Abstract
Background
A light-porous-particle, dry-powder formulation of tobramycin was developed, using PulmoSphere® technology, to improve airway delivery efficiency, substantially reduce delivery time, and improve patient convenience and satisfaction. We evaluated the safety, efficacy and convenience of tobramycin inhalation powder (TIP™) versus tobramycin inhalation solution (TIS, TOBI®) for treating Pseudomonas aeruginosa infection in cystic fibrosis (CF) patients aged ≥6 years.
Methods
In this open-label study, 553 patients were randomized 3:2 to TIP (total 112 mg tobramycin) via the Novartis T-326 Inhaler or TIS 300 mg/5 mL via PARI LC® PLUS nebulizer twice daily for three treatment cycles (28 days on-drug, 28 days off-drug). Safety, efficacy, and treatment satisfaction outcomes were evaluated.
Results
TIP was generally well-tolerated; adverse events were similar in both groups. The rate of cough suspected to be study-drug related was higher in TIP-treated patients (TIP: 25.3%; TIS: 4.3%), as was the overall discontinuation rate (TIP: 26.9%; TIS: 18.2%). Increases in FEV1 % predicted from baseline to Day 28 of Cycle 3 were similar between groups; mean reduction in sputum Pseudomonas aeruginosa density (log10 CFU/g) on Day 28 of Cycle 3 was also comparable between groups. Administration time was significantly less for TIP (mean: 5.6 versus 19.7 minutes, p<0.0001). Treatment satisfaction was significantly higher for TIP for effectiveness, convenience, and global satisfaction.
Conclusions
TIP has a safety and efficacy profile comparable with TIS, and offers a far more convenient treatment option for pseudomonas lung infection in CF.
Keywords: cystic fibrosis, tobramycin inhalation powder (TIP™), pseudomonas aeruginosa, lung infection
Introduction
Individuals with cystic fibrosis (CF) are highly susceptible to endobronchial infection with Pseudomonas aeruginosa (Pa). For most patients, Pa infections begin in childhood and become chronic by early adulthood (1). Chronic Pa infection is associated with an accelerated decline in lung function, and increased morbidity and mortality (2-5). Consequently, effective treatment of Pa infection is crucial to the management of CF.
Tobramycin inhalation solution (TIS (6)) significantly improves lung function and quality of life, and reduces hospitalization rates, in CF patients chronically infected with Pa (7-10). Current treatment guidelines recommend TIS for the treatment of chronic Pa pulmonary infections in CF patients ≥6 years (TOBI® prescribing information; 11-13). Administration time is approximately 15-20 minutes per dose (excluding cleaning and sterilization) (13).
Treatment of Pa infection by inhaled antibiotics is time consuming and places a high burden on CF patients; adherence to treatment is a significant challenge (14-16). An innovative drug-device combination using a new dry-powder formulation of tobramycin has been developed to increase the convenience of administration for patients, which may increase treatment adherence and thereby clinical outcomes (17,18). Tobramycin inhalation powder (TIP™) is formed of light-porous-particles that are manufactured using an emulsion-based spray drying process (19). TIP is delivered via the T-326 Inhaler (Novartis Pharmaceuticals Corporation, USA), a dry powder inhaler (DPI). The device is portable, has no internal or external power source, and is designed to enhance drug delivery to the lung and shorten administration time (13). CF patients as young as 6 years old are capable of inhaling through a device with the internal resistance of the T-326 Inhaler (20). Previous studies showed that single-dose administration of TIP results in more efficient and rapid delivery of tobramycin than TIS in CF patients, while maintaining similar pharmacokinetic characteristics (13,21).
The Establish A new Gold standard Efficacy and safety with tobramycin in cystic fibRosis [EAGER] trial was designed to evaluate the safety, efficacy and convenience of the new inhaled formulation of tobramycin (TIP) versus TIS for treating Pa infection in CF patients.
Methods
This international study was conducted in 127 centers in 15 countries and was approved by an Institutional Review Board or Independent Ethics Committee for each center and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from each patient.
Patients
CF patients aged ≥6 years with forced expiratory volume in one second (FEV1) ≥25 to ≤75% predicted based on Knudson equations (22) and sputum or throat cultures positive for Pa within 6 months of screening (and confirmed at enrollment) were eligible.
