Abstract
Sickle cell disease (SCD) is a debilitating illness that affects the life expectancy of patients. It is possible to test for SCD before birth, to allow for reproductive options to parents. However, under Cameroonian Law, voluntary abortion is a criminal offense and medical abortion is permitted only “…if it is done by an authorized professional and justified by the need to save the mother from grave health jeopardy.” The objective of the present study was to compare the views of Cameroonian doctors, parents with at least one living SCD-affected child, and adult SCD patients, regarding prenatal genetic diagnosis and termination of SCD-affected pregnancy. We conducted a quantitative sociological survey of 110 doctors, 130 parents, and 89 adult patients. The majority accepted the prenatal genetic diagnosis for SCD (78.7%, 89.8%, and 89.2%, respectively). Parents (62.5%) were more in favor of termination of SCD-affected pregnancy, than doctors and adults patients (36.1% and 40.9% acceptance, respectively). Parents and patients who found medical abortion acceptable cited fear to have a SCD-affected child (98.1 and 88.9%) and the poor quality of the affected child's health (92.6% and 81.5%). The data underscore the urgency of policy action to place emphasis on: premarital screening, early detection and care of SCD, socio-economic measures to assist SCD-affected families, appropriateness to consider maternal distress due to fetal anomalies in medical abortion legislation. These novel findings signal potential value-based conflicts on the horizon, and can usefully inform the future policy actions in the African continent as OMICS biotechnologies are increasingly employed in global health. To the best of our knowledge, the present study is the first attempt in sub-Saharan Africa to attempt to triangulate the views of multiple stakeholders towards prenatal diagnosis of SCD and termination of an affected pregnancy.
Introduction
Sickle cell disease (SCD) is a monogenic condition, where homozygote status (e.g., HbSS) results in severely malformed red blood cells. Patients can suffer from anemia, painful episodes, and susceptibility to infection, stroke, and chronic organ damage kidneys, lungs, heart, brain (Bartolucci and Galactéros, 2012). There are five treatment approaches for SCD, namely supportive, symptomatic, preventative, abortive, and curative approaches (Ballas et al., 2012). The supportive approach includes a balanced diet, hydration, and folic acid supplementation; blood transfusions, analgesia, and antibiotics are typed as symptomatic approaches; the preventative approach is taken to preclude the occurrence of disease complications such as pneumonia and influenza vaccination, hydroxyurea for the induction of fetal hemoglobin (HbF), and blood transfusions to avert stroke episodes (Ware and Helms, 2010). Nitric oxide is the only accepted agent for the abortive approach, reported to completely terminate chronic pain episodes in some SCD patients (Atz and Wessel, 1997); lastly, transplantation of hematopoietic stem cells (HSCs) is the only accepted curative treatment for SCD, provided in resource-rich countries (Locatelli et al., 2013) and not available in many sub-Saharan African countries. Management using the above variety of therapies can extend life expectancy to about 45 years in the USA (Platt et al., 1994). When the condition is not managed, patients tend to die in early childhood, as is the case in many countries in Africa (Makani et al., 2011).
The completion of the Human Genome Project has opened opportunities for extensive research, and significant progress in genetic knowledge has led to an increase in the ability for researchers to map and sequence genes for diagnosis, treatment, and prevention of SCD (Norman and Miller, 2011). Indeed, SCD is genetically characterized by a single point mutation, but despite this, there are various genetic modulators that affect the phenotype of this disease and patients can manifest varying degrees of clinical severity. A few genomic loci that have been consistently associated with increased levels of HbF influenced the clinical severity of SCD (Lettre et al., 2008; Thein et al., 2009). In addition, the co-inheritance of α-thalassemia has been associated with a milder phenotype in SCD patients (Belisario et al., 2010; Steinberg and Sebastiani, 2012). Recently, authors provide a study that attempts to link SNP data with mRNA expression in circulating blood cells of patients with SCD with pulmonary hypertension (PH). Examination of expression from patients with SCD reveals that combining the expression patterns of 10 genes can predict the presence of an elevated tricuspid regurgitant jet velocity (TRV) with 100% accuracy (Klings and Morris, 2012). An elevated TRV by Doppler echocardiography is consistent with the risk for PH, to occur in approximately 30% of SCD hemoglobin-SS patients (Klings and Morris, 2012). Although much work still needs to be done, the data provide an interesting framework upon which, it is reasonable to believe, that using genomics to predict specific phenotype in SCD will soon become possible. But, at this point in time, there is no genetic profile that is being used in practice to predict the forthcoming gravity of the disease, and prevention of SCD is still a major priority for developing countries.
