Summary
The spatiotemporal activities of astrocyte Ca2+ signaling in mature neuronal circuits remain unclear. We used genetically encoded Ca2+ and glutamate indicators as well as pharmacogenetic and electrical control of neurotransmitter release to explore astrocyte activity in the hippocampal mossy fiber pathway. Our data revealed numerous localised spontaneous Ca2+ signals in astrocyte branches and territories, but these were not driven by neuronal activity or glutamate. Moreover, evoked astrocyte Ca2+ signaling changed linearly with the number of mossy fiber action potentials. Under these settings astrocyte responses were global, suppressed by neurotransmitter clearance and mediated by glutamate and GABA. Thus, astrocyte engagement in the fully developed mossy fiber pathway was slow and territorial, contrary to that frequently proposed for astrocytes within microcircuits. We show that astrocyte Ca2+ signaling functionally segregates large volumes of neuropil and that these transients are not suited for responding to, or regulating, single synapses in the mossy fiber pathway.
Keywords: astrocyte, calcium, GCaMP, iGluSnFR, mossy fiber, neuron-glia interactions
Introduction
Important progress has been made in understanding the roles of glia in the brain since their discovery over a century ago and following landmark physiological studies (Kuffler, 1967). Astrocytes (a subclass of glia) are known to display dynamic intracellular Ca2+ signals (Agulhon et al., 2008; Li et al., 2013), and it has recently been shown that astrocytes rapidly sense and regulate single synapses (Di Castro et al., 2011; Panatier et al., 2011). In these settings, astrocytes respond to single synapse glutamate release by exhibiting localised intracellular Ca2+ elevations in their main processes a few micrometers from the soma, implying that they are actively involved in microcircuit function (Di Castro et al., 2011; Panatier et al., 2011). However, abolishing one major form of intracellular Ca2+ signal within astrocytes was without obvious consequence for neuronal function (Agulhon et al., 2010; Fiacco et al., 2007; Petravicz et al., 2008). To understand these differences, one needs to explore when astrocytes become excited within neuronal circuits, but this has proven challenging. Unlike neurons, astrocytes are not electrically excitable and evaluations have had to rely on imaging. In particular, available imaging methods that use organic Ca2+ indicator dyes are not ideal for monitoring astrocyte branches, which are the primary sites for interactions with neurons (Reeves et al., 2011; Shigetomi et al., 2013a; Shigetomi et al., 2010; Tong et al., 2012). As a result, the function of astrocyte Ca2+ signaling within neuronal circuits remains incompletely explored (Tong et al., 2012).
We have refined optical and genetic tools in order to study astrocyte branches and territories by building on recent progress with genetically encoded calcium indicators (GECIs) (Tian et al., 2012). GECIs are not a panacea and they have effects such as Ca2+ buffering that are shared with organic dyes. However, under most circumstances they do not obviously perturb neurons or astrocytes and are complementary to other approaches (Chen et al., 2013; Shigetomi et al., 2013a; Shigetomi et al., 2013b; Tian et al., 2009; Zariwala et al., 2012).
To image cytosolic and near membrane Ca2+ we used astrocyte specific expression of cytosolic GCaMP3 or membrane targeted Lck-GCaMP3, respectively (Shigetomi et al., 2013a; Shigetomi et al., 2010; Shigetomi et al., 2011; Tian et al., 2009). To directly image astrocyte cell surface glutamate signals we used a genetically encoded glutamate sensor (Marvin et al., 2013) (iGluSnFR). We also used GCaMP6f, a recent GECI with kinetics similar to the organic Ca2+ indicator dye OGB1-AM (Chen et al., 2013). In order to drive neurotransmitter release selectively from the mossy fiber pathway, we generated novel BAC transgenic “SPRAE” mice that express a drug-activated ion channel within the mossy fiber pathway. Using these optical and pharmacogenetic tools we explored astrocyte signaling in the circuit formed by the dentate gyrus granule cell projection (the mossy fiber pathway) to the CA3 region of the hippocampus (Amaral and Lavenex, 2007). We chose this circuit because mossy fibers are the only feed forward excitatory input to the anatomically well-defined CA3 region of the stratum lucidum (s.l.) (Amaral and Lavenex, 2007; Ruiz and Kullmann, 2012; Spruston and McBain, 2007). Additionally, the anatomical relationship between mossy fibers and postsynaptic neuronal and astrocytic targets has been described by electron microscopy (Rollenhagen and Lübke, 2006; Rollenhagen et al., 2007; Wilke et al., 2013).
Results
We deployed several imaging tools to study astrocytes located in the s.l. of the adult mouse hippocampus (∼P70). The slices were ∼300 μm thick and the imaged astrocytes were located ∼40 μm from the slice surface. The experiments were conducted at room temperature (∼21°C) or at mouse body temperature (34°C), as indicated. The imaging was performed using laser scanning confocal microscopy with a 40X objective lens with a numerical aperture of 0.8. The effective pixel size was 0.2 × 0.2 μm, which is larger than the size of astrocyte branchlets at < 100 nm.
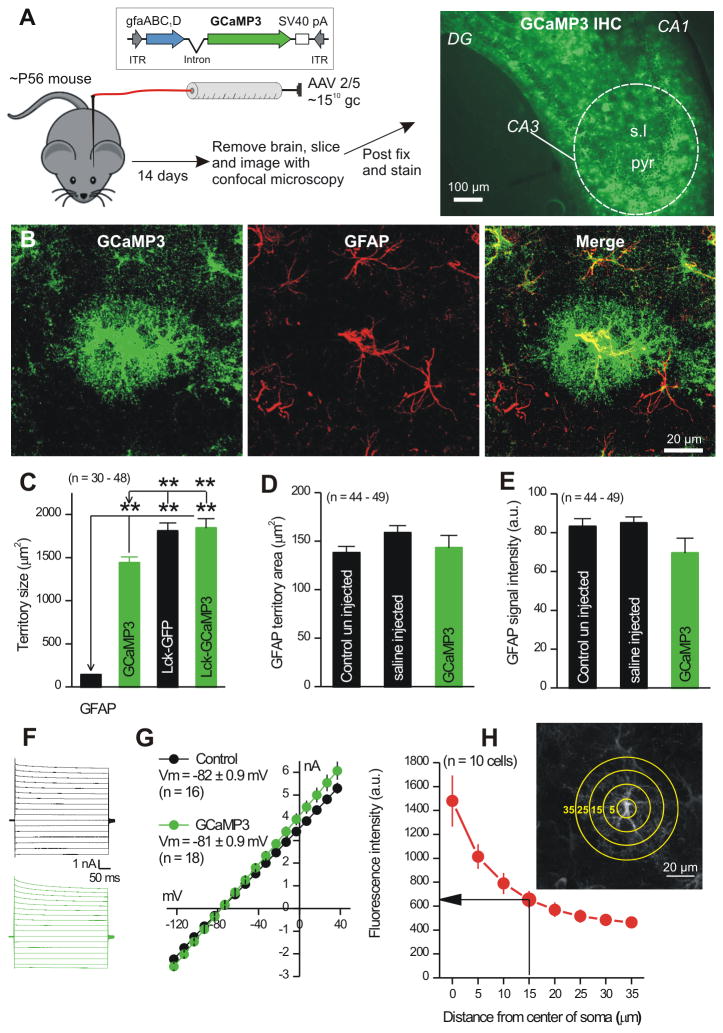
GCaMP3 reveals stratum lucidum astrocyte branches and territories
We could not reliably load hippocampal astrocytes from adult mice with organic Ca2+ indicator dyes. By extending tool development work that showed GCaMP3 is well suited to study astrocytes from adult mice (Shigetomi et al., 2013a; Shigetomi et al., 2013b), we used it, Lck-GCaMP3 and GCaMP6f to explore s.l. astrocyte intracellular Ca2+ signals. For selective expression within astrocytes, we used adeno-associated viruses of the 2/5 serotype (AAV 2/5) and the astrocyte-specific gfaABC1D promoter (Shigetomi et al., 2013a) (Fig 1). GECIs were innocuously expressed within large parts of s.l. astrocyte territories, including their branches. Furthermore, AAV2/5 mediated expression of fluorescent proteins does not alter spontaneous Ca2+ signals in astrocytes (Shigetomi et al., 2013a), which recalls and extends past work with neurons that reported little deleterious effect of GECI expression (Chen et al., 2013; Shigetomi et al., 2013b; Tian et al., 2009; Zariwala et al., 2012). All of the GECIs we used display Ca2+ affinities (∼0.3 μM) similar to organic dyes often used to study astrocytes.
Figure 1. Expression of GCaMP3 in astrocytes.
A. The cartoon illustrates the procedure to inject AAV2/5 capable of expressing GCaMP3 in s.l. astrocytes of P56 mice. The right hand image shows fluorescence signal for GCaMP3 detected by IHC in the CA3 region. B. GCaMP3 and GFAP expression in an astrocyte from the s.l. region. The GCaMP3 expressing astrocyte was GFAP positive and 85 ± 2 % of all GFAP positive astrocytes in the s.l. expressed GCaMP3 (n = 4 mice). C. Maximal projection territory area for GFAP, GCaMP3, Lck-GCaMP3 and Lck-GFP. D-E. GFAP maximal projection territory areas (D) and intensity (E) for astrocytes. F-G. Traces and average data for astrocyte current-voltage relationships (-120 to +40 mV) from control mice or those microinjected with GCaMP3. H. The image shows a representative astrocyte with circles drawn radially (5 μm spacing). Such circles were used to measure the intensity of GCaMP3 expression at increasing distances from the center of the soma (in the graph). The highest intensity was in the soma, which has the largest volume and intensity fell with distance. Average data are shown as mean ± S.EM.
Stratum lucidum astrocytes display slow Ca2+ signals that last seconds
High numbers of Ca2+ signals were observed within s.l astrocytes expressing GCaMP3 (Supp movie 1; Figure 2B-D; n = 13 astrocytes). Eleven ROIs from an astrocyte are shown in Figure 2A,C, illustrating that the soma was relatively silent and that numerous Ca2+ signals occurred in branches. Ca2+ signals in the somata and branches displayed similar amplitudes and second's time scale kinetics (Figure 2B; Supp Table 1). However, ∼8-fold greater numbers of Ca2+ signals were measured within branches than in somata (Figure 2B). Such Ca2+ signals originated from, and were of similar amplitude within, entire territories of single astrocytes (Figure 2D; n = 13 cells). The greatest numbers were detected ∼15 μm from the soma (Figure 2D), perhaps reflecting a region of high astrocyte branching or a larger cytosolic volume per branch at this region. These possibilities cannot be discriminated by light microscopy and further detailed work employing correlated light and electron microscopy is needed. However, the observed result is not due to fortuitous accumulation of GCaMP3 at ∼15 μm (Figure 1H; n = 10 cells).
