Abstract
CYP2A6 metabolizes clinically relevant drugs, including antiretroviral and antimalarial drugs of major public health importance for the African populations. CYP2A6 genotype–phenotype relationship in African populations, and implications of geographic differences on enzyme activity, remain to be investigated. We evaluated the influence of CYP2A6 genotype, geographical differences, gender, and cigarette smoking on enzyme activity, using caffeine as a probe in 100 healthy unrelated Ethiopians living in Ethiopia, and 72 living in Sweden. CYP2A6 phenotype was estimated by urinary 1,7-dimethyluric acid (17U)/1,7-dimethylxanthine or paraxanthine (17X) ratio. The frequencies of CYP2A6*1B, *1D, *2, *4, *9, and *1x2 in Ethiopians were 31.3, 29.4, 0.6, 0.6, 2.8, and 0.3%, respectively. The overall mean±SD for log 17U/17X was 0.12±0.24 and coefficient of variation 199%. No significant difference in the mean log 17U/17X ratio between Ethiopians living in Sweden versus Ethiopia was observed. Analysis of variance revealed CYP2A6 genotype (p=0.04, F=2.01) but not geographical differences, sex, or cigarette smoking as predictors of CYP2A6 activity. Importantly, the median (interquartile range) of 17U/17X ratio in Ethiopians 1.35 (0.99 to 1.84) was 3- and 11-fold higher than the previously reported value in Swedes 0.52 (0.27 to 1.00) and Koreans 0.13 (0.0 to 0.35), respectively (Djordjevic et al., 2013). Taken together, we report here the relevance of CYP2A6 genotype for enzyme activity in this Ethiopian sample, as well as high CYP2A6 activity and unique distribution of the CYP2A6 variant alleles in Ethiopians as compared other populations described hitherto. Because Omics biomarker research is rapidly accelerating in Africa, CYP2A6 pharmacogenetics and clinical pharmacology observations reported herein for the Ethiopian populations have clinical and biological importance to plan for future rational therapeutics efforts in the African continent as well as therapeutics as a global science.
Introduction
Cytochrome P450 2A6 (CYP2A6) is a phase I drug metabolizing enzyme, expressed almost only in the liver. It is involved in the metabolism of steroids and fatty acids as well as activation of procarcinogenes (Tomaszewski et al., 2008; Zhu et al., 2013). CYP2A6 contributes to disposition of nicotine, cotinine, coumarin, and some clinically important drugs including antiretrovirals and antimalarial drugs (Newton et al., 2000; Ogburn et al., 2010; Pelkonen et al., 2000). The CYP2A6 gene is highly polymorphic, and its variant alleles often cause changes in enzyme activity (Oscarson et al., 1999). Moreover, CYP2A6 enzyme activity appears to be affected by concomitant drug use, including rifampicin, dexamethasone, phenobarbital, and ketokonazole (Benowitz et al., 2006; Tomaszewski et al., 2008)
CYP2A6 enzyme activity display pronounced inter-ethnic variations between African Americans, Asians, and Caucasians (Djordjevic et al., 2010; 2013; Kandel et al., 2007; Nakajima et al., 2006), and CYP2A6 genetic variation is commonly considered as the major cause for the observed differences. Various factors such as ethnicity (Djordjevic et al., 2010, 2013; Kandel et al., 2007; Nakajima et al., 2006), genotype (Oscarson et al., 1999; Pitarque et al., 2004), sex (Kandel et al., 2007; Mwenifumbo et al., 2007), age (Johnstone et al., 2006; Sinues et al., 2008), cigarette smoking (Djordjevic et al., 2010; Mwenifumbo et al., 2007), and oral contraceptive use (Sinues et al., 2008) are reported to influence CYP2A6 enzyme activity. However, such findings are often inconsistent (Begas et al., 2007; Djordjevic et al., 2010; Kadlubar et al., 2009; Mahavorasirikul et al., 2009), warranting further investigations. Interestingly, we have previously found a geographical effect related to the CYP2D6 activity where individuals of the same genotype had lower activity in Ethiopia as compared to those in Sweden, which we suggested was inherent in different dietary regimens in the two countries affecting the CYP2D6 activity (Aklillu et al., 2002). A similar geographic difference was also seen for xanthine oxidase (Aklillu et al., 2003), but not for CYP1A2 (Aklillu et al., 2003) or CYP2C19 (Aklillu et al., 2002).