Study design
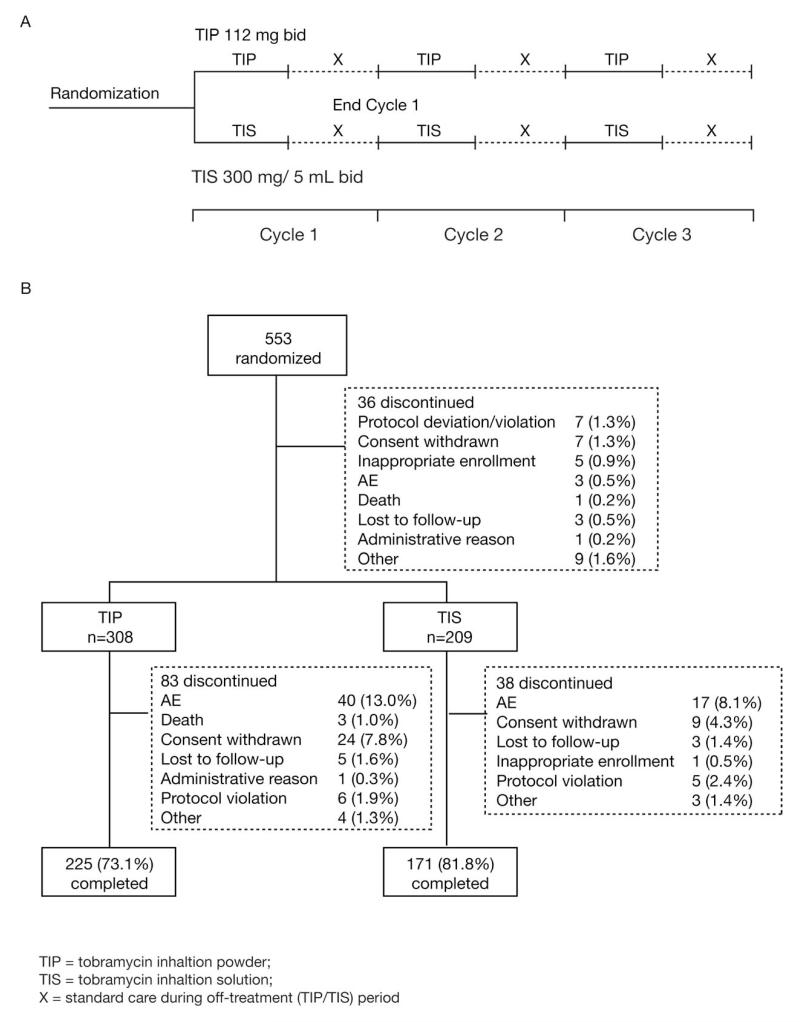
This two-arm, randomized, open-label study comprised three cycles; each cycle consisted of 28 days on-treatment followed by 28 days off-treatment. Total duration of treatment was 24 weeks (Figure 1A). Eligible patients were randomized 3:2 to TIP (four capsules/112 mg tobramycin) twice daily administered via the T-326 Inhaler, or TIS 300 mg/5 mL (TOBI®) twice daily administered via the PARI LC® PLUS jet nebulizer and DeVilbiss PulmoAide compressor or an equivalent alternative.
Figure 1.
Study design (A) and disposition (B).
Study assessments
Safety assessments included the incidence and intensity of all adverse events (AEs) and serious AEs (SAEs), presence of airway reactivity to the drug (post-inhalation drop in FEV1), and changes in hematology, blood chemistry, urine protein, audiology, physical condition and body weight.
The main efficacy measure was relative change in FEV1 % predicted from baseline (pre-dose Day 1) at all scheduled post-treatment visits (Weeks 2, 5, 9, 13, 17, 21 and 25). Other efficacy measures included change in sputum Pa density (log10 CFU/g sputum), tobramycin susceptibility to Pa (assessed using minimum inhibitory concentrations [MIC]), antipseudomonal antibiotic use, and respiratory-related hospitalizations. Serum and sputum pharmacokinetics were assessed in a subset of the population.
Patient’s self-reported treatment satisfaction was measured using the Treatment Satisfaction Questionnaire for Medication (TSQM, a validated instrument) (23), which was modified by adding four study-specific questions: (I) How convenient or inconvenient is it to store the medication?; (II) How easy or difficult is it to put together the parts of the delivery device?; (III) How convenient or inconvenient would it be to use the delivery device away from home?; (IV) How convenient or inconvenient is it for you to take care of the delivery device? (Note: the standard fourteen questions of the TSQM were not altered). The TSQM domain scores were calculated as recommended by the instrument authors; described in detail elsewhere (23). The TSQM domain scores range from 0 to 100 with higher scores representing higher satisfaction for that domain. The term ‘medication’ includes the combination of the medication and delivery device (aerosol machine/nebulizer or inhaler).
Statistical analyses
Sample size was based on the primary variable, safety; inclusion of 300 TIP patients was estimated to provide a 99.8% chance of observing at least one AE with a true incidence of 2% in the TIP group. Inclusion of 500 patients in total (TIP: 300; TIS: 200) would provide 96% power to demonstrate non-inferiority of TIP to TIS with regard to relative change from baseline in FEV1 % predicted after three cycles, based on a non-inferiority margin of 6% and a one-sided significance level of 0.15 (assuming 1% true TIS-TIP treatment difference, and 20% standard deviation).
The primary objective was to evaluate the safety of TIP versus TIS (data are summarized descriptively). A pre-planned non-inferiority analysis compared the efficacy of TIP versus TIS, with regard to relative change in FEV1 % predicted from baseline to the end of Cycle 3 dosing (pre-dose Day 28). Post-hoc sensitivity analyses assessed the impact of patient discontinuation. All randomized patients who received ≥1 dose of study drug were included in the safety and efficacy populations.
Results
A total of 553 patients were randomized; 517 received at least one dose of study medication and were included in the efficacy and safety populations. Baseline demographic and clinical characteristics were similar in the two treatment groups (Table 1). Use of TIS prior to screening was similar in both groups (TIP: 82.1%; TIS: 82.3%). Concomitant therapy was used by 99.7% of TIP-treated and 99.0% of TIS-treated patients.