Figure 2. Properties of Ca2+ signals in s.l. astrocytes.
A. Image of a single s.l astrocyte expressing GCaMP3 with 11 ROIs indicated (Supp movie 1). B. Distributions of astrocyte Ca2+ signal properties (blue bars are for branches and the red bars are for somata). C. Traces for 11 ROIs from Fig 2A. D. Plots Ca2+ signal amplitude (left axis) and number (right axis) in 5 μm bins as a function of distance from the soma. The number of Ca2+ signals in 5 μm bins is shown by the black line. E. Image of an s.l. astrocyte with a yellow line indicating the approximate position of the region chosen for 200 Hz line scan imaging. F. Image of line scan data for the line shown in E, with an expanded region corresponding to a branchlet shown below. In these images, the x-axis is time and the y-axis is distance along the scanned line. G. The trace for the selected region shown in F. H. Distributions showing Ca2+ signal half widths from line scan experiments for somatic and branchlet regions. I-J. Bar graphs summarize Ca2+ signal properties such as dF/F and half width for line scan data. Average data are shown as mean ± S.EM.
To determine if fast Ca2+ signals existed in s.l. astrocytes we used 200 Hz line scan imaging (Figure 2E; n = 10). A representative line scan lasting ∼170 s is shown in Figure 2F, and a branch region is expanded and plotted as a trace (Figure 2G). Ca2+ signals were clearly seen: we repeated the analyses for 10 astrocytes and measured transient properties in branches and somata (Figure 2H-J). These data show that on average the fastest Ca2+ signals last ∼3 s, which is consistent with the frame scan data (Figure 2B,C; Supp Table 1). We also performed a specific set of experiments on single astrocytes to measure astrocyte Ca2+ signals at room temperature and then at 34°C. In these pair wise comparisons we noted significant changes in the properties of Ca2+ signals at warmer temperatures, including acceleration of kinetics in somata, as expected. However, overall the Ca2+ signals lasted seconds (Supp Table 2; n = 7).
Is it possible that fast Ca2+ signals were missed with GCaMP3? To address this we repeated experiments shown in Fig 1 by using GCaMP6f, which displays rapid kinetics and high sensitivity (Chen et al., 2013). GCaMP6f displays larger peak fluorescence changes compared to GCaMP3 (Chen et al., 2013), and in accord using GCaMP6f astrocyte spontaneous Ca2+ signals were 3-fold larger and we observed twice as many ROIs with Ca2+ signals in the branches (Supp Table 1; n = 14). However, the Ca2+ signals detected by GCaMP6f still lasted several seconds (Supp Table 1)
It is conceivable that cytosolic GECIs may miss Ca2+ signals in fine processes. To explore this we used Lck-GCaMP3, which better reports near membrane Ca2+ signals within fine processes (Shigetomi et al., 2013a). However, we found that expression of Lck-GCaMP3 within s.l. astrocytes reported fewer spontaneous Ca2+ signals than GCaMP3 (Supp Table 1; n = 12) even though it was expressed within territory areas larger than GCaMP3 (Fig 1C). These data indicate that s.l. astrocytes do not display a significant number of near-membrane Ca2+ signals in fine processes. We further explored these possibilities in subsequent experiments, but taken together these data suggest that s.l. astrocytes are different from s.r. astrocytes (Shigetomi et al., 2013b) in that fewer signals are mediated by near-membrane Ca2+ dynamics in the s.l. region. This implies diverse astrocyte properties and functions within subfields of the hippocampus.
Stratum lucidum astrocyte Ca2+ signals are not due to mGluRs, NMDARs or action potential firing
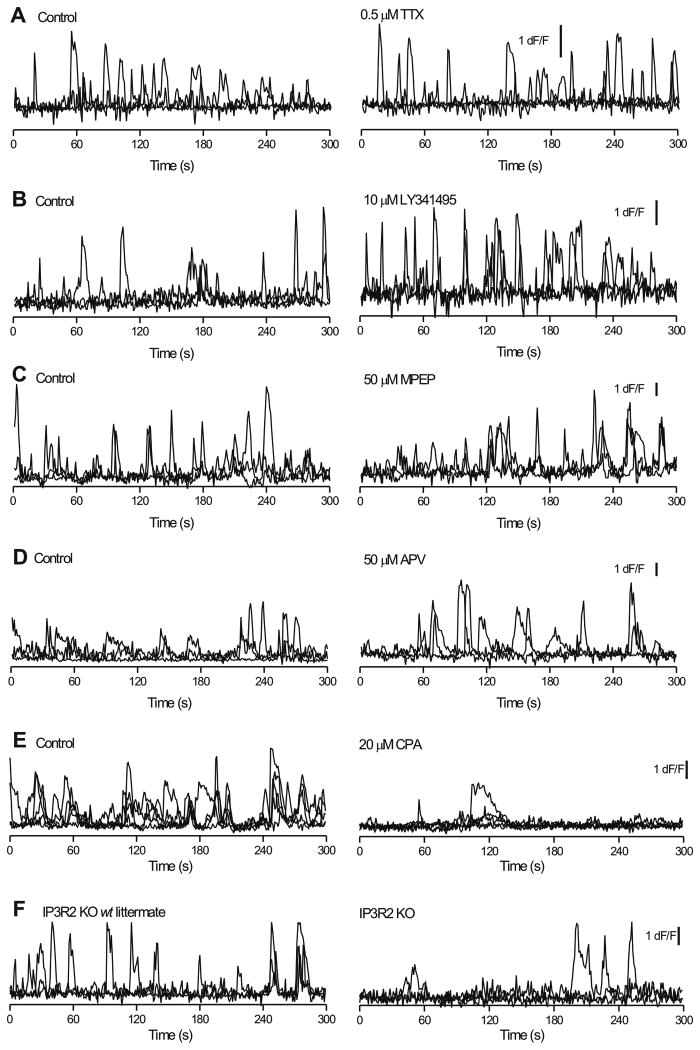
Given the tight association of astrocyte branches and excitatory synapses in the s.l. region (Rollenhagen et al., 2007), we determined if astrocyte Ca2+ signals (Figure 1) were driven by release of glutamate, either tonically or that evoked by action potential firing. We found that application of 0.5 μM tetrodotoxin (TTX), to block action potentials produced no statistically significant change in Ca2+ signal dF/F, frequency or kinetics in somata or branches (Figure 3A; average data in Supp Figure 1A,B; n = 10). Moreover, we found that spontaneous somatic and branch Ca2+ signal amplitudes, frequency and kinetics were not significantly reduced by mGluR5, mGluR2/3 or NMDA receptor antagonists (50 μM MPEP, 10 μM LY341495 and 50 μM APV, respectively; Figure 3B,C,D; Supp Figure 1C-H; n = 10 in each case). These reagents also did not markedly change the basal fluorescence of the astrocytes (Supp Fig 1I-L). However, depletion of intracellular Ca2+ stores with 20 μM cyclopiazonic acid (CPA) for 25 mins significantly (>70%) reduced the numbers of Ca2+ signals in astrocyte somata and branches, thus pointing to their intracellular origin (Figure 3E, Supp Figure 2A,B; n = 5). As expected, CPA significantly elevated the basal fluorescence of astrocytes (Supp Fig 1M). We next used IP3R2 knock-out mice (Agulhon et al., 2010; Petravicz et al., 2008) to explore the role of this channel in controlling release of Ca2+ from stores. Recalling the CPA data, we found that the IP3R2 KO mice displayed significantly reduced numbers of Ca2+ signals compared to WT littermates (by ∼60%; Figure 3F; Supp Figure 2C,D; n = 9 cells).
Figure 3. Stratum lucidum region astrocyte spontaneous Ca2+ signals are not affected when action potentials or glutamate receptors are blocked, but are markedly reduced in number when intracellular Ca2+ stores are depleted or disrupted (IP3R2 KO mice).
A. Four superimposed traces for branchlets under control conditions and then in the presence of 0.5 μM TTX, which had no effect. B-D. As in A, but for experiments when the brain slices were treated with antagonists of mGluR2/3 (10 μM LY341495), mGluR5 (50 μM MPEP) and NMDA receptors (50 μM APV). E. As in A, but for slices treated with cyclopiazonic acid (20 μM CPA) to deplete intracellular Ca2+ stores. F. Representative traces for spontaneous Ca2+ signals recorded from IP3R2 KO mice and their WT littermates. The average data are shown in Supp Fig. 1 & 2
Unexpectedly, the use of GECIs in the IP3R2 KO mice revealed residual Ca2+ signals in s.l. astrocyte somata and mainly in branches (Supp Fig 2C-D). Thus, although Ca2+ signals are reduced in number in the IP3R2 KO mice, they are not abolished when GECIs are deployed to image entire astrocyte territories. Interestingly, the residual Ca2+ signals in s.l astrocytes were not blocked by HC 030031 (Supp Fig 2E,F; n = 8), a selective antagonist for TRPA1 channels. In contrast, functional TRPA1 channels are expressed within s.r. astrocytes (Shigetomi et al., 2013b).
SPRAE mice selectively expressing a drug-activated cation channel in mossy fibers
We considered it important to selectively drive glutamate release from mossy fiber terminals in order to explore astrocyte signaling. As part of an unrelated project (Richler et al., 2008) we created BAC transgenic mice expressing fluorescently-tagged P2X2 receptors (P2X2-YC; YC is Yellow Cameleon 3.1; Supp Figure 3A-C) within mossy fibers, prompting us to characterize and exploit this novel pharmacogenetic tool. P2X2 receptors are cell surface ATPgated cation channels that can be activated by synthetic ATP congeners (e.g. ATPγS). P2X2 receptors enter presynaptic terminals and axons, where their activation increases neurotransmitter release (Khakh and North, 2012). In light of this, we dubbed the mice with mossy fiber expression as “SPRAE” mice, an acronym for Selective P2X Receptor Axoterminal Excitation mice. As reported below, their activation triggered neurotransmitter release from mossy fibers, “spraying” the s.l. region with glutamate.
