principal Investigator and once a year by the NHLBI Project Officer.
Every data form was checked for completeness at the field office. Ambiguous or erroneous items were clarified and corrected. Summary statistics were generated after each examination to identify any peculiar or unreasonable values.
Statistical Analysis Plan
Statistical comparisons between the guided group and the self-managed group will be performed following the principle of intention-to-treat, that is, participants will be analyzed according to the treatment group to which they were randomized, regardless of compliance to assigned intervention.
Measurements of the CVD risk factors and outcome variables at baseline and at the end of the study will be used to test the study hypotheses. For the continuous risk factors and outcome variables, analysis of covariance will be used to compare the differences between the two treatment groups, while adjusting for covariates or baseline differences (Armitage & Berry, 1994). For comparing categorical variables between the two groups, logistic regression models will be used, with gender and age treated as covariates (Hosmer & Lemeshow, 2000). Baseline variables that may affect diabetes incidence or that are significantly different between the two groups will also be adjusted.
Every effort was made to minimize dropouts, and study staff was instructed to complete every question/item on the data form. Imputation methods (Hunsberger, Murray, Davis, & Fabsitz 2001; Little & Rubin, 1987; Little & Schenker, 1995; Myers, 2000) will be used to estimate missing data. Reasons for and the nature (random or not) of missing data will be examined.
Discussion
A CVD risk reduction program was designed with direct community involvement so that when the research project is finished, if proven effective, the intervention program can be adopted by the seven local tribes. The main goals of the Balance Study were to promote healthy lifestyles, to encourage weight loss, and to reduce CVD risk factors. Intervention components designed to improve diet and physical activity were supplemented with elements developed to promote cultural/spiritual and emotional/motivational balance, in an effort to follow the traditional AI concept of health. To our knowledge, only one previous intervention program has included these four elements of health (Stefanich et al., 2005). Our intervention materials were primarily developed and delivered by local AI experts and trained professionals. In accordance with accepted community-based participatory research practices, our program was designed to allow for gradual changes and improvements and allowed modifications to be made according to suggestions from our participants. We also tried to maximize compliance by holding the intervention meetings in the evenings and during lunch hours.
In addition to many personal responsibilities, such as caring for grandchildren or other family members, the lack of transportation for the participants to attend our activities was a major barrier and challenge. Because of limited resources, we were not able to control the diet and physical activity of each individual participant in the guided group. Another limitation was the exclusion of individuals who were already participating in the IHS Special Diabetes Prevention Initiative, which might have led to a selection bias. That is, the pool of eligible participants might have had a higher proportion of individuals who were less interested in behavior change, thus decreasing the number of participants achieving full participation in our prevention activities and consequently reducing the study’s ability to define a difference in an outcome at the end of the intervention.
Conclusion
The Balance Study is unique in that it provides the space and time for participants to get away from the stress and pace of everyday life and immerse themselves in a supportive atmosphere that promotes self-efficacy and holistic wellness, including the body, mind, emotions, and spirit. Because of the direct and heavy community involvement, when the research project is finished, if proven effective, we anticipate that the seven local tribes will be readily able to adopt the program. We believe this study provides a basis for continued implementation by the tribes, and with time and additional efforts it will prove to be a useful addition to existing programs.
Acknowledgments
We thank the AI tribes (Apache, Caddo, Comanche, Delaware, Fort Sill Apache, Kiowa, and Wichita) and communities in southwestern Oklahoma for their support, cooperation, and assistance. We also thank the Indian Health Service Oklahoma City Area Office, the Lawton Indian Hospital and its clinics at Anadarko and Carnegie and the Balance Study staff for their support and diligent work. The opinions expressed in this article are those of the authors and do not necessarily reflect the view or official position of the Indian Health Service or the National Heart, Lung, and Blood Institute. The Balance Study is funded by a cooperative agreement between the National Heart, Lung, and Blood Institute, National Institutes of Health (Grant U01HL087354) and the University of Oklahoma Health Sciences Center.
Footnotes
Conflict of interest The authors do not have any financial disclosures.
Contributor Information
Elisa T. Lee, Email: elisa-lee@ouhsc.edu, Center for American Indian Health Research, College of Public Health, University of Oklahoma Health Sciences Center, P.O. Box 26901, Oklahoma City, OK 73126-0901, USA.
Jared B. Jobe, Division of Cardiovascular Sciences, National Heart, Lung and Blood Institute, Bethesda, MD, USA
Jeunliang Yeh, Center for American Indian Health Research, College of Public Health, University of Oklahoma Health Sciences Center, P.O. Box 26901, Oklahoma City, OK 73126-0901, USA.