Table 1.
Baseline demographic and clinical characteristics (Safety population).
| TIP (n=308) | TIS (n=209) | |
|---|---|---|
| Age (years), mean (SD) | 26 (11.4) | 25 (10.2) |
| Age group, n (%) | ||
| ≥6 to <13 years | 28 (9.1) | 18 (8.6) |
| ≥13 to <20 years | 66 (21.4) | 48 (23.0) |
| ≥20 years | 214 (69.5) | 143 (68.4) |
| Sex, n (%) | ||
| Males | 171 (55.5) | 115 (55.0) |
| Females | 137 (44.5) | 94 (45.0) |
| Race, n (%) | ||
| Asian | 2 (0.6) | 2 (1.0) |
| Black | 3 (1.0) | 1 (0.5) |
| Caucasian | 279 (90.6) | 189 (90.4) |
| Hispanic | 20 (6.5) | 17 (8.1) |
| Other | 4 (1.3) | 0 (0.0) |
| Region, n (%) | ||
| North America | 195 (63.3) | 131 (62.7) |
| Europe and rest of world1 | 104 (33.8) | 71 (34.0) |
| Latin America | 9 (2.9) | 7 (3.4) |
| Body mass index (kg/m2), mean (SD) | 20.7 (4.0) | 20.4 (3.5) |
| Screening FEV1 % predicted, n (%)2 | ||
| ≥25 to <50% | 128 (41.6) | 89 (42.6) |
| ≥50 to <75% | 180 (58.4) | 120 (57.4) |
| Baseline FEV1 % predicted, mean (SD)2 | 53 (14.2) | 53 (15.9) |
| Chronic macrolide use, n (%) | 187 (60.7) | 125 (59.8) |
| Last use of antipseudomonal antibiotics prior to first dose (month), n (%) |
||
| =1 | 78 (25.3) | 46 (22.0) |
| >1–3 | 171 (55.5) | 112 (53.6) |
| >3–6 | 33 (10.7) | 24 (11.5) |
| >6 | 11 (3.6) | 9 (4.3) |
| Never used | 15 (4.9) | 18 (8.6) |
| P.aeruginosa tobramycin MIC, n (%)3 | ||
| >8 μg/mL | 68 (22.1) | 48 (23.0) |
Rest of world including Australia and Israel
Randomization is based on screen FEV1% predicted. Baseline FEV1 was defined as the last measurement prior to the first dose of study drug
Maximum MIC of all P. aeruginosa phenotypes; TIP = tobramycin inhalation powder; TIS = tobramycin inhalation solution; SD = standard deviation; FEV1 = forced expiratory volume in one second; MIC = minimum inhibitory concentration.
The main cause for discontinuation was AEs (Figure 1B); more patients discontinued in Cycle 1 than in Cycles 2 and 3 for both groups. Adherence to therapy (based on the number of doses actually received versus the possible maximum) was generally high (>90% of doses) over all 3 cycles in both groups. All patients who completed the study without major protocol deviations were included in the per protocol (PP) population (TIP: 60.8%; TIS: 66.5%); failure to take at least 80% of the study drug was the most frequent reason for exclusion.
Safety
A higher percentage of TIP-than TIS-treated patients reported AEs (90.3% versus 84.2%, p<0.05) (Table 2). Most AEs were mild or moderate in intensity (TIP: 73.4%; TIS: 68.5%). The percentage of patients with AEs was highest in Cycle 1, in both groups (77.9% versus 66.5%), and decreased with each successive cycle (Cycle 2: 67.0% versus 66.3%; Cycle 3: 65.8% versus 58.5%).
Table 2.
Most common (≥5% in any group) adverse events occurring in Cycles 1–3 (Safety population).
| n (%) | TIP (n=308) | TIS (n=209) |
|---|---|---|
| Any adverse event | 278 (90.3) | 176 (84.2) |
| Cough | 149 (48.4) | 65 (31.1) |
| Lung disorder† | 104 (33.8) | 63 (30.1) |
| Productive cough | 56 (18.2) | 41 (19.6) |
| Dyspnea | 48 (15.6) | 26 (12.4) |
| Pyrexia | 48 (15.6) | 26 (12.4) |
| Oropharyngeal pain | 43 (14.0) | 21 (10.5) |
| Dysphonia | 42 (13.6) | 8 (3.8) |
| Hemoptysis | 40 (13.0) | 26 (12.4) |
| Headache | 35 (11.4) | 25 (12.0) |
| Nasal congestion | 25 (8.1) | 15 (7.2) |
| Nausea | 23 (7.5) | 20 (9.6) |
| Rales | 22 (7.1) | 13 (6.2) |
| Rhinorrhea | 22 (7.1) | 15 (7.2) |
| Pulmonary function test decreased | 21 (6.8) | 17 (8.1) |
| Upper respiratory tract infection | 21 (6.8) | 18 (8.6) |
| Wheezing | 21 (6.8) | 13 (6.2) |
| Chest discomfort | 20 (6.5) | 6 (2.9) |
| Fatigue | 20 (6.5) | 10 (4.8) |
| Vomiting | 19 (6.2) | 12 (5.7) |
| Sinusitis | 18 (5.8) | 15 (7.2) |
| Pulmonary congestion | 17 (5.5) | 9 (4.3) |
TIP = tobramycin inhalation powder; TIS = tobramycin inhalation solution.