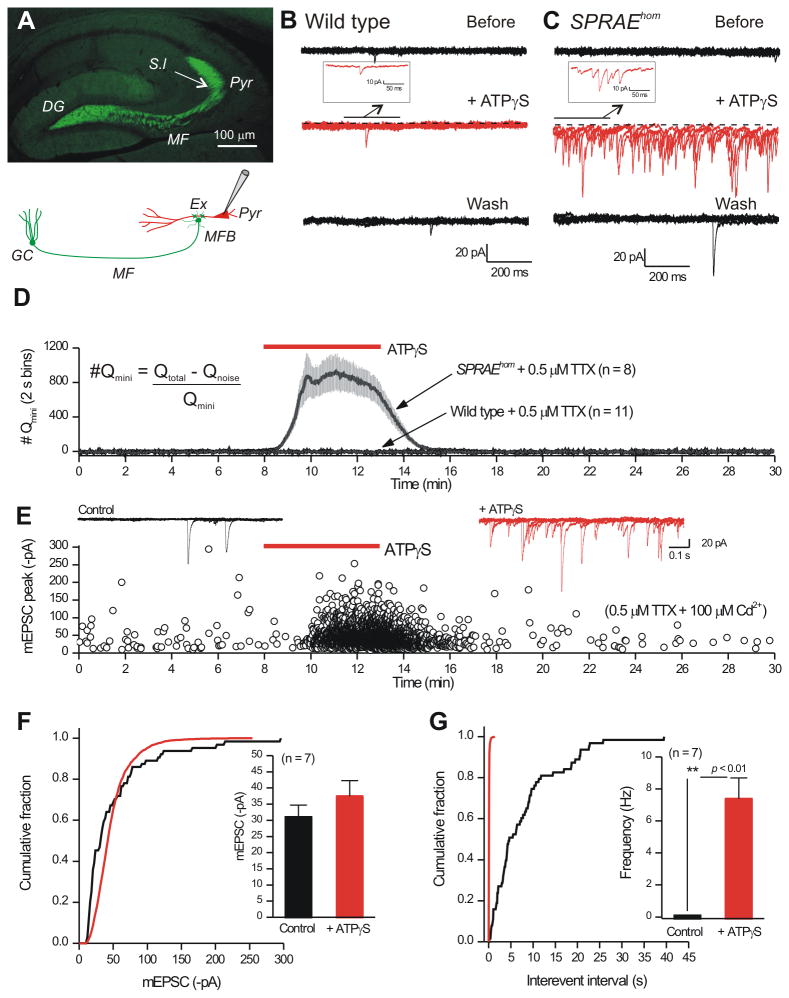
We found remarkably specific P2X2-YC expression within mossy fibers of SPRAE mice (Figure 4A), but not in WT mice (Supp Figure 3D,E; n = 5). Striking expression was observed in the mossy fiber pathway, particularly within the terminal regions located in the s.l. (Supp Figure 3G-I). We also evaluated the basic properties of granule cells from SPRAE mice in comparison to WT littermates and found no obvious differences (Supp Figure 4A-B; n = 15-31). However, despite the absence of detectable P2X2-YC immunostaining within granule cell bodies, we did detect small somatic P2X2-YC mediated currents in SPRAE mice (Supp Figure 4C,D; n = 15-23; Supp Figure 4E,F; n = 5,7). Overall, SPRAE mice display P2X2-YC expression in the mossy fibers, a feature we exploited to drive neurotransmitter release.
Figure 4. Activation of P2X2-YC channels in the mossy fiber pathway of SPRAE mice increases glutamate release onto CA3 pyramidal neurons.
A. P2X2-YC expression (green), from anti-GFP primary antibody and Alexa 488-conjugated secondary antibody, in the mossy fiber pathway. B. Five 1 s traces superimposed showing mEPSCs recorded from a CA3 pyramidal neuron from a WT mouse before, during and after 100 μM ATPγS application. C. As in B, but for recordings from SPRAEhom mice. D. Quantification of experiments such as those shown in B and C. The y-axis plots the approximate number of quanta in 2 s bins (Qmini was measured from mEPSCs before the application of ATPγS, Qnoise was measured from silent periods and Qtotal was measured in 2 s bins). E. Traces and graphs show an exemplar neuron, showing an increase in frequency in the presence of ATPγS, but no increase in amplitude. Measuring all mEPSCs in 7/11 cells showed no increase in mEPSC amplitude (F), whereas frequency was increased (G). Experiments for CA3 interneurons are reported in Supp Fig. 5. Average data are shown as mean ± S.EM.
SPRAE mice permit selective glutamate release from the mossy fiber pathway
Mossy fiber boutons form synapses with CA3 pyramidal neurons via thorny excrescence spines in the s.l. (Amaral and Lavenex, 2007; Spruston and McBain, 2007) (Figure 4A). By monitoring glutamate mediated miniature (i.e. spontaneous) excitatory postsynaptic currents (mEPSCs) onto CA3 pyramidal neurons (in 0.5 μM TTX), we found that application of drug to activate P2X2-YC channels (ATPγS; 100 μM) dramatically increased their frequency. Similar responses were never observed in WT mice (Figure 4B). The large frequency increase made it difficult to measure all mEPSCs individually (Figure 4C), prompting us to measure charge transfer (nC). We measured charge transfer (nC) in 2 s bins during 30 min recordings before, during and after drug applications for SPRAE and WT mice (Figure 4D), revealing that ATPγS robustly triggered the release of glutamate onto pyramidal neurons from SPRAE mice, but had no effect in WT mice (Figure 4D; n = 11, 8). In 7/11 cells in the presence of TTX (0.5 μM) and Cd2+ (100 μM; Supp Figure 4G; n = 11), all mEPSCs could be measured individually (in 4 cells they could not), revealing that ATPγS increased mEPSC frequency, but not amplitude (Figure 4E-G). In this experiment, the combination of TTX and Cd2+ was used to block action potential and voltage-gated Ca2+ channel dependent release.
In order to determine if mossy fiber filopodial terminals onto interneurons also express P2X2-YC in SPRAE mice, we repeated experiments shown in Figure 4 during recordings from s.l. interneurons. We found that ATPγS reliably increased glutamate release onto all interneurons examined in the SPRAE mice (Supp Figure 5; n = 5,9). We also evaluated if activation of P2X2-YC in SPRAE mice released glutamate onto areas approximating the size of s.l. astrocytes by using 50 μm diameter glutamate biosensor electrodes, and found that it did (Supp Figure 5). Thus, SPRAE mice are a robust pharmacogenetic tool to selectively evoke glutamate release from hippocampal mossy fibers.
Astrocytes display prolonged Ca2+ signals when release from mossy fibers is increased
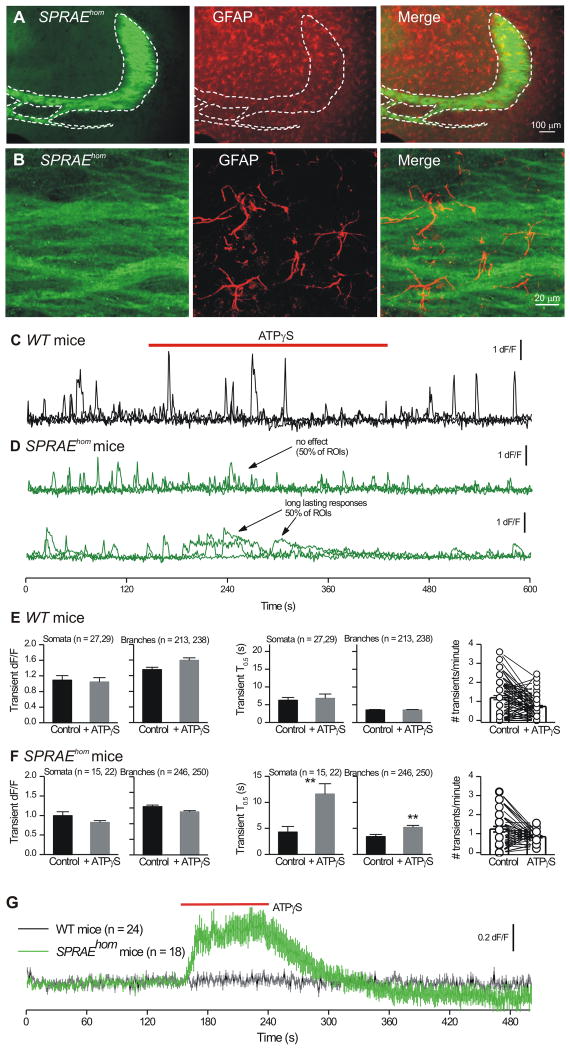
The combination of our findings with GCaMPs and SPRAE mice (Figs. 1-4) presented an opportunity to directly determine if s.l. astrocytes (Figure 5A,B) display Ca2+ signals when neurotransmitter release from mossy fibers is elevated. We microinjected AAV2/5 for GCaMP3 into WT and SPRAE mice and monitored Ca2+ signals in s.l. astrocytes before, during and after ATPγS applications to increase mossy fiber neurotransmitter release (Figure 5C,D). During control periods in both WT and SPRAE mice we detected equivalent Ca2+ signals (Figure 5E-F) and as expected for WT mice (Figure 3), ATPγS produced no effect (Figure 5C,E; n = 9 cells). If astrocytes respond to quantal-like glutamate release, we would expect to observe more or prolonged Ca2+ signals in SPRAE mice during ATPγS applications. We found that ATPγS did not significantly increase astrocyte Ca2+ signal frequency or amplitude (Figure 5D,F; n = 10). However, ATPγS resulted in prolonged Ca2+ signals in ∼50% of ROIs (Figure 5D), and this effect was statistically significant across all ROIs for SPRAE mice (Figure 5F; p < 0.01). The prolonged ATPγS-evoked events in SPRAE mice were significantly reduced in frequency in slices treated with CPA (Supp Fig 7A; n = 7), implying store-mediated Ca2+ release. Given that ATPγS in SPRAE mice reliably elevates quantal-like glutamate release onto two neuronal targets of mossy fibers and broadly into the neuropil, this result provides evidence that high rates of glutamate release from mossy fiber terminals resulted in prolonged store-mediated astrocyte Ca2+ signals, which lasted up to 13 s (Figure 5F). This could happen because astrocytes respond to ambient levels of transmitter or to more frequent quantal-like events that result in the appearance of prolonged signals. In future work, these possibilities may be fruitfully explored with kinetic modelling when all the necessary parameters are known (Rusakov et al., 2011).