The concept of prevention also extends to early detection before birth. In fact, prenatal genetic diagnosis (PND) offers reproductive options to at-risk parents, who could choose, whether to opt for medical abortion if the fetus is affected. Under various legislations, voluntary abortion is generally defined as the deliberate interruption of pregnancy on the patient's request, while medical abortion is generally defined as induced interruption of pregnancy by a qualified physician to safeguard the health of the mother or indicated by parental distress due to a fetal anomaly (Boland and Katzive, 2008). Under Cameroonian Law, voluntary abortion is a criminal offense and medical abortion is permitted only ‘…if it is done by an authorized professional and justified by the need to save the mother from grave health jeopardy’ (Act 339; exception 1; the Cameroonian Penal Code). Parental distress related to fetus pathology like SCD is not specifically considered. Following various surveys, ethical/legal debates about maternal health/rights, and the desirability of termination of affected pregnancies (TAP), PND of SCD was introduced in Cameroon (Wonkam et al., 2011a, 2011b). Nevertheless, the success of such a program is dependent on the education and attitudes of health professionals (Mountcastle-Shah and Holtzman, 2000; Wertz and Knoppers, 2002; Wonkam et al., 2006; Adeola Animasahun et al., 2012), since they will have to become more involved in providing genetic testing, guide patients to a clinical genetic service, and manage the outcomes of patients' choice when tested (Giardiello et al., 1997). In addition, genetic counseling for, and uptake of PND or TAP, are also dependent on the severity of the disease, but also on the social and cultural values of the affected populations, specifically affected families (Wonkam et al., 2011a).
Purpose of the present study
We conducted a structured quantitative sociological survey on 110 doctors, 130 parents with one living child with SCD, and 89 adult patients suffering from SCD, in Cameroon. The objective was to compare their diverse attitudes regarding prenatal genetic diagnosis and termination of SCD-affected pregnancy. There were three major research questions: (1) whether they agreed with PND and pregnancy termination in general, 2) whether they agreed with PND and TAP for SCD, and 3) the reasons for their attitudes.
Methods
Cameroon: the setting of the study
Cameroon has 20 million inhabitants and a population growth of almost 3% per annum. In 2002, approximately 47% of the population was urban; poverty in Cameroon affects up to 30% of the urban population (World Bank, 2010). There is no universal medical insurance coverage in Cameroon, and care of patients is heavily dependent on the capacity of families to afford the hospitals' bills. This country is also facing an epidemiologic transition with an increasing burden of chronic noncommunicable diseases, such as SCD (Wonkam et al., 2013). In developing countries, it has been estimated that hemoglobinopathies alone represent a health burden comparable to that of all communicable diseases combined (Weatherall, 2008). Due to the high carrier frequency ranging from 8% to 34% (Weatherall and Clegg, 2001), Cameroon has developed a national control program for SCD; however, this program remains largely unimplemented and care for SCD patients is still inadequate as there is not yet a specialized center for SCD and lifelong medical care and surveillance are not yet available; in addition, provision of healthcare services is hampered by major economic difficulties (Wonkam et al., 2013).
Ethics approval
Approval for the study was granted by the National Ethical Committee of the Ministry of Public Health, Republic of Cameroon N° 033/CNE/DNM/07. Informed consent was obtained from all participants prior to their enrollment in this study.
Design
This research was a quantitative social science study with structured administered questionnaires. A detailed description of the study methods was reported elsewhere (Wonkam et al., 2006, 2011a, 2013b). For the purposes of this article, we only provide a summary of the study methods.
Sample population and eligibility criteria
The sampling methods used included both purposeful and convenience sampling. For medical doctors' recruitment, we seized the opportunity of the 20th Cameroon National Medical Conference, an annual scientific congress for postgraduate continuing medical education, to randomly sample physicians, irrespective of their practice location or specialties. The completed questionnaires were collected at the end of the each of the 3 days of the conference. The response rate was 36.7% (110/300).
For parents and SCD patients' participants, in an attempt to ensure inclusion of adult SCD-affected patients and their parents reflecting the entire spectrum of severity in this illness, we issued a call for participation using national Cameroonian media. We also approached two SCD patients' associations in Cameroon. Participants needed to be at least 18 years old with a diagnosis of SCD that was confirmed by a laboratory documentation of hemoglobin electrophoresis.
Questionnaire format
The data were collected by means of a structured questionnaire consisting of three sections of closed-ended questions. These were (1) Socio-demographic characteristics; (2) Attitudes towards SCD screening policies; and (3) Attitudes about principles of SCD-related prenatal diagnosis and termination of an affected pregnancy if the participant's unborn child was proven to be affected. Response options were “Yes”, “No,” or “Undecided”.
Research setting and data collection
The study was conducted in Yaoundé, the capital city of Cameroon. For doctors, before administering the survey questionnaire at the reception desk of the medical conference, an introductory explanation stated the purpose of the survey.
For parents and patients' participants, an introductory explanation informed parents and SCD patients about the purpose of the study. Informed consent was also obtained at this stage. In addition to the introductory explanation, each patient and parent's participant was given full nondirective genetic counseling with neutral information concerning PND and its reproductive options. Images were used to explain the obstetric procedure of PND and risks (specifically 1% induced miscarriages). Face-to-face questionnaires were administered immediately after the counseling session, in a private consultation room by two investigating physicians: a medical geneticist and a general practitioner. Due to the sensitive nature of some questions, a follow-up visit was proposed if the participants felt it was needed. None of the parents or patients requested this option.
Data analysis
Data were analyzed using SPSS (Statistical Package for Social Sciences, Chicago, version 21). Descriptive statistics were used to measure proportion, mean/median of participants' variables. Because of the nonrandom sampling, relationship between two or more variables was evaluated by nonparametric tests (H test of Kruskal-Wallis or Z test of Kolmogorov-Smirnov, when applicable). The p values were considered significant below 0.05.