Lung disorders were generally reported by the investigator as a pulmonary or cystic fibrosis exacerbation
Cough (not including productive cough) was the most frequently reported AE throughout the entire study period (TIP: 48.4%; TIS: 31.1%). As a baseline symptom, cough was present in a high proportion of patients (42%) in both groups. Most cough events were mild or moderate in intensity. The frequency of severe cough events was low and balanced between groups (2.6% versus 1.9%). Less than 4% (12/308) of TIP-treated patients discontinued due to cough versus 1% (2/209) of TIS-treated patients. Cough events were suspected by the investigator as being related to study drug in 25.3% and 4.3% of patients in the TIP and TIS group, respectively. Other treatment-related AEs more commonly reported in the TIP group were dysphonia (13.6% versus 3.8%) and dysgeusia (3.9% versus 0.5%).
Clinically significant bronchospasm (defined as an acute relative change of ≥20% decrease in FEV1 % predicted from pre-dose to 30-minutes post-dose) in any cycle was experienced by 5.2% and 5.3% of TIP- and TIS-treated patients, respectively.
The incidence of SAEs was similar in both groups (TIP: 27.4%; TIS: 29.2%). Lung disorders were the most commonly reported SAE in both groups (TIP: 19.5%; TIS: 18.7%). SAEs were reported by fewer patients in both groups, with each successive treatment cycle (Cycle 1: 15.5%; Cycle 2: 9.3%; Cycle 3: 7.4%). Three deaths were reported during the study. All were in the TIP group, although none was related to the study drug.
There were no clinically relevant changes from baseline to pre-specified time points for vital signs, and biochemical or hematological measures. There were few reports (≤4%) of renal function changes as measured by increases in serum creatinine, blood urea nitrogen, or proteinuria in both groups. The incidence of AEs related to renal and urinary disorders, including proteinuria, dysuria, polyuria and nephrolithiasis, was ≤1% in both groups.
Audiology was performed in a subpopulation of patients (TIP: 78 [25.3%]; TIS: 45 [21.5%]). Twenty (25.6%) TIP-treated and 7 (15.6%) TIS-treated patients experienced a decrease from baseline in any audiology test frequency at any visit; the decrease was of a similar degree in both groups. Using the criteria for either ear of 10 dB loss at three consecutive frequencies, 15 dB loss at two consecutive frequencies and 20 dB loss at any frequency, 3 (0.97%) TIP-treated and 2 (0.96%) TIS-treated patients were considered to have clinically significant hearing loss. Hearing complaints tended to be intermittent and transient.
Efficacy
Spirometry
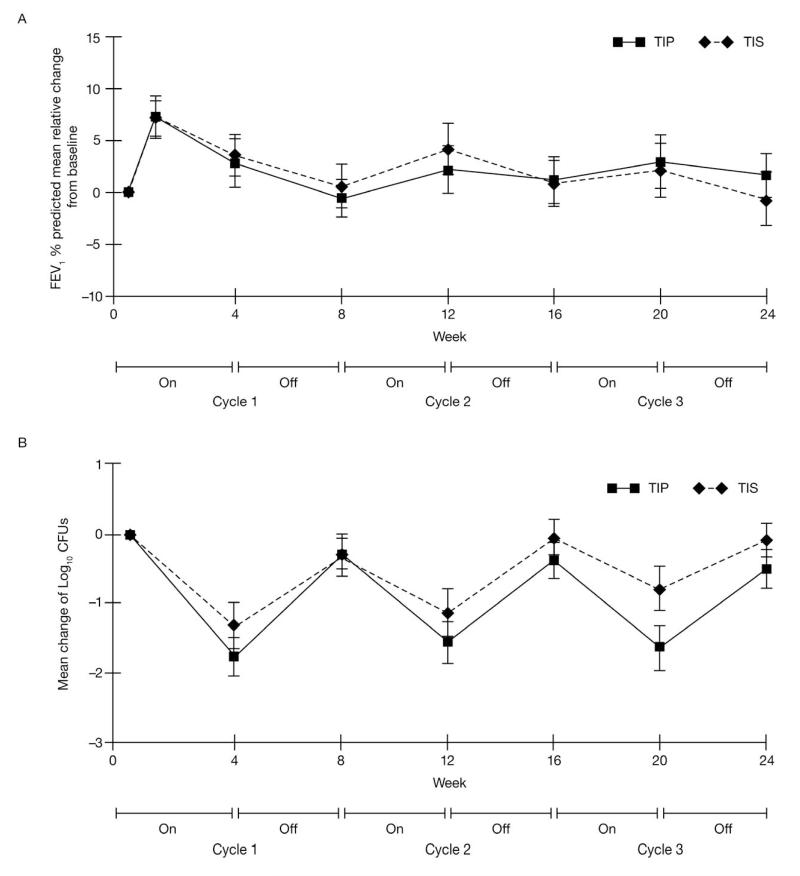
Increases in FEV1 % predicted from baseline to Day 28 of Cycle 3 were similar between groups (least squares [LS] mean difference 1.1% relative change [SE 1.75], Figure 2A). The lower limit (−0.67%) of the one-sided 85% [CI] (equivalent to 70% two-sided) was within the predefined 6% margin for predefined non-inferiority indicating that TIP was non-inferior to TIS. LS mean values from the PP population supported this analysis (LS mean difference in FEV1 of 1.2%, lower limit of the one-sided 85% CI was −1.02%).