Figure 5. Stratum lucidum region astrocyte Ca2+ responses during glutamate release from mossy fibers in SPRAEhom mice.
A. IHC for P2X2-YC in the mossy fibers (green) along with staining for astrocytes using GFAP (red); higher magnification views shown in panel B. C. Three representative traces superimposed for ROIs from astrocyte branches from a WT mouse before, during and after 100 μM ATPγS applications. D. As in C, but for astrocytes located in the s.l. region and imaged from SPRAEhom mice. E. Properties of astrocyte Ca2+ signals before and during ATPγS applications in WT mice. F. Properties of astrocyte Ca2+ signals before and during ATPγS applications in SPRAE mice. However, there was no significant difference in the duration of the spontaneous Ca2+ transients in WT and SPRAE mice before ATPγS. For somata, in WT mice their T0.5 was 6.2 ± 0.9 s and in SPRAE mice the T0.5 was 4.3 ± 1.1 s (n = 27 and 15; p = 0.16 with an unpaired t test). For branches, in WT mice their T0.5 was 3.5 ± 0.2 s and in SPRAE mice the T0.5 was 3.4 ± 0.4 s (n = 213 and 246; p = 0.83 with an unpaired t test). G. S.l. region astrocyte cell surface glutamate imaging with iGluSnFR in WT and SPRAEhom mice. Average data are shown as mean ± S.EM.
Astrocyte cell surface glutamate imaging with iGluSnFR in SPRAE mice
Increasing neurotransmitter release from mossy fiber terminals caused prolonged Ca2+ signals in astrocyte somata and branches (Figure 5). To explore this, we used a genetically encoded glutamate sensor (Marvin et al., 2013) called iGluSnFR, expressed within astrocytes. The glutamate affinity of iGluSnFR at ∼5 μM (Supp Figure 8A-C; n = 9) is in the range of the EC50 values for mGluRs and therefore it is a good indicator of whether or not mGluRs would be activated by the concentration of glutamate that reaches the astrocyte membrane. We made several observations with iGluSnFR. First, it was robustly expressed on the surface of astrocytes in the s.l. (Supp Figure 8D). Second, we did not detect spontaneous iGluSnFR fluorescence increases in astrocytes from WT or SPRAE mice (Supp Figure 8E-G; n = 24, 18), extending data in Figure 3. Third, in every astrocyte examined in SPRAE mice, we measured robust increases in iGluSnFR fluorescence over astrocyte territories in response to ATPγS applications (Figure 5G; Supp Figure 8E-G; n = 18). Similar responses were never observed in WT mice (Figure 5G; Supp Figure 8E-G; n = 24). These data provide direct evidence that micromolar glutamate is released from the mossy fibers onto astrocytes. Glutamate release is expected to be pulsatile from the mossy fibers themselves, but is it ambient or pulsatile in relation to much larger astrocyte territories that are distanced from release sites? Our data with biosensor electrodes show that glutamate levels are elevated within large areas of neuropil equivalent to whole astrocytes in SPRAE mice during ATPγS applications (Supp Figure 6). In accord, our modeling of the kinetics of iGluSnFR also indicate that the baseline increase shown in Figure 9C represents an ambient increase in glutamate (Supplementary note 1).
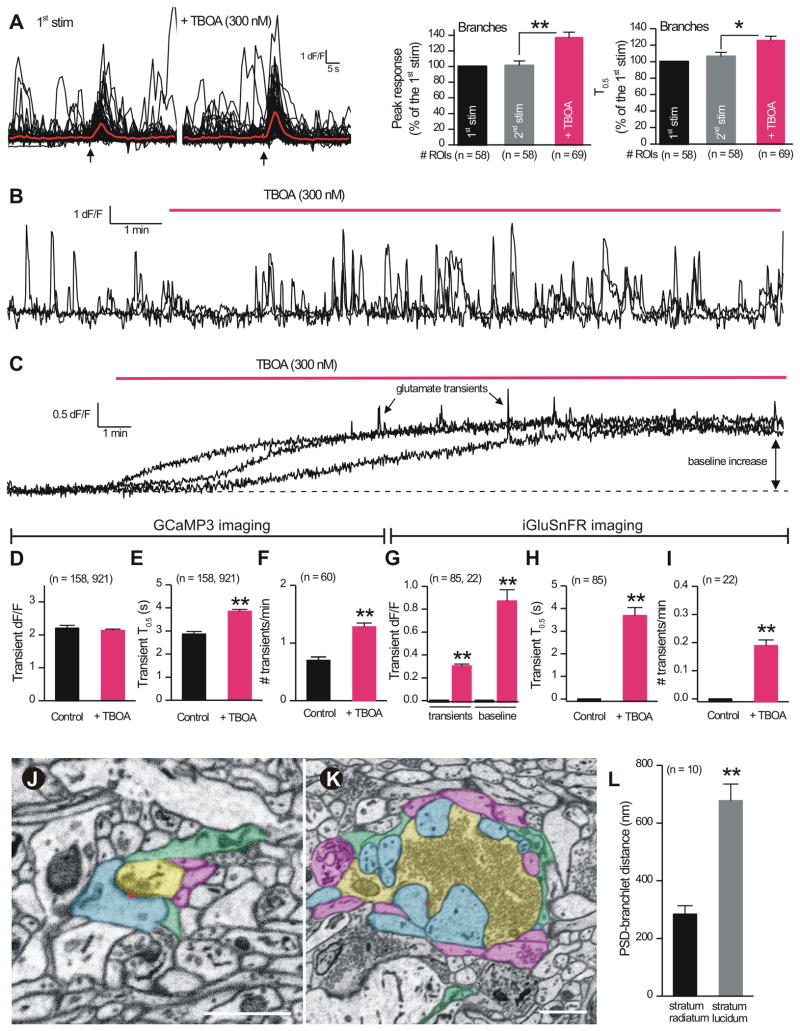
Figure 9. Glutamate clearance regulates EFS-evoked and spontaneous Ca2+ signals in astrocytes.
A. Traces for EFS-evoked astrocyte Ca2+ signals in branches before and during applications of TBOA (0.3 μM). The bar graphs to the right show average data, indicating that TBOA increased and prolonged EFS-evoked signals. B. Traces for spontaneous Ca2+ signals in astrocyte branches before and during TBOA. C. As in B, but for iGluSnFR glutamate signals. Note in the presence of TBOA the baseline increases and iGluSnFR transients are observed. D-F. Quantification of experiments such as those shown in B. G-I. Bar graphs show quantification of experiments such as those shown in C. J-K. Images of the arrangement of cellular elements around boutons located in the s.r. (J) and s.l. (K). Postsynaptic spines (blue) form a larger, more complex synaptic complex with boutons (yellow) in the stratum lucidum. Red dots indicate location of a PSD. Astrocyte processes (green) are generally more distal from PSDs in stratum lucidum than in stratum radiatum. Neighboring axons not forming boutons also surround synapse in both areas (purple). Scale bars = 1 micron. L. Graph summarizes the shortest distances from a PSD to an astrocyte branchlet for the stratum radiatum and stratum lucidum. Average data are shown as mean ± S.EM.
Astrocytes display Ca2+ signaling during electrical field stimulation (EFS)
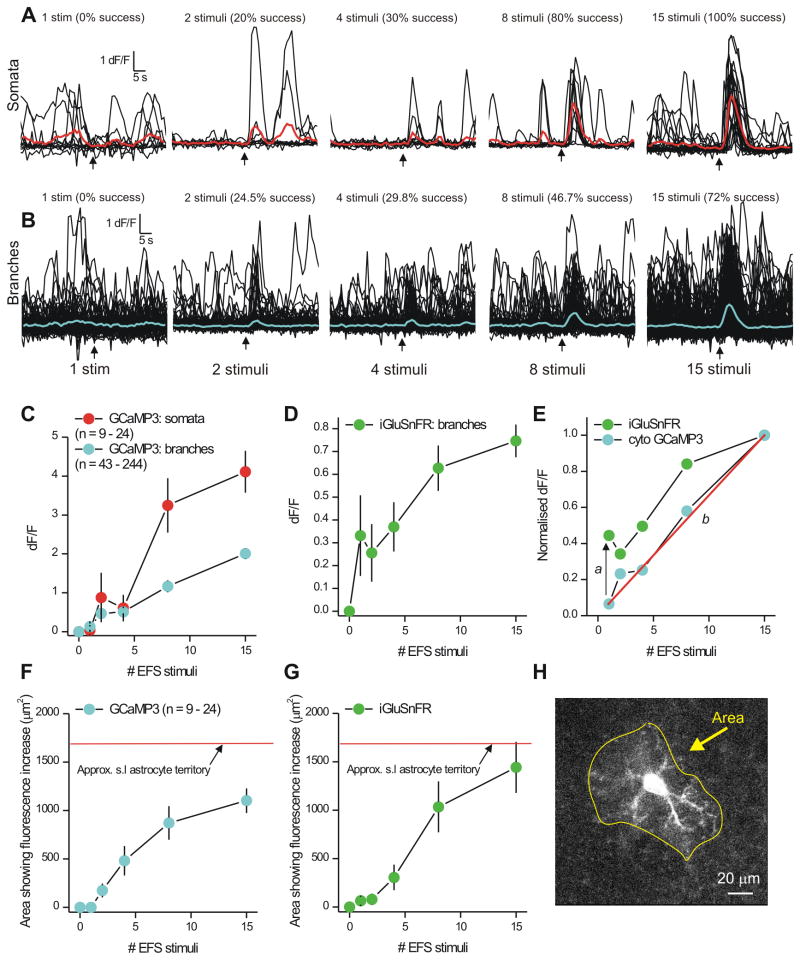
To explore neurotransmitter mechanisms, we used local electrical field stimulation (EFS) with a glass microelectrode to evoke APs in the mossy fibers while imaging s.l. astrocytes. We found that single stimuli failed to evoke GCaMP3-observed Ca2+ signals in astrocyte branches or somata (Figure 6A-C; n = 9-24) and that two stimuli were the minimum required to observe significant Ca2+ signals (Figure 6A,B; n = 9-24). Robust responses were seen for 8 stimuli and the relationship between stimulus number and somatic Ca2+ signals levelled out at 15 stimuli (Figure 6C; n = 9-24). By analysing data for somata and branches separately, we found that the largest Ca2+ signals were seen for somata, and that the relationship between EFS and branch Ca2+ signals was approximately linear (Figure 6C; n = 9-24). This linearity was due to an EFS-dependent increase in the occurrence of Ca2+ signals, which we measured as success rate (in % in Fig 6A,B). To determine if one stimulus did in fact release glutamate onto astrocyte branches, we performed parallel experiments with iGluSnFR. We found that the relationship between astrocyte iGluSnFR signals and EFS was different to that observed for Ca2+ signals measured with GCaMP3 (Figure 6D,E; n = 6). First, one stimulus was sufficient to evoke iGluSnFR signals, but not Ca2+ signals, which required at least two stimuli (a in Figure 6E). Second, the relationship between stimulus number and Ca2+ signals was approximately linear (b in Figure 6E). Third, during brief trains when astrocytes responded reliably to local EFS with iGluSnFR and Ca2+ signals, they did so quite globally covering large areas of the astrocyte territory (e.g. 8 stimuli; Figure 6F-H).