Results
Sociodemographic characteristics
As shown in Table 1, the majority of participants lived in urban areas, had attended secondary or tertiary education, and defined themselves as Christian. Contrary to patients and parents' participants, the majority of doctors were male (Table 1).
Table 1.
Differential Participants' Socio-Demographic Characteristics
| N (%) | ||||
|---|---|---|---|---|
| Participants characteristics | Doctors (N =110) | Parents with SCD children (N=130) | Adult SCD patients (N=89) | P values |
| Gender | ||||
| Male | 81.1 | 20.0 | 57.3 | 0.001 |
| Female | 19.9 | 80.0 | 42.7 | |
| Mean age (year±sem) | 34.7±0.8 | 39.6±1 | 23±2.1 | 0.001 |
| Practice/living Location | ||||
| Urban | 76.5 | 89.0 | 84.3 | 0.5 |
| Rural | 33.5 | 11.0 | 15.7 | |
| Religion | ||||
| Christian | 88.3 | 93.0 | 95.5 | 0.5 |
| Muslim | 1.9 | 5.4 | 3.0 | |
| Traditionala/others | 9.8 | 1.6 | 1.5 | |
| Level of education (N=128) | ||||
| No formal | NA | 2.3 | 2.9 | |
| Primary | NA | 15.6 | 15.7 | |
| Secondary | NA | 60.9 | 52.9 | |
| University | 100 | 21.1 | 28.6 | 0.00 |
| Employment status (N=128) | ||||
| Employed | 100 | 61.7 | 18.5 | 0.00 |
| Unemployed /never work/ student | NA | 38.3 | 81.5 | |
Traditional refers to practice of indigenous African ancestral culture; NA, not applicable.
The majority in the doctors' group identified themselves as general practitioners (GP): 42.6% (46/108). The distribution of other medical specialties was uneven: gynecologist and obstetricians or residents in gynecology 13.9% (15/108) and surgery 13.9% (15/108) were the most represented groups.
Participants' family history of SCD
A proportion of 13.5% of doctor participants indicated to have a family member affected with SCD. Amongst parent participants, 12.3% had at least two living affected SCD-children; and 16.8% (n=15) of the patient participants had at least one sibling also living with SCD. In addition, 25.7% of the parent participants revealed that they have had at least one child death, due to, or believed to be due to, SCD, and about one-third of the patient participants (29.6%, n=23) indicated that one or more of their siblings had died due to causes believed to be related to SCD.
Attitudes and practice regarding SCD screening in families
The majority of participants were very supportive of preventive genetic practice as evidenced by the fact that 100% of doctors, 100% of parents, and 84.4% of patients' participants indicated that they supported genetic counseling for SCD. In addition, parents and patients were favorable to premarital screening (92% and 100%, respectively), retrospective cascade screening (84.8% and 90.3%, respectively), antenatal care systematic screening in pregnant women (93.7% and 91.4%, respectively), and neonatal screening (95.3% and 97.1%, respectively), all of which could lead to prevention of SCD, by reducing the number of at-risk couples and ultimately the incidence, or to improve the early detection and care of SCD-affected children.
Attitudes about SCD prenatal genetic diagnosis and termination of an affected pregnancy
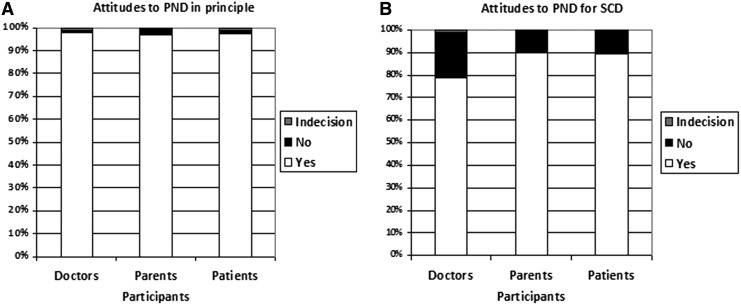
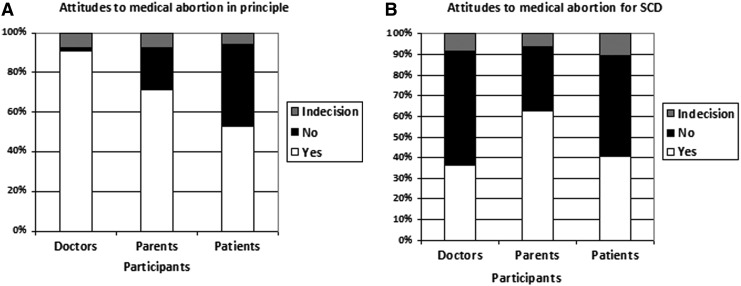
The majority of participants in the three groups supported the general principle of PND (Fig. 1A) and PND for SCD (Fig. 1B). However, while the majority will accept the principle of termination of pregnancies in general (Fig. 2A), the proportion was significantly lower amongst parents and patients as compared to doctors (Fig. 2B). A lower proportion of doctors and patients' participants will consider TAP from SCD as compare to the majority of parents' participant (Fig. 2B).