Figure 2.
Overall efficacy: A) relative change in FEV1 % predicted from baseline over three cycles (Efficacy population); B) change from baseline in Pseudomonas aeruginosa sputum density (Efficacy population).
Microbiology
Mucoid and non-mucoid Pa sputum density showed a decrease from baseline in both groups at all time points (Figure 2B presents combined mean of all phenotypes). The mean change from baseline was greater in the TIP versus TIS group on Day 28 of Cycle 3 (mean change: −1.6 versus −0.92 log10 CFU/g for mucoid phenotype and – 1.77 versus −0.73 log10 CFU/g for non-mucoid phenotype, respectively). On Day 28 of Cycle 3, 11.6% of TIP and 9.9% of TIS patients had negative Pa cultures.
The MIC of tobramycin against Pa increased from baseline to Day 28 of Cycle 3 in both groups (≥4-fold increase: 67/199 [33.7%] versus 42/154 [27.3%]; ≥2-fold increase: 97/199 [48.7%] versus 61/154 [39.6%]).For patients with Pa isolates (all phenotypes) with an MIC <8 g/ml at baseline (TIP, 22.1%; TIS, 23.0%), 19.1% versus 14.9% of TIP- and TIS-treated patients had an MIC >8 g/ml at the end of Cycle 3.
Antipseudomonal antibiotic use and hospitalizations
The proportion of patients requiring any new antipseudomonal antibiotic was significantly higher with TIP than TIS (64.9% versus 54.5%, p=0.0148). However, the average number of days of antibiotic usage tended to be less in the TIP group (mean [SD]: 30.9 [23.34] versus 33.4 [24.42] days, p=0.2039). Most newly used antibiotics were administered orally; oral antibiotics were used in 55.5% and 39.7% of patients in the TIP and TIS groups, respectively. Ciprofloxacin was the most frequently used new anti-pseudomonal antibiotic during the study (47.7% versus 34.0%).
The number of patients hospitalized for respiratory-related events was similar in the TIP versus TIS group (24.4% versus 22.0%). The proportion of hospitalized patients receiving antibiotics (excluding inhaled antibiotics) was well matched between groups (22.1% versus 21.5%).
Administration time
Mean duration of administration was significantly less for TIP (5.6 minutes) than TIS (19.7 minutes, excluding cleaning and sterilization) at each study visit and overall (p<0.0001).
Patient-reported treatment satisfaction
Overall, patient satisfaction was higher with TIP (Table 3). LS mean scores were significantly higher (p<0.0001) with TIP than TIS at all visits for effectiveness (a higher score indicates higher satisfaction for that domain); this takes into account patients’ assessment of the ability of their medication to treat or prevent their condition and relieve symptoms, as well as the time it takes to start working. Patients also reported treatment with TIP to be significantly more convenient than TIS for the entire study (p<0.0001); this takes into account patients’ assessment of the overall convenience of treatment (e.g. ease of use and time). Furthermore, global satisfaction was reported to be statistically greater for TIP than TIS at all visits (p=0.0018). No difference in side effect ratings (patients’ assessment of how bothersome the side effects are and their perception of the impact of the side effects on their physical and mental abilities) was reported between groups during the study (p=0.6833). The four supplementary questions relating to the use and maintenance of the inhalation devices (i.e. storage, device assembly, use of device away from home and device caretaking) all showed a significant difference in favor of TIP for the entire study period (p<0.0001).
Table 3.
Patient-reported treatment satisfaction from modified Treatment Satisfaction Questionnaire for Medication (Safety population).
| Average of LS means over visits |
LS mean difference (SE) |
p value | ||
|---|---|---|---|---|
| TIP (n=308) | TIS (n=209) | |||
| Effectiveness | 74.8 | 65.4 | 9.36 (1.460) | <0.0001 |
| Side effects | 92.1 | 92.6 | −0.50 (1.218) | 0.6833 |
| Convenience | 82.7 | 58.4 | 24.35 (1.547) | <0.0001 |
| Global satisfaction | 76.2 | 71.0 | 5.20 (1.655) | 0.0018 |
LS mean = least squares mean; TIP = tobramycin inhalation powder; TIS = tobramycin inhalation solution; SE = standard error. LS mean, LS mean difference (TIP–TIS), and p values are calculated from repeated measures model with treatment, baseline FEV1 % predicted, age, chronic macrolide use, region, visit, visit-by-treatment interaction in the model. A higher score indicates higher satisfaction for that domain.