Genetic variation in the CYP2A6 gene and genotype–phenotype relationship has been well investigated in Whites, Asians, and African Americans, but not in African populations, where it may have clinical relevance, particularly in the field of HIV/AIDs and malaria therapy because of the involvement of CYP2A6 in the metabolism of efavirenz and artesunate, respectively (Newton et al., 2000; Ogburn et al., 2010). Caffeine has been used as an in vivo probe to determine the relative levels of CYP2A6 activity, since the formation of 1,7 dimethylurate (17U) from 1,7 dimethylxanthine (17X) is mainly catalyzed by CYP2A6, and the ratio of 17U/17X is used as an index for CYP2A6 activity.
To our knowledge, there has been no systematic CYP2A6 genotype–phenotype correlation studies published from Africa. Furthermore, sub-Saharan African populations display wide genetic heterogeneity and hence data from one geographic region may not be extrapolated directly to others with in the continent (Aklillu et al., 2007). In the present study, we investigated the effect of CYP2A6 genotype, sex, smoking, and geographical differences on CYP2A6 enzyme activity using caffeine as a probe in unrelated healthy Ethiopians living in Ethiopia or Sweden. We also compared CYP2A6 genotype and phenotype in Ethiopian population versus other populations reported previously using similar genotype–phenotype procedures. Besides conforming relevance of CYP2A6 genotype for enzyme activity, our result indicates remarkably high CYP2A6 activity and unique distribution of CYP2A6 variant alleles in Ethiopians compared to whites, Asian, or other black populations.
Materials and Methods
Subjects
The study involved healthy unrelated subjects of Ethiopian origin living in Ethiopia (n=100) or in Sweden (n=72), previously participating in similar studies (Aklillu et al., 2002, 2003). Genomic DNA and 0–8 h urine samples collected post caffeine administration from these studies were further analyzed to investigate CYP2A6 genotype and enzyme activity. In brief, participants from Sweden were recruited among two groups: subjects adopted as small children by Swedish parents and now about 20 to 30 years of age (n=5), and subjects who left their home country and lived in Sweden for more than 10 years (n=30), 5 to 10 years (n=31), and 3 to 5 years (n=7). Using a detailed questionnaire, all subjects gave information about 1) their ethnic group, 2) time of arrival to Sweden, 3) health status, 4) dietary habits, 5) drug intake, and 6) smoking habits. The Human Ethics Committees at Huddinge University Hospital, Karolinska Institutet, Stockholm, Sweden, and the National Ethics committees at Ethiopian Science and Technology commission, Addis Ababa, Ethiopia, approved the study.
CYP2A6 Phenotyping
Phenotyping was performed using caffeine urinary test according to Carrillo et al. (2000), with modifications as previously described (Aklillu et al., 2003). Subjects were instructed to abstain from methylxanthine-containing foods and beverages (i.e., coffee, tea, chocolate, cola drinks) for at least 24 h before and throughout the study. Subjects received a 100-mg oral dose of caffeine (Koffein; ACO AB, Helsingborg, Sweden) after emptying their bladder before bedtime, and 0- to 8-h urine was collected. The volume and pH of the urine collected were measured, pH was adjusted to 3.5 with 0.1 M HCl, and 20 mL aliquots were stored at −20°C until analysis. Molar concentrations of caffeine metabolites 17X (1,7-dimethylxanthine or paraxanthine) and 17U (1,7-dimethyluric acid) were determined using high-performance liquid chromatography, and the CYP2A6 enzyme activity was assessed using 17U/17X ratio (Djordjevic et al., 2010; 2013; Nowell et al., 2002).
CYP2A6 Genotyping
Using QIAamp DNA Mini Kit (QIAGEN GmbH, Hilden, Germany), genomic DNA was isolated from whole blood samples. Genotyping for gene conversion in 3′ region (CYP2A6*1B), gene deletion (CYP2A6*4), gene duplication (CYP2A6*1x2), −1013A>G (CYP2A6*1D), −48T>G (CYP2A6*9) and 479T>A (CYP2A6*2) were performed using an allele-specific PCR method, as previously described (Djordjevic et al., 2013; Oscarson et al., 1999; Pitarque et al., 2001; Rao et al., 2000). All amplifications were performed on GeneAmp PCR System 2700 (Applied Biosystems, Foster City, CA), using the reagents purchased from Invitrogen, Carlsbad, CA, or Roche Applied Science, Mannheim, Germany. Haplotype analysis was performed using the population genetic software program Arlequin, version 3.11 (http://cmpg.unibe.ch/software/arlequin3).