FIG. 1.
Different attitudes to prenatal genetic diagnosis. The majority of participants in the three groups supported the general principle of prenatal diagnosis in principle (A) and prenatal diagnosis for sickle cell anemia (B).
FIG. 2.
Different attitudes to termination of an affected pregnancy. However, while the majority in the three groups will accept the principle of termination of pregnancies in general (A), the proportion was significantly lower amongst parents and patients participants as compared to doctors (B). The rate of acceptance of TAP among patients is considerably lower than that of parents of a child affected by SCD and slightly higher than that of physicians (B).
In the three groups, when we questioned participants about PND and TAP for SCD specifically, a significantly lower proportion (p<0.01) of interviewed doctors, parents, and patients accepted the principle of PND for SCD and medical termination of an SCD-affected pregnancy as compare to the general principles (Fig. 1 and Fig. 2).
Amongst parents, the acceptance of the principle of medical termination for SCD increased with unemployment status (p<0.01) and single marital status (p<0.05), but not amongst patients. In the three groups, attitudes about PND and TAP were not significantly influenced by gender, religion, or family history (i.e., Number of affected children/sibling, and affected-family member in extended family).
Reasons for attitudes about SCD prenatal genetic diagnosis and termination of an affected pregnancy
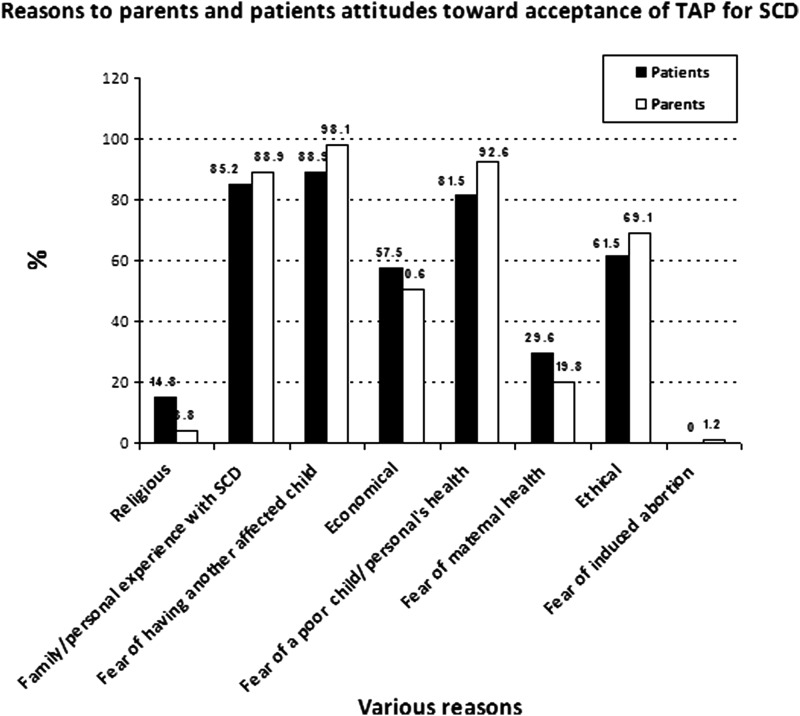
We examined responses specific to parents and patients willing to accept TAP for SCD [62.5% (n=81) and 40.9% (n=36), respectively]. For both groups, negative personal life experience of SCD, fear of having an affected child, fear of the poor quality of the child's health, ‘ethical’ issues (without any further specification), and economics were the main reasons given to explain their attitudes (Fig. 3).
FIG. 3.
Different reasons of parents and adult SCD patients toward acceptance of termination of an affected pregnancy for SCD. We examined responses specific to parents and patients willing to accept TAP for SCD (62.5% (n=81) and 40.9% (n=36), respectively). For both groups, negative personal life experience of SCD, fear of having an affected child, fear of the poor quality of the child's health, ‘ethical’ issues (without any further specification), and economics were the main reasons given to explain their attitudes.
We examined responses of parents (31.3%; n=38) and patients (48.5%; n=43) willing to reject termination of an affected pregnancy. Parents and patients rejecting TAP claimed mostly ethical (69.4% and 78.1%, respectively) and religious considerations (58.8% and 45.5%, respectively). Interestingly, ethical reasons were cited by the majority to justify TAP (69.1% and 61.5%) as well as to reject it (69.4% and 78.1 %). The research design could not allow the possibility to unpack the category ‘ethical’ with our research participants.
Discussion
To the best of our knowledge, the present study is the first attempt in sub-Saharan Africa to attempt to triangulate the views of medical doctors, parents, and adult patients' attitudes towards prenatal diagnosis and termination of an affected pregnancy. The study signals potential value-based conflicts among the three groups regarding medical abortion for SCD that could be partly embedded in the major burden of SCD on patients and families in an environment challenged by socio-economic difficulties and ill health system (Wonkam et al., 2013, 2014b).