Figure 6. EFS evokes Ca2+ signaling in astrocytes during bursts of stimuli.
A. Traces from astrocyte somata located in the s.l. region during local EFS of the mossy fiber pathway. The red traces are averages of the individual black traces. Signals were seen only for greater than two stimuli. B. As in A, but for traces from branch ROIs; blue is an average of the individual black traces. The numbers above each set of traces indicate the success rate for each stimulus. C. Summary graph from experiments such as those shown in A and B. D. The graph shows the increase in cell surface iGluSnFR fluorescence as a function of the number of local stimuli delivered to the mossy fiber terminals with EFS. E. Plots the branchlet signals for Ca2+ and iGluSnFR: note the two plots do not overlap. In this plot, a indicates a threshold shift, b indicates that the relationship between Ca2+ signals in astrocyte branches and the number of stimuli was approximately linear. F. Plots the area of the astrocyte Ca2+ signals as a function of EFS stimuli and in relation to the territory of an s.l. astrocyte (Fig. 1C). G. As in F, but for iGluSnFR signals. H. Image of an s.l. astrocyte expressing GCaMP3 taken at the peak of 15 stimuli, showing Ca2+ elevation in most of its territory. Average data are shown as mean ± S.EM.
We also performed a series of experiments to compare astrocyte Ca2+ responses to 1 and 15 EFS (Figure 7). Thus we found that astrocytes from IP3R2 KO mice expressing GCaMP3 failed to respond to 1 or 15 EFS (Figure A,E; n = 9). Moreover, even with GCaMP6f we failed to detect astrocyte responses to 1 EFS, whereas 15 EFS worked reliably (Figure 7C, E; n = 9). We next used membrane targeted Lck-GCaMP3 to explore the possibility that cytosolic GCaMP3 may have missed signals in near membrane regions of processes. We found no evidence for any Lck-GCaMP3-detectable Ca2+ signals as a result of 1 EFS (Figure 7D, E; n = 11). Interestingly, using Lck-GCaMP3 we found that astrocyte reliability to 15 EFS was significantly reduced from 100% with GCaMP3 (Figure 6A, 6B) to 27% with Lck-GCaMP3 (Figure 7D; n = 3 of 11 cells; p < 0.01 using unpaired Fisher's Exact test). These data are consistent with those reported earlier (Supp Table 1) and indicate that the majority of the spontaneous and EFS-evoked Ca2+ signals in s.l. astrocytes are mediated by intracellular stores. Thus overall, a variety of approaches (Figs 6-7) show that astrocyte branches “see” glutamate released following one EFS, but that they do not detectably respond with an elevation of Ca2+ until bursts of stimuli are used. The astrocyte Ca2+ response to a single stimulus is undetectable with the full range of the methods that we employed or it does not exist.
Figure 7. Evaluations of astrocyte responses to 1 and 15 stimuli in IP3R2 KO mice and with various GECIs.
A-D. Ca2+ imaging traces for somata and branches before, during and after EFS with 1 or 15 stimuli under the various conditions indicated. In the case of IP3R2 KO mice, comparative measurements were made with WT littermates (B). E. Bar graphs summarize average data from the experiments shown in A-D. Average data are shown as mean ± S.EM.
Astrocyte Ca2+ signaling during action potential bursts is due to glutamate and GABA
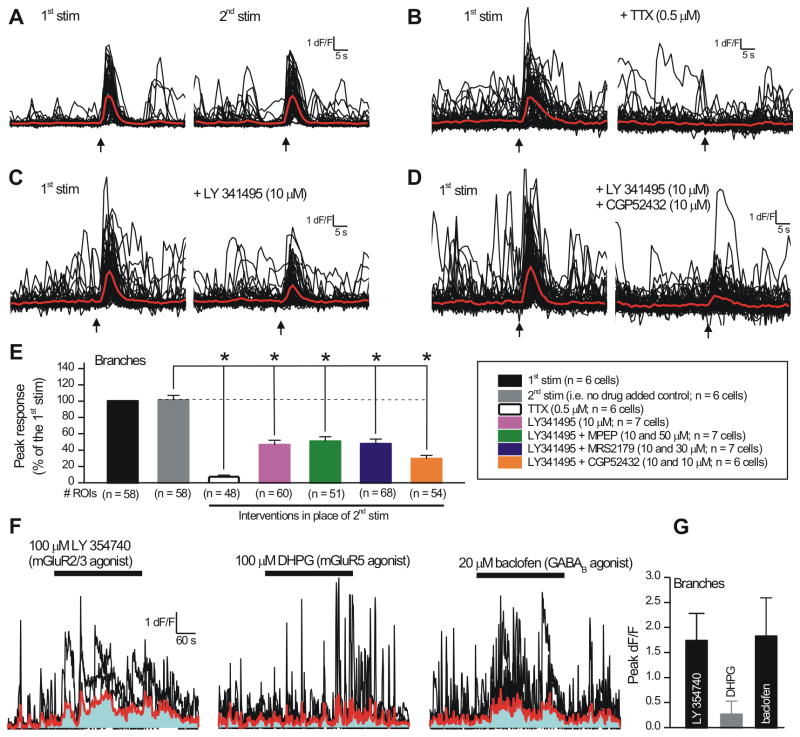
Having established that bursts of 8 and 15 stimuli evoke robust astrocyte GCaMP3-observed Ca2+ signals, we next explored the underlying neurotransmitter mechanisms by focussing on the branches, i.e. the sites of interaction with mossy fiber terminals. We used a two pulse protocol, whereby 8 or 15 stimuli were applied twice to the mossy fibers, 8 min apart. This gave reproducible responses (Figure 8A,E; n = 6) and inter-pulse application of TTX (0.5 μM) abolished the second response (Figure 8B,E; n = 6). Because mossy fibers release glutamate onto astrocytes (Figure 6D,G) and because mature astrocytes express mGluR2/3 receptors (Sun et al., 2013), we began by applying the mGluR2/3 antagonist LY341495 (10 μM) and found that it significantly reduced EFS-evoked Ca2+ responses in astrocyte branches (Figure 8C,E; n = 7). However, even in the presence of LY341495, a residual EFS-evoked response persisted and was larger than that in the presence of TTX (p < 0.05 using Dunnett's ANOVA). This prompted us to explore a role for mGluR5 receptors: we found that a combination of LY341495 (10 μM) and MPEP (50 μM) did not reduce the responses any more than LY341495 alone (Figure 8E; n = 7). Taken together these data suggest that glutamate release from mossy fibers evokes Ca2+ signals in astrocyte branches; ∼50% are controlled via mGluR2/3 (Sun et al., 2013), with mGluR5 having a negligible contribution.
Figure 8. Electrical field stimulation evokes Ca2+ signals in astrocytes that are mediated by glutamate and GABA.
A.Traces show the protocol: two local EFS stimuli were delivered to the mossy fiber terminals 8 min apart. B. Application of TTX before the second EFS stimulation abolished the astrocyte Ca2+ signals. C. As in B, but for applications of the mGluR2/3 receptor antagonist LY341495. D. As in B, but for applications of the mGluR2/3 and GABAB receptor antagonists together (LY341495 and CGP52432). E. Summary bar graph for astrocyte branches from experiments such as those shown in A-D, and for additional evaluations as indicated. The differences were analyzed using a Dunnett's ANOVA test, whereby all the treatments were compared to the control 2nd stimulation response. F. Representative traces (black) and average data (red) for agonist evoked Ca2+ signals in astrocyte branches. The area under the curve is shown in blue. G. Summary data for experiments such as those in F. Average data are shown as mean ± S.EM.
A variety of approaches show that mossy fibers also contain the molecular machinery to release GABA (Caiati, 2013; Gutiérrez, 2005; Walker et al., 2002), and ATP is thought to be released from nerve terminals in many CNS areas. In light of these facts, we evaluated if the residual response in the presence of LY341495 (Figure 8C,E) was mediated by P2Y or GABAB receptors that are known to elevate astrocyte Ca2+ signals in other areas of the hippocampus. We found that application of the P2Y1 antagonist MRS2179 (30 μM) was without effect (Figure 8E; n = 7). However, we found that application of the GABAB receptor antagonist CGP52432 (10 μM) together with LY341495 reduced the EFS-evoked responses to levels below those observed with LY341495 alone (Figure 8D,E; n = 7). This was more clearly seen from ANOVA tests; in the presence of CGP52432 and LY341495, the EFS-evoked response was not statistically different to that in the presence of TTX (p > 0.05, Dunnett's ANOVA). Overall, these data indicate that astrocytes respond to glutamate and GABA release from mossy fiber terminals via mGluR2/3 and GABAB receptors, revealing a hitherto unknown potential physiological role for mossy fiber GABA release within this circuit. Consistent with this result, mGluR2/3 and GABAB receptor agonists elevated Ca2+ levels in s.l. astrocytes branches (in TTX), whereas an agonist of mGluR5 receptors did not (Figure 8F,G). Finally, in the SPRAE mice a combination of CGP52432 and LY341495 also significantly reduced the number, peak amplitude and duration of prolonged Ca2+ signals triggered by ATPγS applications (Supp Figure 7B; n = 6).