Pharmacokinetics
Pharmacokinetic assessments were performed in 30 TIP-treated and 14 TIS-treated patients. Serum tobramycin concentrations were comparable for TIP and TIS. Sputum levels were generally higher for TIP 30-minutes post-dose (mean±SD on Day 28, Cycle 3: TIP, 1979±2770 μg/g; TIS, 1074±1182 μg/g). At baseline, more than 91.2% of TIP patients had Pa isolates with an MIC at least 20 times lower (64 g/mL or less) than the mean sputum concentration observed within 30 minutes of the first dosing in Cycle 1. At the end of Cycle 3, 86.4% of TIP patients had Pa isolates (all phenotypes) with an MIC at least 30 times lower (64 g/mL or less) than the mean sputum concentration observed 30-minutes post-dose.
Discussion
This study demonstrates that the safety profile of a new drug-device combination of TIP delivered via the T-326 Inhaler, is similar to TIS, especially with regard to systemic safety. The results of safety analyses are supportive of previously published reports showing an acceptable safety profile for TIP, which is generally well-tolerated in most patients with CF (13, 18).
The dose of TIP (112 mg tobramycin) used in this study was chosen based on a previous study that showed it is pharmacokinetically equivalent to the licensed formulation of TIS (TOBI®) (16). In the present study, pharmacokinetic analysis confirmed that serum tobramycin concentrations were comparable for TIP and TIS post-inhalation. Importantly, systemic levels were low relative to those associated with producing toxicity with intravenous tobramycin (10-12 g/mL) (24).
AEs were similar in both groups; except for the incidence of cough, dysphonia and dysgeusia, which was higher in patients receiving TIP. Cough is a frequent event with all inhaled therapies (13). It is possible that the comparatively high powder ‘payload’ may underlie the higher reported cough rate in TIP-versus TIS-treated patients (as well as the local effect of dysphonia and dysgeusia). It is unclear if the high level of prior TIS use in these patients and the open-label design had an influence on the propensity to report cough as an AE for each treatment. Most cough events were mild or moderate in intensity in both groups. The frequency of cough was similar in patients using TIP, regardless of lung disease severity at baseline. While the majority of patients in the study were adults, it appeared that children and adolescents tended to cough more with TIP than adults, whereas the opposite was true for TIS. However, none of the children discontinued TIP treatment due to cough events. Cough in the absence of other AEs (i.e. cough not associated with other features suggestive of an exacerbation) led to discontinuation of five of the 308 patients randomized to TIP. Post-hoc analyses showed that cough did not appear to be related to bronchospasm; additionally, the number of patients experiencing a ≥20% decline in FEV1 % predicted within 30-minutes of inhalation was similar in both groups. The incidence of SAEs was similar in TIP- and TIS-treated patients. TSQM side effect ratings showed no difference between groups, indicating that unwanted effects of TIP and TIS had a similar impact on patients’ lives.
The overall discontinuation rate was higher with TIP (26.9%) than TIS (18.2%); however, there is no clear reason for the difference in discontinuation rates between groups. The main reason for discontinuation in both groups was an AE; most frequently cough and lung disorders (reported as pulmonary or CF exacerbations), although relatively few patients in either group discontinued due to cough or lung disorders.
The present study was also powered to assess the non-inferiority of TIP to TIS with regards to efficacy. The pre-planned efficacy analyses show that TIP was not inferior to TIS, in terms of maintenance of lung function. Importantly, the difference in discontinuation rates did not influence the non-inferiority conclusion, based on various sensitivity analyses. The results support those of a recent placebo-controlled trial that show TIP significantly improves lung function in CF patients (21).
In accordance with previous studies (6, 17), TIP and TIS both decreased sputum Pa densities (mucoid and non-mucoid phenotypes). Conversely, MICs increased from Day 1 to 28 in all 3 cycles. Exposure of bacterial populations to antibiotics may select for microbes with reduced susceptibility to these drugs over time (25). However, tobramycin susceptibility thresholds with parenteral administration might not be relevant to inhaled therapy, as drug concentrations achieved at the site of infection with inhaled antibiotics, including tobramycin, can be significantly higher than systemic concentrations (13). At the end of Cycle 3, most TIP patients (86.4%) had an MIC at least 30 times lower than the mean sputum concentration observed 30-minutes post-dose. The results of the pharmacokinetic analyses also showed that sputum concentrations were generally greater for TIP; however, the level of inter-subject variability was high, similar to previous studies. Based on these data we can conclude that TIP administration appears to result in lung exposure that is comparable to TIS.
The use of new anti-pseudomonal antibiotics was significantly greater in TIP-versus TIS-treated patients during the study. The difference does not appear to have been driven by events of a higher clinical intensity. In both groups, the majority of newly used anti-pseudomonal antibiotics were oral, with ciprofloxacin the most frequently used. These data suggest the difference is largely driven by oral ciprofloxacin use for milder events (i.e. those not needing intravenous antibiotics and/or hospitalization). It is possible that the higher incidence of cough in the TIP group may have driven the greater use of other antibiotics. Again, the influence of the open-label design cannot be excluded. Post-hoc sensitivity analyses suggest that the use of new anti-pseudomonal antibiotics did not alter the conclusion regarding the non-inferior efficacy of TIP versus TIS.