Failure to provide adequate services for SCD in Cameroon and the parental distress
The low acceptance of medical abortion by the group of doctors' participant contrasted with that of the majority of parents with an SCD-affected child. The proportion of doctors who accepted TAP for SCD (40.9%) could be still considered high as well. But a more interesting thing is that the proportion is similar to support for voluntary abortion that we reported earlier (Wonkam and Hurst, 2007), and much lower than the support doctors gave to medical abortion in principle (90.1%; Fig. 2A). Another interpretation here could be that doctors are conceptualizing abortion for SCD to be more similar to voluntary abortion than to medical abortion in general.
Views of doctors could be influenced by the progress in pathogenesis and prospects of new emerging treatments of SCD such as hydroxyurea (Sheth et al., 2013), as well as possible cure with stem cell (cord blood or bone marrow) transplantation has been successful in some patients in many settings (Locatelli et al., 2013). However, hydroxyurea is seldom used by patient in Cameroon and stem cell transplantation is not available (Wonkam et al. 2013, 2014b). Regardless, we previously anticipated that if doctors are not supportive of the idea, then implementation of a prenatal testing program in Cameroon could be seriously hindered (Wonkam et al., 2006). In practice, since the introduction of PND in Cameroon, demands from parents have been increasing and the majority of parents with affected fetuses have elected to terminate SCD-affected pregnancies (Wonkam et al., 2011c). The strong motivation of parents regarding TAP for SCD and the surprisingly high (40.9%) rate of acceptability of TAP amongst patients, may well reflect the failure of professional stakeholders to provide adequate care services to patients with SCD in Cameroon, leading to severe clinical manifestation in patients and a higher perceived burden in affected families (Wonkam et al., 2014a, 2014b). This is not necessarily surprising, but could have prospective major policy action implications. Indeed, if the criterion of parental distress, which may be due to SCD, was considered in the Cameroonian Law as criteria to allow legal termination of pregnancy, it is likely that distress would be based on many aspects, not just the predicted suffering of the child, but also the burden of raising such a child and this would include financial concerns. This is a difficult ethical topic everywhere (Boland and Katzive, 2008). The parental distress of caring for children affected with SCD in the Cameroonian context seems to have led some of them to the painful choice of illegal voluntary abortion for fear of having another SCD-affected child (Wonkam et al., 2011b). Moreover, two-thirds of the surveyed parents' participants (52/72) had chosen not to have further children for fear of producing subsequent SCD-affected individuals (Wonkam et al., 2011a). This is further confirmed by the relatively high proportion of adult SCD patients themselves who are in favor of medical abortion for SCD (Fig. 2B) due to the poor perception of their quality of life (Wonkam et al., 2014b). It is possible that some SCD patients had such poor quality of life that they did not find their own lives worth living, and their parents and themselves did not want another child to have the same experience (Wonkam et al., 2013b, 2014b). This is an alarming finding that requires urgent attention of policy makers in Cameroon.
In a completely different context, we reported on the increasing burden of SCD and the introduction of PND for SCD in clinical practice in Cape Town, South Africa (Wonkam et al., 2012). Following telephone interviews involving 15 parents with at least one SCD-affected child (mean age: 36.2 years; eight female; 50% unemployed), regarding PND and TAP for SCD, we found that although 12 out of 15 respondents said they will like to take the option of PND for SCD in future; however, 10 out of 15 disagreed with the idea of TAP for SCD (unpublished data). The major difference between the Red Cross War Memorial Hospital in Cape Town, where the SCD-affected children of these group of parents were attended, and the Central Hospital in Yaoundé in Cameroon, is a provision in Cape Town of a specialized free of charge comprehensive clinic for SCD that offers most of the available care and therapeutic options to families (Wonkam et al., 2012). It could be possible that, in the South African context, a favorable social environment and hospital services available to parents have contributed to alleviate the burden of SCD on parents and therefore, improved their ability and power to take care, leading to a lower desirability of TAP for SCD.
“Power to cure” of doctors vs. “power to care of parents” in Down syndrome and SCD
Interestingly, we previously reported on the paradox between the perception of severity of SCD that was higher than that of Down syndrome (DS) amongst Cameroonian doctors and the contrasting higher acceptance of TAP for DS by the same group, as compared to the acceptance of TAP for SCD (Wonkam et al., 2006). Furthermore, we recently reported on the attitudes of a group of South African parents with a preschool child with DS towards PND and termination of a DS-affected pregnancy; the participants had a positive attitude towards PND and felt that it was every parent's right to have the option. However, all of them were totally opposed to the termination of a DS-affected pregnancy due to their personal experience (Scott et al., 2013).
Despite the difference in the studies' settings, participants, and methodologies, these data seem to indicate that the intellectual disability associated with DS for which the medical profession cannot provide any cure, reflects on the attitudes of Cameroonian doctors (Wonkam et al., 2006), and challenge their “power to cure.” In contrast, DS does not seems to challenge the “power to care” of South African parents in their day-to-day life (Scott at al., 2013). It could tempting to argue that parental distress could be higher for SCD as compared to Down syndrome, in the African context (Wonkam et al., 2013b); nevertheless, as described in the preliminary data above, the low acceptance of TAP for SCD by parents who have a child with SCD in Cape Town, indicates that socio-economic factors and the quality of health services are possible critical determinants of parents' “power to care” and, ultimately critical determinants of parents' attitudes to TAP in Africa. This possibility is also supported by data from Nigeria where parents with SCD-affected children disclosed a high burden of SCD on their families (Brown et al., 2010).