Astrocyte Ca2+ signaling in the mossy fiber pathway is gated by glutamate clearance
Hippocampal astrocytes express high levels of GLT-1 and lower levels of GLAST glutamate transporters (Regan et al., 2007; Rothstein et al., 1996). In order to explore roles for glutamate clearance we applied the specific GLT-1/GLAST blocker TFB-TBOA (Huang et al., 2004) (300 nM) and found that it significantly increased EFS-evoked Ca2+ signals and duration in astrocyte branches (Figure 9A). These data provide further evidence that EFS-evoked astrocyte Ca2+ signals are mediated by glutamate (Figure 8) acting via mGluR2/3 receptors in astrocyte branches that appear not to be saturated. To further explore astrocyte engagement in the mossy fiber circuit, and its relation to glutamate uptake (Huang et al., 2004), we applied TBOA and monitored spontaneous Ca2+ signals in astrocyte branches, as well as astrocyte cell-surface glutamate signals with iGluSnFR (Figure 9). To our surprise, in the case of Ca2+ signals, we found that TBOA significantly increased their duration and frequency (Figure 9B, D-F). TBOA also significantly increased the baseline iGluSnFR fluorescence of astrocytes, implying increased glutamate in the neuropil (Figure 9C, G), and it caused the appearance of brief pulsatile glutamate transients that could be easily observed with iGluSnFR (Figure 9C, 9G-I). Given that glutamate release does not contribute to spontaneous astrocyte Ca2+ or iGluSnFR signals (Figs. 3 & 5), these data in the presence of TBOA provide compelling evidence that astrocyte engagement within the mossy fiber pathway is tightly gated by glutamate transporters.
We next used electron microscopy to explore proximity between astrocyte branches and mossy fiber synapses. We compared proximity between astrocytes and post-synaptic densities in the s.l. and at classical synaptic spines in the s.r. We observed that the relationship between postsynaptic densities and astrocyte branches was different for these two regions of the hippocampus. For the s.l. region, astrocyte branchlets surrounded the body of the large mossy fiber terminals and were peripherally located in relation to those for s.r. synapses (Figure 8J,K). We found that the shortest distance between a PSD and the nearest branchlet was twice as long for the s.l. as compared to the s.r. (Figure 9L; n = 10, p < 0.05). In the simplest interpretation that is also supported by our physiological measurements (Figures. 2-9), astrocyte branchlets are located peripherally at mossy fiber synapses (Figure 9K,L) and are perhaps too distanced to detect transmitter release resulting from single stimuli. Our electron microscopy recalls past work (Rollenhagen and Lübke, 2006; Rollenhagen et al., 2007; Wilke et al., 2013).
Discussion
We explored when and how astrocytes display Ca2+ signaling using novel optical and genetic tools in a mature model circuit with well defined anatomy. We made several observations that may be portentous of circuits in general and that contribute to our understanding of astrocyte signaling in the mossy fiber pathway.
New insights and their relation to past work
First, as far as we know, our study is the first exploration of astrocyte Ca2+ signaling in the mossy fiber pathway, which is a crucial limb of the hippocampal trisynaptic circuit. Our data form the basis to explore how astrocytes are engaged within, and contribute to, the function of the hippocampal circuit. As such, our work extends insights from the dentate gyrus (Di Castro et al., 2011) and s.r. regions (Panatier et al., 2011). In particular the similarities and differences are important to note because they may be physiologically relevant to each hippocampal region.
Second, s.l. region astrocyte Ca2+ signals can be studied using AAV2/5 viruses to express GCaMP3, which is an excellent GECI for studying astrocyte intracellular signals (Shigetomi et al., 2013a). Past experiments have reported Ca2+ signals in thick astrocyte branches a few micrometers from the soma in the dentate gyrus and s.r. (Di Castro et al., 2011; Panatier et al., 2011). However, by using GECIs we observed a panorama of spontaneous and localized Ca2+ signals within almost entire astrocyte territories.
Third, astrocyte spontaneous Ca2+ signals were not driven by action potential firing or endogenous glutamate release, which is different to astrocytes in the dentate gyrus and s.r. (Di Castro et al., 2011; Panatier et al., 2011). We also did not detect spontaneous glutamate release onto astrocytes using iGluSnFR (Marvin et al., 2013), although we could readily measure neuronal evoked glutamate release onto astrocytes. These observations are consistent with the fact that granule cells have strongly negative resting membrane potentials and low firing rates at rest (Ruiz and Kullmann, 2012). Moreover, these data also imply that glutamate release from s.l. astrocytes themselves is minimal.
Fourth, using transgenic SPRAE mice that allow the selective increase of neurotransmitter release from the mossy fibers, we found that astrocytes responded with prolonged Ca2+ signals that were significantly reduced by antagonists of mGluR2/3 and GABAB receptors.
Fifth, astrocytes responded to synaptic glutamate release during electrical stimulation of the mossy fibers, but only during bursts of stimuli. Under these circumstances (i.e. bursts) astrocyte Ca2+ signals covered large territory areas. This is different to astrocytes in the dentate gyrus and s.r. where astrocytes respond very locally to sparse action potentials and possibly even single vesicles (Di Castro et al., 2011; Panatier et al., 2011).
Sixth, astrocyte Ca2+ responses evoked by electrical stimulation were due to glutamate acting mainly via mGluR2/3 receptors and not via mGluR5 receptors as in the s.r. of younger rats (Panatier et al., 2011). Our data are consistent with recent realizations that mGluR5 receptors are not expressed in adult astrocytes (Sun et al., 2013). Interestingly, a small component of the electrically-evoked response was mediated by GABA acting at GABAB receptors. This is relevant in the context of the mossy fiber pathway, because GABA is released from the mossy fibers (Caiati, 2013; Walker et al., 2002) and astrocytes express Ca2+ mobilizing GABAB receptors (Kang et al., 1998).
Seventh, astrocyte Ca2+ signaling was tightly gated by glutamate clearance. Thus, when glutamate uptake was blocked we observed enhanced spontaneous Ca2+ signals, enhanced iGluSnFR glutamate signals onto astrocytes, and elevated electrically evoked astrocyte responses. Our data do not simply show that glutamate transporters regulate spontaneous Ca2+ signals, rather they unexpectedly show that glutamate transporters gate the engagement of astrocytes and spontaneous Ca2+ signals. Glutamate transporters were not considered in past work (Di Castro et al., 2011; Panatier et al., 2011).
Eighth, by monitoring astrocyte Ca2+ signaling and glutamate signals during electrical stimulation of the mossy fibers, we found that astrocytes sense glutamate reliably even during a single stimulus to the mossy fibers, but translate this to Ca2+ signaling less efficiently. Moreover, when Ca2+ signaling is observed, it increases over entire territories.
Ninth, a study on the adult cortex showed that astrocyte Ca2+ responses to whisker stimulation were partly mediated by synaptic release of glutamate acting on astrocyte mGluR5 receptors (Wang et al., 2006), whereas a recent study concluded that glutamatergic signaling is insufficient to trigger astrocyte Ca2+ signaling (Sun et al., 2013). Our data extend these findings by showing that s.l. astrocytes respond to synaptic glutamate release during bursts of activity or during high amounts of quantal like release in SPRAE mice (via mGluR2/3 and GABAB receptors). The use of GECIs may permit similar studies of other brain regions and prepare us for the possibility that astrocytes may have microcircuit specific properties, emphasizing the need for caution in generalizing from one brain region to another.
Tenth, and more broadly for evaluations of astrocytes throughout the brain, our studies provide a tool kit that is useful to explore physiological astrocyte responses in detail. As recently discussed (Li et al., 2013), these tools do not replace organic Ca2+ indicator dyes, but are demonstrably better for exploring astrocyte territories and branches.
Ca2+ signal properties and kinetics
Using GCaMP3 imaging we found evidence for numerous spontaneous Ca2+ signals throughout astrocytes. By pooling data across identical control conditions, our summary data show that Ca2+ signals in somata and branches display T0.5 values of 4.4 ± 0.16 and 3.1 ± 0.04 s, respectively when imaged by GCaMP3 (329 and 3757 events from 65 cells). Ca2+ signals also lasted seconds (∼3 s) when detected by GCaMP6f and we found no evidence for faster Ca2+ signals. Interestingly, we observed fewer signals with Lck-GCaMP3, implying that Ca2+ signals in s.l. astrocytes are mainly intracellular in origin. In accord, the signals were significantly reduced in mice that lacked IP3R2s. Thus differences exist between s.l. and s.r. astrocytes (Shigetomi et al., 2013a; Shigetomi et al., 2013b), likely reflecting the existence of astrocyte heterogeneity in different hippocampal regions. Irrespectively, all the spontaneous signals we observed in s.l. astrocytes were slow, revealing fundamental constraints on how quickly astrocytes can track to neuronal input in this pathway. Moreover, our data showed that a statistically insignificant number of astrocyte spontaneous Ca2+ signals were due to action potential firing or endogenous glutamate release, which is in accord with the known electrical properties of the mossy fiber pathway (Ruiz and Kullmann, 2012).
A recent proposal is that astrocytes respond to and regulate single synapses in the dentate gyrus and s.r. (Di Castro et al., 2011; Panatier et al., 2011). A key line of evidence was the finding that astrocytes displayed localised Ca2+ signals when quantal release probability was elevated using high osmotic strength sucrose (Di Castro et al., 2011), implying that astrocytes respond during quantal-like glutamate release. Cognizant of these findings and their implications for the function of microcircuits, we took advantage of SPRAE mice generated in our laboratory. Activation of P2X2-YC channels with drug increased mEPSCs onto all CA3 pyramidal neurons, all s.l. interneurons, broadly into the s.l. neuropil, and resulted in prolonged astrocyte Ca2+ signals in SPRAE mice. The glutamate released from mossy fibers readily reached astrocyte branches, because we could detect it using iGluSnFR where it increased the duration of Ca2+ signals. More generally, SPRAE mice may be useful in future work to explore outstanding questions in mossy fiber physiology (Ruiz and Kullmann, 2012).