Delivery of antibiotics via inhalation is an attractive option for treating chronic lung infection in CF patients. However, aerosolized antibiotics such as TIS requires a compressor and nebulizer, and are associated with prolonged drug administration times (13). TIS is approved for use with the PARI-LC® PLUS nebulizer only, which requires 15-20 minutes to nebulize a dose. Faster nebulizers than the PARI-LC® PLUS have been developed since the approval of TIS and are available in some countries; however, they have not been adequately studied to demonstrate efficacy and safety with TIS. In the present study, administration times for TIP were approximately 14 minutes faster than TIS (excluding time required to set up, clean and disinfect the TIS nebulizer), consistent with previously reported administration times (13). This resulted in a 28 minute saving per day, equivalent to 13 hours per 4-week cycle, in addition to the reduced time required to set up and clean the T-326 Inhaler versus the PARI LC® PLUS nebulizer and compressor. Reducing administration time may have beneficial effects on adherence and clinical outcomes. The ease of use and convenience of TIP were highlighted in the treatment satisfaction responses obtained via the TSQM. Mean patient assessments for effectiveness, convenience and global satisfaction domains were significantly greater for TIP than TIS; importantly, this difference was sustained over the course of the study. The four additional questions relating to the use and maintenance of the inhalation devices also showed a significant difference in favor of TIP.
In summary, the safety profile of TIP was comparable with the currently approved formulation of tobramycin, TIS, with the exception of cough, dysphonia and dysgeusia. Patient-reported satisfaction was significantly higher with TIP than TIS, which was related to the ease of use and convenience of TIP. Importantly, non-inferiority was demonstrated for TIP with regard to efficacy. Thus, the new drug-device combination for TIP could offer CF patients a much more convenient option for treating Pa infections, without compromising safety or efficacy.
Acknowledgments
The study principal investigators are: L Sindel, M Woo, C Lew, G Shay, B Morrissey, M Schechter, D Caplan, G Sharma, T Spencer, T Martin, D Waltz, P Sammut, J Acton, M Konstan, K McCoy, G Graff, P Flume, K Schultz, R Amaro-Galvez, D Froh, G Albers, C Conrad, P Radford, C Ren, A Dozer, N Amin, S Millard, S Nasr, B Nickerson, M Guill, P Hiatt, T Lahiri, T Murphy, M Wall, S Fiel, R Fink, K Jones, S McColley, K Meyer, N Kraynack, F Quijano, D Stokes, A Stone, D Toder, D Geller, K Moffett, D Roberts, J Rosen, D Schellhase, J Sharp, M McCarthy, S Reyes, R Kravitz, M Sherman, W Sexauer, J Akhter, J Biller, F Adler-Shohet, J Lieberman, M Howenstine, J Smith, T Ferkol, S Boas, L Lester, N Turcios, M Berdella, J Nick, R Silver, R Cohen, P Fornos, C Orozco, D Brown, M Pian, R Zanni, B Chatfield, D Hayes, J Royall, D Bisberg, D Willey-Courand, V Antony, P Vauthy, M Light, D Schaeffer (USA); P Fernandez (Chile); M Calabria, A Cardona (Columbia); A Bustamante (Mexico); S Dominique, R Chiron, A Munck, D Hubert (France); H Rabin (Canada); T Nüßlein, M Griese, M Ballmann, T Wagner, E Rietschel, H-G Posselt, D Staab, H-E Heuer, T Welte (Germany); K Hyurkovits, K Holics (Hungary); M Tamm (Switzerland); S Quattricci, R Casciaro, R Gagliardini, F Pardo, D Salvatore, C Braggion (Italy); H Tiddens, LM van den Toorn; C Magis-Escurra (Netherlands); S Gartner, C Vaquez Cordero, J Perez Frias, C Prados Sanchez, A Sole (Spain); C Taylor, L Kuitert, S Conway, S Elborn, J Whitehouse (United Kingdom); H Greville, D Serisier, P Middleton (Australia); H Blau, E Kerem, L Bentur, M Aviram (Israel); M Fotoulaki (Greece).
The authors would like to acknowledge the efforts of the Cystic Fibrosis Foundation Data Safety Monitoring Board and the Cystic Fibrosis Therapeutics Development Network during this study. The authors would also like to acknowledge the efforts of Francis Jones, Simon Piggott and Pearl Kho during this study. Writing and editorial assistance was provided by Melanie Stephens (ACUMED®, London, UK). This assistance was funded by Novartis Pharma AG (Basel, Switzerland).
Research funding support: This study was funded by Novartis Pharma AG, Basel, Switzerland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Classification: 90: Pulmonology
Clinical trial registration: NCT00388505
Conflict of interest
Dr Konstan has received income from Aradigm Corp, Boehringer Ingelheim, CSL Behring, Genentech, Inc., GlaxoSmithKline, Gilead Sciences, Inc., NanoBio, Nektar Therapeutics, Novartis Pharmaceuticals, PTC Therapeutics, Transave, Inc., and Vertex Pharmaceuticals, Inc. for consulting activities. Dr Flume has received income from AstraZeneca in the form of lecture fees, and from Nanobio for consulting activities. Dr Kappler has received income from Novartis in the form of consulting and lecture fees. Dr Chiron has no conflicts to report. Dr Geller has received income from Aradigm, CSL Behring, Discovery Labs, Genentech, MAP Pharmaceuticals, NanoBio, Novartis Pharmaceuticals, and Teva for consulting activities. Mark Higgins, Florian Brockhaus, Jie Zhang, Gerhild Angyalosi and Ellie He are employees of Novartis Pharmaceuticals.