Indeed, in Nigeria, 92% of the SCD heterozygous carrier mothers and 85% of female SCD patients favored PND, but 63% of parents as compared to 35% of SCD female patients indicated they would opt for termination of an affected pregnancy (Durosinmi et al., 1995). However, a sample of Nigerian doctors would accept termination of an affected pregnancy for SCD in only 21.4% (Adeyemi and Adekanle, 2007). A more recent study amongst health professional in Nigeria [44% (n=167) were medical doctors], equally confirmed the trend reported in the present study: only 33% of the respondents will allow preventive termination of pregnancy if prenatal screening confirms SCD (Animasahun et al., 2012). It could be argued in the case of SCD, that perception of the “power to cure” of both Cameroonian and Nigerian doctors is higher that of the “power to care” of Cameroonian and Nigerian parents. In both Cameroon and Nigeria, relevant to the issue of parental distress, the unpredictable nature of vaso-occlusive crisis with potential fatal consequences, previous difficult experience in management of SCD (Fig. 3), and a high financial and economic burden were the factors that affected coping ability of parents with SCD the most (Durosinmi et al., 1995; Brown et al., 2010; Wonkam et al. 2014a). It would be interesting to report on another sub-Saharan African setting such as Benin, where SCD patients seem to receive appropriate care (Rahimy et al., 2009), in order to explore whether an equivalent number parents of well-treated SCD patients and adult SCD patients themselves, would also consider TAP. As shown by preliminary research in Cape Town summarized above, it is possible that with better care and improvement of their “power to care”, parents and patients would be less inclined to embrace TAP for SCD; this hypothesis will require further investigations to help in adopting policy actions that will allow the parents to opt freely for reproductive options that do not conflicts their societal Ethics.
Potential societal ethical conflict regarding TAP for SCD in Cameroon?
Concerning induced abortion of fetuses with birth defects, literature has warned that decisions of TAP constitute a problematic value judgment about what is considered a life worth living, and possibly a step in the direction of eugenics (Shakespeare, 1998; Garel et al., 2002). These concerns were raised by authors in Ghana who considered that “PND and selective abortion appears to be applying capital punishment to the unborn child for “crimes” only the parents can be responsible for” (Kyerewaa Edwin et al., 2011). Cameroonian doctors could be sensitive to this, especially for a potentially treatable condition like SCD. It is critical to comment that even in countries where abortion is legal, fetal ‘indications’ are not always recognized (Boland and Katzive, 2008), as this decreases the freedom of the parents who may want to keep a pregnancy, and can be considered an eugenic policy (Shakespeare, 1998).
The different views of patients, physicians, and parents also indicate a potential ethical conflict between various segments of the Cameroonian society regarding TAP for SCD. Additional studies among various groups may provide detailed insight into identifying different values, priorities, or understandings of the situation faced by SCD patients. This could help in shaping the perception of the community regarding genetic technology and the use of prenatal diagnosis and its reproductive options in Cameroon. The postgenome era promises can offer new insights into the nature of SCD, its prevention and treatment; sub-Saharan Africa needs urgent societal and policies adjustment to this. The present study provides additional clues to support the statement that the successful use of genetic testing for prevention and care of SCD will require a close relationship with researchers, the medical profession, families, and patients to overcome the psychosocial and ethical challenges faced by health care providers, individuals, family members, social support systems, and their community (Norman and Miller, 2011).
Limitations and Research Recommendations
Doctors who attended conferences and/or worked in an urban environment in Yaoundé were most likely to be recruited, and the views presented here cannot be extended to that of the majority. Moreover, their response rate was relatively low. In addition the gender imbalance did not reveal attitudes of female doctors who are not represented here; female doctors represent almost 40% of the Cameroonian doctors' population. The reasons explaining the views of doctors also need further investigations. Parents and patients' participants with access to mass media would have been more likely to be recruited in this study; patients' support groups, which were also involved in participant recruitment, were more likely to be utilized by city dwellers; this could have excluded more rural and poorer participants. The population of Yaoundé and its surroundings is predominantly of Christian faith. Geographical access from the North of Cameroon to Yaoundé, where the study occurred, would have been limited for Muslims, living mostly in the North (World Bank, 2010). Thus, the results of this study could only be considered applicable to the relatively resourced, urbanized population groups in Cameroon.
Another possible limitation of our study is our choice of quantitative instead of qualitative methods. Since we did not engage with research participants in interviews or focus group discussions, we are not in a position to offer deep insight into what motivated their choices. We see the contribution of this study as demonstrating trends for further investigation through qualitative social science research that could bring more in-depth insight into the analysis and understanding of various views.