Can the kinetics of the Ca2+ signals we measured with GCaMP3, GCaMP6f and Lck-GCaMP3 be directly compared to the kinetics of signals measured with organic Ca2+ dyes in other parts of the hippocampus (Di Castro et al., 2011; Panatier et al., 2011)? Although the affinities of the indicators employed are similar, we still suggest caution in this regard because it is well established that the same Ca2+ dynamics will be reported differently by different Ca2+ indicators depending on their concentration and affinity. Hence, kinetic modeling is needed to explore precisely how astrocyte Ca2+ signals appear as observables with distinct imaging approaches. As recently highlighted, the field of astrocyte biophysics lags behind similar studies of neurons by decades (Rusakov et al., 2011). From this perspective, our work lays the foundations for future experimental and modeling work.
Evoked responses and tight gating of astrocyte engagement by glutamate transporters
Using EFS of mossy fibers, we found that s.l. region astrocytes responded to bursts of stimuli rather than a single stimulus when we used GCaMP3 or GCaMP6f. This is reminiscent of past work (D'Ascenzo et al., 2007; Gordon et al., 2009). S.l. astrocytes responded unreliably to all examined forms of EFS when imaged using Lck-GCaMP3, which supports the view that the Ca2+ signals have an intracellular origin. Our use of iGluSnFR directly showed that single stimuli to the mossy fibers release sufficient amounts of glutamate to reach astrocyte branches, implying that s.l. astrocytes are ineffective postsynaptic responders to sparse action potentials. Moreover, with bursts of stimuli, astrocytes appear to respond proportionately to the number of stimuli applied to the mossy fibers, with increases in Ca2+ that covered entire territories and large areas of neuropil. This may be a powerful entrainment mechanism to control release of substances from astrocytes. On the basis of these data, we conclude that the physiological role of astrocyte Ca2+ signaling in the s.l. region is to respond to bursts of activity and functionally segregate large areas of neuropil over a time course of seconds. We suggest that the most likely consequence for neuronal circuit activity is increased local blood flow (Attwell et al., 2010), homeostatic regulation via release of slow neuromodulators such as adenosine, D-serine and ions and perhaps release of synaptogenic factors broadly in the vicinity of synapses that need them. In future work, these possibilities need to be systematically and painstakingly explored, but most usefully once methods to selectivity/completely block and precisely mimic physiological astrocyte Ca2+ signals have been developed.
The receptors on s.l. region astrocyte branches that respond to neurotransmitter release from mossy fibers appear to be mGluR2/3 and GABAB receptors. Although mGluR2/3 and GABAB are coupled to Gi/o G-proteins, the βγ-subunits mediate Ca2+ release from intracellular stores by activating phospholipase C directly or by interaction with IP3 receptors (Zeng et al., 2003). Interestingly, a previous study employing mGluR2/3 receptor agonists to study cortical astrocytes using bulk loading of Ca2+ indicator dyes was not able to evaluate Ca2+ signals in branches (Sun et al., 2013). Using GCaMP3 we were able to monitor astrocyte branches directly in adult brain slices and show that mGluR2/3 receptors mediate neuron-astrocyte functional interactions on the time scale of seconds and on distance scales of whole astrocyte territories (∼1500 μm2), implying astrocytes do not act as sensors of single synapses or sparse activity.
Finally, our data show that spontaneous and evoked astrocyte Ca2+ signaling is tightly gated by glutamate uptake via GLT-1 and GLAST glutamate transporters (Rothstein et al., 1996). Glutamate uptake may be expected to regulate astrocyte Ca2+ signals, but our data unexpectedly show that the function of glutamate transporters acts as a gate for spontaneous Ca2+ signals. Given that glutamate uptake is compromised in many brain disorders (Kanai et al., 2013), our data suggest that the contribution of glutamate-mediated astrocyte Ca2+ signaling for the function of neuronal circuits is likely to be particularly manifest during disease (Agulhon et al., 2012). This realisation also suggests an explanation for why abolishing astrocyte intracellular store mediated Ca2+ signals was without any major consequence in healthy mice. If so, astrocyte roles need to be explored in disease models (Agulhon et al., 2012) and astrocytes may represent targets for therapeutic development to treat neurological and psychiatric disorders.
Experimental procedures
Molecular biology and adenovirus (AAV 2/5) generation
Viruses were made as described (Shigetomi et al., 2013a). Our virus constructs have been deposited at Addgene in the Khakh lab repository (http://www.addgene.org/Baljit_Khakh). The AAVs are also available from the UPenn Vector Core (http://www.med.upenn.edu/gtp/vectorcore).
Surgery and in vivo microinjections of AAV 2/5
Postnatal day 56 to 63 (P56-P63) male and female C57BL/6 or SPRAE mice were used in all experiments in accordance with institutional guidelines. All surgical procedures were conducted under general anesthesia using continuous isoflurane (induction at 5%, maintenance at 1–2.5% vol/vol). Following induction of anesthesia, the mice were fitted into a stereotaxic frame with their heads secured by blunt ear bars and their noses placed into an anesthesia and ventilation system (David Kopf Instruments, Tujunga CA). Mice were administered 0.05 ml of buprenorphine (Buprenex®, 0.1 mg/ml) subcutaneously prior to surgery. The surgical incision site was then cleaned 3 times with 10% povidone iodine and 70% ethanol. Skin incisions were made, followed by craniotomies of 2-3 mm in diameter above the left parietal cortex using a small steel burr (Fine Science Tools) powered by a high-speed drill (K.1070, Foredom). Saline (0.9%) was applied onto the skull to reduce heating caused by drilling. Unilateral viral injections were carried out by using a stereotaxic apparatus (David Kopf Instruments) to guide the placement of bevelled glass pipettes (1B100-4, World Precision Instruments) into the left hippocampus (2 mm posterior to bregma, 2 mm lateral to midline, 2.2 mm from the pial surface). Either 2 μl of AAV2/5 gfaABC1D Lck-GCaMP3 (1.2 × 1013 gc/ml), 2 μl of AAV2/5 GfaABC1D GluSnFr (4.2 × 1012 gc/ml), 1.5μl of AAV2/5 gfaABC1D Lck-GFP (2.41 × 1013 gc/ml), 1.5 μl of AAV5 gfaABC1D GCaMP6f (2.4 × 1013 gc/ml) or 1.5 μl of AAV2/5 gfaABC1D GCaMP3 (1.5 × 1013 gc/ml) was injected by using a syringe pump (Pump11 PicoPlus Elite, Harvard Apparatus). Glass pipettes were left in place for at least 10 mins. Surgical wounds were closed with single external 6-0 nylon sutures. Following surgery, animals were allowed to recover overnight in cages placed partially on a low-voltage heating pad. Buprenorphine was administered 2 times per day for up to 2 days after surgery. In addition, Trimethoprim / Sulfamethoxazole (40 and 200 mg, respectively per 500 ml water) was dispensed in the drinking water for one week. Mice were sacrificed 14-20 days post-surgery for imaging (typically 14-16 days).
Mice
The generation of SPRAE mice is described in detail in the Supplementary information. IP3R2 KO mice were obtained from Dr. Ju Chen at UCSD and maintained as a heterozygous line (Li et al., 2005). Homozygotes and WT littermates were used for experiments when they reached age P56-80.
Preparation of brain slices and Ca2+ imaging
Coronal slices of hippocampus (300 μm) were cut in solution comprising (mM): 87 NaCl, 25 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 25 D-glucose, 75 sucrose, 7 MgCl2 and 0.5 CaCl2 saturated with 95% O2 and 5% CO2 (pH 7.4). Slices were incubated at ∼34°C for 30 min and subsequently stored at room temperature in artificial cerebrospinal fluid (aCSF) comprising (mM): 126 NaCl, 2.5 KCl, 1.3 MgCl2, 10 D-glucose, 2.4 CaCl2, 1.24 NaH2PO4, and 26 NaHCO3 saturated with 95% O2 and 5% CO2 (pH 7.4). All other slice procedures were exactly as described (Shigetomi et al., 2008). All our imaging was performed using commercially available confocal microscopes. In brief, cells were imaged using an Olympus Fluoview 300 confocal microscope with a 40X water immersion objective lens with a numerical aperture of 0.8 or with the Olympus Fluoview 1000 confocal microscope using the same lens. We used the 488 nm line of an Argon laser, with the intensity adjusted to 5-10% of the maximum output, which was 16.9 mW in the case of the Fluoview 300 and 10 mW in the case of the Fluoview 1000. The emitted light pathway consisted of an emission high pass filter (>510 nm) before the photomultiplier tube. Framescans were recorded at 1 frame/second and linescans at 200 Hz. For electrical stimulation a glass pipette (1B 150-4, WPI) with a tip resistance of 3-5 MΩ was filled with a solution comprising (mM): 130 NaCl, 30 HEPES, 5 Glucose, adjusted to pH 7.3 with NaOH and connected to a Grass S88 Stimulator via the Stimulus Isolator A360 (WPI). The tip of the electrode was placed in the mossy fiber pathway at 30-80 μm (usually 40-50 μm) distance from the astrocyte. Individual pulses were 1 ms in duration and stimuli were delivered at 40 μA with 1, 2, 4, 8 or 15 pulses per second at a rate of 15 Hz. All imaging experiments with SPRAE mice were performed in the presence of TTX (0.5 μM) as well as MR2179 (30 μM) and DPCPX (10 μM) to block P2Y1 and adenosine A1 receptors, respectively on astrocytes (Supp. Figure 4H).
Electron microscopy
Serial block-face scanning electron microscope (SBEM) volumes of mouse CA3 stratum lucidum and CA1 stratum radiatum were used for analyzing astrocyte-PSD distances. The original data set was collected as part of a recent study (Wilke et al., 2013), but reanalyzed here to specifically measure the distances. Briefly, a mouse (P14) was perfused with 2.5% glutaraldehyde / 2.0% paraformaldehyde in cacodylate buffer and the tissue was sectioned and stained for SBEM imaging as previously described (Deerinck et al., 2010). The IMOD software package was used to perform analysis of astrocyte branchlet-to-PSD distances (Kremer et al., 1996). For both stratum lucidum and stratum radiatum, PSDs were initially marked with a point at their center. All astrocytic branchlets in the surrounding neuropil were manually segmented to generate 3D surfaces. The IMOD tool mtk was used to measure the distance from the PSD to each astrocyte surface.
Supplementary Material
Highlights.