References
- 1.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Repir Crit Care Med. 2003;168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 2.Henry RL, Mellis CM, Petrovic L. Mucoid Pseudomonas aeruginosa is a marker of poor survival in cystic fibrosis. Pediatr Pulmonol. 1992;12:158–161. doi: 10.1002/ppul.1950120306. [DOI] [PubMed] [Google Scholar]
- 3.Ballmann M, Rabsch P, von der Hardt H. Long-term follow up of changes in FEV1 and treatment intensity during Pseudomonas aeruginosa colonisation in patients with cystic fibrosis. Thorax. 1998;53:732–737. doi: 10.1136/thx.53.9.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosorok MR, Zeng L, West SE, et al. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatr Pulmonol. 2001;32:277–287. doi: 10.1002/ppul.2009.abs. [DOI] [PubMed] [Google Scholar]
- 5.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 6.TOBI® (Tobramycin) [Accessed September 9, 2009];Prescribing information. Available from: http://www.tobitime.com/info/tools/prescribing.jsp.
- 7.Ramsey BW, Pepe MS, Quan JM, et al. Cystic Fibrosis Inhaled Tobramycin Study Group Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med. 1999;340:23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 8.Moss RB. Long-term benefits of inhaled tobramycin in adolescent patients with cystic fibrosis. Chest. 2002;121:55–63. doi: 10.1378/chest.121.1.55. [DOI] [PubMed] [Google Scholar]
- 9.Murphy TD, Anbar RD, Lester LA, et al. Treatment with tobramycin solution for inhalation reduces hospitalizations in young CF subjects with mild lung disease. Pediatr Pulmonol. 2004;38:314–320. doi: 10.1002/ppul.20097. [DOI] [PubMed] [Google Scholar]
- 10.Quittner AL, Buu A. Effects of tobramycin solution for inhalation on global ratings of quality of life in patients with cystic fibrosis and Pseudomonas aeruginosa infection. Pediatr Pulmonol. 2002;33:269–276. doi: 10.1002/ppul.10074. [DOI] [PubMed] [Google Scholar]
- 11.Flume PA, O’Sullivan BP, Robinson KA, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957–969. doi: 10.1164/rccm.200705-664OC. [DOI] [PubMed] [Google Scholar]
- 12.Döring G, Hoiby N. Consensus Study Group. Early intervention and prevention of lung disease in cystic fibrosis: a European consensus. J Cyst Fibros. 2004;3:67–91. doi: 10.1016/j.jcf.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Geller DE, Konstan MW, Smith J, Noonberg DB, Conrad C. Novel tobramycin inhalation powder in cystic fibrosis subjects: pharmacokinetics and safety. Pediatr Pulmonol. 2007;42:307–313. doi: 10.1002/ppul.20594. [DOI] [PubMed] [Google Scholar]
- 14.Dodd ME, Webb KA. Understanding non-compliance with treatment in adults with cystic fibrosis. J R Soc Med. 2000;93:2–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: challenges to disease self-management. J Cyst Fibros. 2009;8:91–96. doi: 10.1016/j.jcf.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zemanick ET, Harris JK, Conway S, et al. Measuring and improving respiratory outcomes in cystic fibrosis lung disease: Opportunities and challenges to therapy. J Cyst Fibros. 2010;9:1–16. doi: 10.1016/j.jcf.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geller DE. The science of aerosol delivery in cystic fibrosis. Pediatr Pulmonol. 2008;43:S5–S17. [Google Scholar]
- 18.Konstan M, Geller DE, Minić P, Brockhaus F, Zhang J, Angyalosi G. Tobramycin inhalation powder for Pseudomonas aeruginosa infection in cystic fibrosis: the EVOLVE trial. Ped Pulm. 2010 doi: 10.1002/ppul.21356. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haynes A, Nakamura J, Heng C, et al. Respiratory Drug Delivery 2010. Davis Healthcare Intl Publ; River Grove, IL: 2010. pp. 701–706. [Google Scholar]
- 20.Tiddens HA, Geller DE, Challoner P, et al. Effect of dry powder inhaler resistance on the inspiratory flow rates and volumes of cystic fibrosis patients of six years and older. J Aerosol Med. 2006;19:456–465. doi: 10.1089/jam.2006.19.456. [DOI] [PubMed] [Google Scholar]
- 21.Newhouse MT, Hirst PH, Duddu SP, et al. Inhalation of a dry powder tobramycin pulmosphere formulation in healthy Volunteers. Chest. 2003;124:360–366. doi: 10.1378/chest.124.1.360. [DOI] [PubMed] [Google Scholar]
- 22.Knudson RJ, Lebowtiz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 23.Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12. doi: 10.1186/1477-7525-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sweetman SC. Martindale: The Complete Drug Reference. Pharmaceutical Press; London: [Accessed 29 Jun 2009]. [online) Available from: http://www.medicinescomplete.com. [Google Scholar]
- 25.Davies JC, Bilton D. Bugs, biofilms, and resistance in cystic fibrosis. Respir Care. 2009;54:628–640. doi: 10.4187/aarc0492. [DOI] [PubMed] [Google Scholar]