Policy and Practice Implications
The findings indicate the urgent need to develop and implement specific policy actions at least four levels:
1) the implementation of the national control program of SCD with screening policies that put emphasis on premarital detection to, hopefully, reduce the number of cases that will be referred to PND; indeed, 97.7% (n=127) of the parents surveyed were unaware before marriage that they were at risk of having a child with SCD (Wonkam et al., 2011a);
2) to implement policies and practices that improve the early detection and care of SCD, for example, neonatal screening and the use of hydoxyurea; and to develop specialized centre for affordable care for SCD patients;
3) to develop a clear social policies for birth defects and disabilities with inclusion of socio-economic measures to assist affected families and alleviate the burden of SCD e.g. SCD could be formally recognised as a disability; this implies the provision of adequate financial and educational support to families;
4) to reopened the discussion the appropriateness of the Cameroonian abortion laws to the increasingly used OMICS technology in early detection of birth defects. Specifically, to discuss the possibility to include maternal distress, due to foetal anomalies like SCD, as acceptable justification for medical abortion.
Conclusions
Taken together, these three groups, the Cameroonian doctors, parents, and SCD patients, appear to accept the principles of preventive genetic medicine, including SCD prenatal diagnosis and in a surprisingly high proportion, of termination of an affected pregnancy amongst parents and patients. The study provides some clues that parental and patients' distress, which could have motivated their views, could be related to the difficulties to care and to live with SCD, in an environment challenged by economical difficulty and poor provision of heath care. The study signals potential value-based conflicts among the three groups regarding medical abortion for SCD and can inform concrete future policy interventions as OMICS biotechnologies are increasingly employed in African countries.
Acknowledgments
For their input, we thank the patients' association, “Globule Rouge”, and the parents who participated in the survey. This study was supported by the Commission for Humanitarian Affairs of the Geneva University Hospitals, Geneva, Switzerland, and the NIH no. 1UO1HG007459-01.
Author Disclosure Statement
The authors declare there are no conflicts of interest.
References
- Adeola Animasahun B, Nwodo U, and Njokanma OF. (2012). Prenatal screening for sickle cell anemia: Awareness among health professionals and medical students at the Lagos University Teaching Hospital and the concept of prevention by termination. J Pediatr Hematol Oncol 34, 252–256 [DOI] [PubMed] [Google Scholar]
- Atz AM, and Wessel DL. (1997). Inhaled nitric oxide in sickle cell disease with acute chest syndrome. Anesthesiology 4, 988–990 [DOI] [PubMed] [Google Scholar]
- Ballas SK, Kesen MR, Goldberg MF, et al. (2012). Beyond the definitions of the phenotypic complications of Sickle Cell Disease: An update on management. Sci World J 2012, 949535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolucci P, and Galactéros F. (2012). Clinical management of adult sickle-cell disease. Curr Opin Hematol 19, 149–155 [DOI] [PubMed] [Google Scholar]
- Belisário AR, Rodrigues CV, Martins ML, Silva CM, and Viana MB. (2010). Coinheritance of α-thalassemia decreases the risk of cerebrovascular disease in a cohort of children with sickle cell anemia. Hemoglobin 34, 516–529 [DOI] [PubMed] [Google Scholar]
- Boland R, and Katzive L. (2008). Developments in laws on induced abortion: 1998–2007. Int Fam Plan Perspect 34, 110–120 [DOI] [PubMed] [Google Scholar]
- Brown BJ, Okereke JO, Lagunju IA, Orimadegun AE, Ohaeri JU, and Akinyinka OO. (2010). Burden of health-care of carers of children with sickle cell disease in Nigeria. Health Soc Care Community 18, 289–295 [DOI] [PubMed] [Google Scholar]
- Durosinmi MA, Odebiyi AI, Adediran IA, Akinola NO, Adegorioye DE, and Okunade MA. (1995). Acceptability of prenatal diagnosis of sickle cell anaemia (SCA) by female patients and parents of SCA patients in Nigeria. Soc Sci Med 41, 433–436 [DOI] [PubMed] [Google Scholar]
- Garel M, Gosme-Seguret S, Kaminski M, and Cuttini M. (2002). Ethical decision-making in prenatal diagnosis and termination of pregnancy: A qualitative survey among physicians and midwives. Prenat Diagn 22, 811–817 [DOI] [PubMed] [Google Scholar]
- Giardiello FM, Brensinger JD, Petersen GM, et al. (1997). The use and interpretation of commercial APC gene testing for familial adenomatous polyposis. N Engl J Med 336, 823–827 [DOI] [PubMed] [Google Scholar]
- Klings ES, and Morris CR. (2012). Making it personal: Using genomics to predict pulmonary hypertension in sickle cell disease. Am J Respir Crit Care Med 186, 304–305 [DOI] [PubMed] [Google Scholar]