Astrocyte branches/territories were studied using optical and pharmacogenetic tools
Spontaneous astrocyte Ca2+ signals were not due to action potentials or glutamate
Evoked astrocyte Ca2+ signals were slow, territorial and triggered by spike trains
Astrocyte Ca2+ signaling was tightly gated by neurotransmitter clearance
Acknowledgments
Most of this work was supported by NIH grant NS060677 and partly by NIH grants MH099559 and MH104069 (BSK). O J-W was partly supported by T32 NS007101. X.W.Y. was supported by the NIH grants NS049501 and NS074312. TS, MHE and EAB were supported by an award from NIH/NIGMS P41GM103412 which funds the National Center for Microscopy and Imaging Research. Special thanks to A Ghosh and S Wilke for sharing SBEM data sets which were reanalyzed here. Thanks to R Serrano for help analyzing line scan data. Thanks to MV Sofroniew for sharing equipment. Thanks also to R Huckstepp for help with setting up the glutamate biosensors. Many thanks to Ju Chen (UCSD) for sharing IP3R2 KO mice. Thanks to current and former members of the Khakh lab for their input and help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agulhon C, Fiacco TA, McCarthy KD. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science. 2010;327:1250–1254. doi: 10.1126/science.1184821. [DOI] [PubMed] [Google Scholar]
- Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, McCarthy KD. What is the role of astrocyte calcium in neurophysiology? Neuron. 2008;59:932–946. doi: 10.1016/j.neuron.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agulhon C, Sun MY, Murphy T, Myers T, Lauderdale K, Fiacco TA. Calcium Signaling and Gliotransmission in Normal vs. Reactive Astrocytes. Front Pharmacol. 2012 doi: 10.3389/fphar.2012.00139. Epub 2012 Jul 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D, Lavenex P. Hippocampal neuroanatomy. In: Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J, editors. The Hippocampus Book. Chapter 3. Oxford University Press; 2007. pp. 37–114. [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiati MD. Is GABA co-released with glutamate from hippocampal mossy fiber terminals? J Neurosci. 2013;33:1755–1756. doi: 10.1523/JNEUROSCI.5019-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, Moss SJ, Haydon PG. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci U S A. 2007;104:1995–2000. doi: 10.1073/pnas.0609408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deerinck TJ, Bushong EA, Lev-Ram V, Shu X, Tsien RY, Ellisman MH. Enhancing serial block-face scanning electron microscopy to enable high resolution 3-D nanohistology of cells and tissues. Microsc Microanalysis. 2010;16(Suppl 2):1138–1139. [Google Scholar]
- Di Castro MA, Chuquet J, Liaudet N, Bhaukaurally K, Santello M, Bouvier D, Tiret P, Volterra A. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat Neurosci. 2011;10:1276–1284. doi: 10.1038/nn.2929. [DOI] [PubMed] [Google Scholar]
- Fiacco TA, Agulhon C, Taves SR, Petravicz J, Casper KB, Dong X, Chen J, McCarthy KD. Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron. 2007;54:611–626. doi: 10.1016/j.neuron.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Iremonger KJ, Kantevari S, Ellis-Davies GC, MacVicar BA, Bains JS. Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron. 2009;64:391–403. doi: 10.1016/j.neuron.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez R. The dual glutamatergic-GABAergic phenotype of hippocampal granule cells. Trends Neurosci. 2005;28:297–303. doi: 10.1016/j.tins.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Huang YH, Sinha SR, Tanaka K, Rothstein JD, Bergles DE. Astrocyte glutamate transporters regulate metabotropic glutamate receptor-mediated excitation of hippocampal interneurons. J Neurosci. 2004;24:4551–4559. doi: 10.1523/JNEUROSCI.5217-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Clémençon B, Simonin A, Leuenberger M, Lochner M, Weisstanner M, Hediger MA. The SLC1 high-affinity glutamate and neutral amino acid transporter family. Mol Aspects Med. 2013;34:108–120. doi: 10.1016/j.mam.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;8:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Khakh BS, North RA. Neuromodulation by extracellular ATP and P2X receptors in the CNS. Neuron. 2012;76:51–69. doi: 10.1016/j.neuron.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of threedimensional image data using IMOD. J Struct Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- Kuffler SW. Neuroglial cells: physiological properties and a potassium mediated effect of neuronal activity on the glial membrane potential. Proc R Soc Lond B Biol Sci. 1967;168:1–21. doi: 10.1098/rspb.1967.0047. [DOI] [PubMed] [Google Scholar]
- Li D, Agulhon C, Schmidt E, Oheim M, Ropert N. New tools for investigating astrocyte-to-neuron communication. Front Cell Neurosci. 2013;7:193. doi: 10.3389/fncel.2013.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zima AV, Sheikh F, Blatter LA, Chen J. Endothelin-1-induced arrhythmogenic Ca2+ signaling is abolished in atrial myocytes of inositol-1,4,5-trisphosphate(IP3)-receptor type 2-deficient mice. Circ Res. 2005;96:1274–1281. doi: 10.1161/01.RES.0000172556.05576.4c. [DOI] [PubMed] [Google Scholar]
- Marvin JS, Borghuis BG, Tian L, Cichon J, Harnett MT, Akerboom J, Gordus A, Renninger SL, Chen TW, Bargmann CI, et al. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat Methods. 2013;10:162–170. doi: 10.1038/nmeth.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier A, Vallée J, Haber M, Murai KK, Lacaille JC, Robitaille R. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell. 2011;146:785–798. doi: 10.1016/j.cell.2011.07.022. [DOI] [PubMed] [Google Scholar]
- Petravicz J, Fiacco TA, McCarthy KD. Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci. 2008;28:4967–4973. doi: 10.1523/JNEUROSCI.5572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves A, Shigetomi E, Khakh BS. Bulk loading of calcium indicator dyes to study astrocyte physiology: key limitations and improvements using morphological maps. J Neurosci. 2011;31:9353–9358. doi: 10.1523/JNEUROSCI.0127-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan MR, Huang YH, Kim YS, Dykes-Hoberg MI, Jin L, Watkins AM, Bergles DE, Rothstein JD. Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J Neurosci. 2007;27:6607–6619. doi: 10.1523/JNEUROSCI.0790-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richler E, Chaumont S, Shigetomi E, Sagasti A, Khakh BS. Tracking transmitter-gated P2X cation channel activation in vitro and in vivo. Nat Methods. 2008;5:87–93. doi: 10.1038/nmeth1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollenhagen A, Lübke JH. The morphology of excitatory central synapses: from structure to function. Cell Tissue Res. 2006;326:221–237. doi: 10.1007/s00441-006-0288-z. [DOI] [PubMed] [Google Scholar]
- Rollenhagen A, Sätzler K, Rodríguez EP, Jonas P, Frotscher M, Lübke JH. Structural determinants of transmission at large hippocampal mossy fiber synapses. J Neurosci. 2007;27:10434–10444. doi: 10.1523/JNEUROSCI.1946-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Ruiz AJ, Kullmann DM. Ionotropic receptors at hippocampal mossy fibers: roles in axonal excitability, synaptic transmission, and plasticity. Front Neural Circuits. 2012;6:112. doi: 10.3389/fncir.2012.00112. Epub 2013 Jan 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusakov DA, Zheng K, Henneberger C. Astrocytes as regulators of synaptic function: a quest for the Ca2+ master key. Neuroscientist. 2011;5:513–523. doi: 10.1177/1073858410387304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Bowser DN, Sofroniew MV, Khakh BS. Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J Neurosci. 2008;28:6659–6663. doi: 10.1523/JNEUROSCI.1717-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Bushong EA, Haustein MD, Tong X, Jackson-Weaver O, Kracun S, Xu J, Sofroniew MV, MH E, Khakh BS. Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J Gen Physiol. 2013a;141:633–647. doi: 10.1085/jgp.201210949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Jackson-Weaver O, Huckstepp RT, O'Dell TJ, Khakh BS. TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J Neurosci. 2013b;33:10143–10153. doi: 10.1523/JNEUROSCI.5779-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Kracun S, Sofroniew MV, Khakh BS. A genetically targeted optical sensor to monitor calcium signals in astrocyte processes. Nature Neuroscience. 2010;13:759–766. doi: 10.1038/nn.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nature Neuroscience. 2011;15:70–80. doi: 10.1038/nn.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N, McBain C. Structural and functional properties of hippocampal neurons. In: Andersen, Morris, Amaral, Bliss, O'Keefe, editors. The hippocampus book. Oxford University Press; 2007. [Google Scholar]
- Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, Lovatt D, Han X, Smith Y, Nedergaard M. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science. 2013;339:197–200. doi: 10.1126/science.1226740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Akerboom J, Schreiter ER, Looger LL. Neural activity imaging with genetically encoded calcium indicators. Prog Brain Res. 2012;196:79–94. doi: 10.1016/B978-0-444-59426-6.00005-7. [DOI] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Shigetomi E, Looger LL, Khakh BS. Genetically encoded calcium indicators and astrocyte calcium microdomains. Neuroscientist. 2012;19:274–291. doi: 10.1177/1073858412468794. [DOI] [PubMed] [Google Scholar]
- Walker MC, Ruiz A, Kullmann DM. Do mossy fibers release GABA? Epilepsia. 2002;43:196–202. doi: 10.1046/j.1528-1157.43.s.5.6.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. Astrocytic Ca(2+) signaling evoked by sensory stimulation in vivo. Nat Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- Wilke SA, Antonios JK, Bushong EA, Badkoobehi A, Melek E, Hwang M, Terada M, Ellisman MH, Ghosh A. Deconstructing complexity: serial block-face electron microscopic analysis of the hippocampal mossy fiber synapse. J Neurosci. 2013;33:507–522. doi: 10.1523/JNEUROSCI.1600-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zariwala HA, Borghuis BG, Hoogland TM, Madisen L, Tian L, De Zeeuw CI, Zeng H, Looger LL, Svoboda K, Chen TW. A Cre-Dependent GCaMP3 Reporter Mouse for Neuronal Imaging In Vivo. J Neurosci. 2012;32:2131–2141. doi: 10.1523/JNEUROSCI.4469-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Mak DO, Li Q, Shin DM, Foskett JK, Muallem S. A new mode of Ca2+ signaling by G protein-coupled receptors: gating of IP3 receptor Ca2+ release channels by Gbetagamma. Curr Biol. 2003;13:872–876. doi: 10.1016/s0960-9822(03)00330-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.