Tauqeer Ali, Center for American Indian Health Research, College of Public Health, University of Oklahoma Health Sciences Center, P.O. Box 26901, Oklahoma City, OK 73126-0901, USA.
Everett R. Rhoades, Center for American Indian Health Research, College of Public Health, University of Oklahoma Health Sciences Center, P.O. Box 26901, Oklahoma City, OK 73126-0901, USA
Allen W. Knehans, Department of Nutritional Sciences, College of Allied Health, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA
Diane J. Willis, Center for American Indian Health Research, College of Public Health, University of Oklahoma Health Sciences Center, P.O. Box 26901, Oklahoma City, OK 73126-0901, USA
Melanie R. Johnson, Center for American Indian Health Research, College of Public Health, University of Oklahoma Health Sciences Center, P.O. Box 26901, Oklahoma City, OK 73126-0901, USA
Ying Zhang, Center for American Indian Health Research, College of Public Health, University of Oklahoma Health Sciences Center, P.O. Box 26901, Oklahoma City, OK 73126-0901, USA.
Bryce Poolaw, Lawton Indian Hospital, Lawton, OK, USA.
Billy Rogers, Native Workshops, Norman, OK, USA.
References
- Appel LJ, Champagne CM, Harsha DW, et al. Writing Group of the PREMIER Collaborative Research Group. Effects of comprehensive lifestyle modification on blood pressure control: Main results of the PREMIER clinical trial. Journal of the American Medical Association. 2003;289:2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- Armitage P, Berry G. Statistical methods in medical research. 3. Oxford, England: Blackwell Sciences; 1994. [Google Scholar]
- Armstrong DL. A community diabetes education and gardening project to improve diabetes care in a northwest American Indian tribe. The Diabetes Educator. 2000;26:113–120. doi: 10.1177/014572170002600112. [DOI] [PubMed] [Google Scholar]
- Beresford SA, Johnson KC, Ritenbaugh C, Lasser NL, Snetselaar LG, Black HR, et al. Low-fat dietary pattern and risk of colorectal cancer: The Women’s Health Initiative randomized controlled Dietary Modification trial. Journal of the American Medical Association. 2006;295:643–654. doi: 10.1001/jama.295.6.643. [DOI] [PubMed] [Google Scholar]
- Casper ML, Denny CH, Coolidge JN, William GI, Jr, Crowell A, Galloway JM, et al. Atlas of heart disease and stroke among American Indians and Alaska Natives. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and Indian Health Service; 2005. [Google Scholar]
- Chow S-C, Liu J-P. Design and analysis of clinical trials: Concepts and methodologies. 2. New York, NY: Wiley; 2004. [Google Scholar]
- Denny CH, Holtzman D, Cobb N. Surveillance for health behaviors of American Indians and Alaska Natives: Findings from the Behavioral Risk Factor Surveillance System, 1997–2000. Morbidity and Mortality Weekly Report. 2003;52(SS-7):1–13. [PubMed] [Google Scholar]
- Fleiss J. The design and analysis of clinical experiments. New York, NY: Wiley; 1986. [Google Scholar]
- Gilliland SS, Azen SP, Perez GE, Carter JS. Strong in body and spirit: Lifestyle intervention for Native American adults with diabetes in New Mexico. Diabetes Care. 2002;25:78–83. doi: 10.2337/diacare.25.1.78. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Brewer HB, Jr, Cleemen JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- Hodge FS, Pasqua A, Marquez CA, Geishirt-Cantrell B. Storytelling to promote wellness in American Indian communities. Journal of Transcultural Nursing. 2002;13(1):6–11. doi: 10.1177/104365960201300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz CR, Robinson M, Seifer S. Community-based participatory research from the margin to the mainstream: Are researchers prepared? Circulation. 2009;119:2633–2642. doi: 10.1161/CIRCULATIONAHA.107.729863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskin M, Begay S, Bolin P, Hermes J, Ingraham LE, Killean T, et al. Providing lifestyle interventions in Native American communities. Obesity Management. 2005;1:251–255. [Google Scholar]
- Hosmer DW, Lemeshow S. Applied logistic regression. 2. New York, NY: Wiley; 2000. [Google Scholar]
- Howard BV, Lee ET, Cowan LD, Devereux RB, Galloway JM, Go OT, et al. Rising tide of cardiovascular disease in American Indians. The Strong Heart Study. Circulation. 1999;99:2389–2395. doi: 10.1161/01.cir.99.18.2389. [DOI] [PubMed] [Google Scholar]
- Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, et al. Low-fat dietary pattern and risk of cardiovascular disease: The Women’s Health Initiative Randomized Controlled Dietary Modification Trial. Journal of the American Medical Association. 2006;295:655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- Hu G, Lindström J, Valle TT, Eriksson JG, Jousilahti P, Silventoinen K, et al. Physical activity, body mass index, and the risk of type 2 diabetes in patients with normal or impaired glucose regulation. Archives of Internal Medicine. 2004;164:892–896. doi: 10.1001/archinte.164.8.892. [DOI] [PubMed] [Google Scholar]
- Hunsberger S, Murray D, Davis CE, Fabsitz RR. Imputation strategies for missing data in a schoolbased multi-centre study: The Pathways study. Statistics in Medicine. 2001;20:305–316. doi: 10.1002/1097-0258(20010130)20:2<305::aid-sim645>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Indian Health Service Program Statistics Team. Trends in Indian health. Rockville, MD: Office of Public Health, U.S. Department of Health and Human Services; 1999. [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriska AM, Saremi A, Hanson RL, Bennett PH, Kobes S, Williams DE, et al. Physical activity and the incidence of type 2 diabetes in a high-risk population. American Journal of Epidemiology. 2003;158:669–675. doi: 10.1093/aje/kwg191. [DOI] [PubMed] [Google Scholar]
- Lee ET, Cowan LD, Welty TK, Sievers M, Howard WJ, Oopik A, et al. All-cause mortality and cardiovascular disease mortality in three American Indian populations, aged 45–74 years, 1984–1988. The Strong Heart Study. American Journal of Epidemiology. 1998;147:995–1008. doi: 10.1093/oxfordjournals.aje.a009406. [DOI] [PubMed] [Google Scholar]
- Lee ET, Welty TK, Cowan LD, Wang W, Rhoades DA, Devereux R, et al. Incidence of diabetes in American Indians of three geographic areas: The Strong Heart Study. Diabetes Care. 2002;25:49–54. doi: 10.2337/diacare.25.1.49. [DOI] [PubMed] [Google Scholar]
- Lichtenstein AH, Appel LJ, Brands M, et al. American Heart Association Nutrition Committee. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. New York, NY: Wiley; 1987. [Google Scholar]
- Little RJA, Schenker N. Handbook of statistical methods—missing data. New York, NY: Plenum Press; 1995. [Google Scholar]
- Macaulay AC, Paradis G, Potvin L, Cross EJ, Saad-Haddad C, McComber A, et al. The Kahnawake Schools Diabetes Prevention Project: Intervention, evaluation, and baseline results of a diabetes primary prevention program with a native community in Canada. Preventive Medicine. 1997;26:779–790. doi: 10.1006/pmed.1997.0241. [DOI] [PubMed] [Google Scholar]
- Miller ER, 3rd, Erlinger TP, Young DR, Jehn M, Charleston J, Rhodes D, et al. Results of the diet, exercise, and weight loss intervention trial (DEW-IT) Hypertension. 2002;40:612–618. doi: 10.1161/01.hyp.0000037217.96002.8e. [DOI] [PubMed] [Google Scholar]
- Myers WR. Handling missing data in clinical trials: An overview. Drug Information Journal. 2000;34:525–533. [Google Scholar]
- Stefanich CA, Witmer JM, Young BD, Benson LE, Penn CA, Ammerman AS, et al. Development, adaptation, and implementation of a cardiovascular health program for Alaska Native women. Health Promotion Practice. 2005;6:472–481. doi: 10.1177/1524839904263725. [DOI] [PubMed] [Google Scholar]
- The Diabetes Prevention Program Research Group. The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22:623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. New England Journal of Medicine. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- Wallerstein NB, Duran B. Using community-based participatory research to address health disparities. Health Promotion Practice. 2006;7:312–323. doi: 10.1177/1524839906289376. [DOI] [PubMed] [Google Scholar]
- Wang W, Lee ET, Fabsitz RR, Devereux R, Best L, Welty TK, et al. A longitudinal study of hypertension risk factors and their relation to cardiovascular disease: The Strong Heart Study. Hypertension. 2006;47:403–409. doi: 10.1161/01.HYP.0000200710.29498.80. [DOI] [PubMed] [Google Scholar]
- Xu J, Lee ET, Best LG, Begum M, Knowler WC, Fabsitz RR, et al. Association of albuminuria with all-cause and cardiovascular disease mortality in diabetes: The Strong Heart Study. British Journal of Diabetes & Vascular Disease. 2005;5:334–340. [Google Scholar]