- Kyerewaa Edwin A, Edwin F, and Etwire V. (2011). Controlling sickle cell disease in Ghana—Ethics and options. Pan Afr Med J 10,14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettre G, Sankaran VG, Bezerra MA, et al. (2008). DNA polymorphisms at the BCL11A, HBS1L-MYB, and beta-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc Natl Acad Sci USA 105, 11869–11874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locatelli F, Kabbara N, Ruggeri A., et al. (2013). Outcome of patients with hemoglobinopathies given either cord blood or bone marrow transplantation from an HLA-identical sibling. Blood 122, 1072–1078 [DOI] [PubMed] [Google Scholar]
- Makani J, Cox SE, Soka D, et al. (2011). Mortality in sickle cell anemia in Africa: A prospective cohort study in Tanzania. PLoS One 6, e14699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle-Shah E, and Holtzman NA. (2000). Primary care physicians' perceptions of barriers to genetic testing and their willingness to participate in research. Am J Med Genet 94, 409–416 [DOI] [PubMed] [Google Scholar]
- Norman BJ, and Miller SD. (2011). Human genome project and sickle cell disease. Soc Work Public Health 26, 405–416 [DOI] [PubMed] [Google Scholar]
- Platt OS, Brambilla DJ, Rosse WF, et al. (1994). Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med 330, 1639–1644 [DOI] [PubMed] [Google Scholar]
- Rahimy MC, Gangbo A, Ahouignan G, and Alihonou E. (2009). Newborn screening for sickle cell disease in the Republic of Benin. J Clin Pathol 62, 46–48 [DOI] [PubMed] [Google Scholar]
- Scott CJ, Futter M, and Wonkam A. (2013). Prenatal diagnosis and termination of pregnancy: Perspectives of South African parents of children with Down syndrome. J Community Genet 4, 87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakespeare T. (1998). Choices and rights: Eugenics, genetics and disability equality. Disability Society 13, 665–681 [DOI] [PubMed] [Google Scholar]
- Sheth S, Licursi M, and Bhatia M. (2013). Sickle cell disease: Time for a closer look at treatment options? Br J Haematol 162, 455–464 [DOI] [PubMed] [Google Scholar]
- Steinberg MH, and Sebastiani P. (2012). Genetic modifiers of sickle cell disease. Am J Hematol 87, 795–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thein SL, Menzel S, Lathrop M, and Garner C. (2009). Control of fetal hemoglobin: New insights emerging from genomics and clinical implications. Hum Mol Genet 18(R2), R216–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware RE, and Helms RW. (2010). Stroke with transfusion changing to hydroxyurea (SWiTCH): A phase 3 randomized clinical trial for treatment of children with sickle cell anemia, previous stroke, and iron overload. Blood 116, 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherall DJ. (2008). Hemoglobinopathies worldwide: Present and future. Curr Mol Med 8, 592–599 [DOI] [PubMed] [Google Scholar]
- Weatherall DJ, and Clegg JB. (2001). Inherited haemoglobin disorders: An increasing global health problem. Bull World Health Organ 79, 704–712 [PMC free article] [PubMed] [Google Scholar]
- Wertz DC, and Knoppers BM. (2002). Serious genetic disorders: Can or should they be defined? Am J Med Genet 108, 29–35 [DOI] [PubMed] [Google Scholar]
- Wonkam A, de Vries J, Royal CD, Ramesar R, and Angwafo FF., 3rd. (2013). Would you terminate a pregnancy affected by sickle cell disease? Analysis of views of patients in Cameroon. J Med Ethics. doi: 10.1136/medethics-2013-101392 [DOI] [PubMed] [Google Scholar]
- Wonkam A, and Hurst SA. (2007). Acceptance of abortion by doctors and medical students in Cameroon. Lancet 369, 1999. [DOI] [PubMed] [Google Scholar]
- Wonkam A, Mba CZ, Mbanya D, Ngogang J, Ramesar R, and Angwafo FF., 3rd. (2014a). Psychosocial burden of sickle cell disease on parents with an affected child in Cameroon. J Genet Couns 23, 192–201 [DOI] [PubMed] [Google Scholar]
- Wonkam A, Mba CZ, Mbanya D, Ngogang J, Ramesar R, and Angwafo FF., 3rd. (2014b). Psychosocial stressors of sickle cell disease on adult patients in Cameroon. J Genet Couns DOI: 10.1007/s10897-014-9701-z [DOI] [PubMed] [Google Scholar]
- Wonkam A, Ngongang Tekendo C, Zambo H, and Morris MA. (2011c). Initiation of prenatal genetic diagnosis of sickle cell anaemia in Cameroon (sub-Saharan Africa). Prenat Diagn 31, 1210–1212 [DOI] [PubMed] [Google Scholar]
- Wonkam A, Njamnshi AK, and Angwafo FF., III. (2006). Knowledge and attitudes concerning medical genetics amongst physicians and medical students in Cameroon (sub-Saharan Africa). Genet Med 8, 331–338 [DOI] [PubMed] [Google Scholar]
- Wonkam A, Njamnshi AK, Mbanya D, Ngogang J, Zameyo C, and Angwafo FF., 3rd. (2011a). Acceptability of prenatal diagnosis by a sample of parents of sickle cell anemia patients in Cameroon (sub-Saharan Africa). J Genet Couns 20, 476–485 [DOI] [PubMed] [Google Scholar]
- Wonkam A, Tekendo CN, Sama DJ, et al. (2011b). Initiation of a medical genetics service in sub-Saharan Africa: Experience of prenatal diagnosis in Cameroon. Eur J Med Genet 54, e399–404 [DOI] [PubMed] [Google Scholar]
- World Bank. (2010). Education for all-fast track initiative: Support to the education sector. Report no. 48373, 1–2 [Google Scholar]