Statistical analysis
Chi-square test or Fisher exact test were used to compare observed and expected allele frequencies according to Hardy-Weinberg equilibrium, as well as to compare genotype and haplotypes frequencies between Ethiopians living in Ethiopia and Ethiopians living in Sweden. The 17U/17X ratio was log-transformed before statistical analyses. Consistency of the data with the normal distribution was assessed by Shapiro-Wilk test. The effects of gender, cigarette smoking, and environment on enzyme activity were evaluated using independent sample t-test. Levene's test was applied to determine variance homogeneity. Main effect ANOVA was used to identify factors associated with between subject variability in CYP2A6 enzyme activity. Graphical representation and statistical analyses were performed using Statistica, version 10 (StatSoft Inc, Tulsa, OK, USA) and SPSS Statistics (IBM Corporation, Somers, NY) software, version 22.0, respectively; p<0.05 was considered as significant.
Results
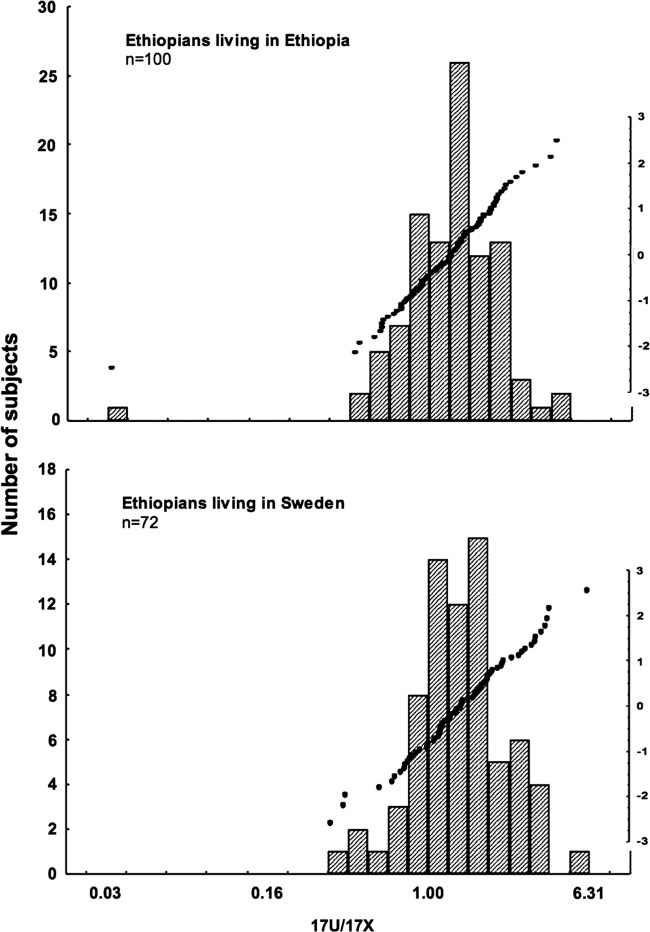
The overall log-transformed 17U/17X ratio ranged from −1.54 to 0.70 (mean±SE=0.122±0.02; Median (IQR)=0.12 (−0.01 to 0.27), Coefficient of variation=199%). The frequency distributions and probit plots of the log-transformed 17U/17X ratio in Ethiopians living in Ethiopia and Ethiopians living in Sweden are presented in Figure 1. The log 17U/17X ratio for one subject was extremely low, and data from this outlier was excluded. The distribution of 17U/17X ratio was unimodal and normally distributed (SW-W=0.99, p>0.75). Comparison of log 17U/17X ratio based on country of residence, sex differences, and smoking habit is presented in Table 1. There was no significant difference in the mean log 17U/17X ratio between Ethiopians living in Ethiopia and Ethiopians living in Sweden. Similarly no significant difference in the mean log 17U/17X ration between male and female subjects or between smokers and non-smokers was observed (Table 1).
FIG. 1.
The frequency distributions and probit plots of the log transformed 17U/17X ratio in Ethiopians living in Ethiopia and Ethiopians living in Sweden.
Table 1.
Comparison of Mean±SD of Log Urinary 17U/17X Ratio Based on Country of Residence, Gender, and Smoking Habit among Healthy Ethiopian Volunteers Using Independent t-Test
| Parameters | n | Range | Coefficient of variation | Mean±SD | Difference between means | 95% CI for the mean difference | P |
|---|---|---|---|---|---|---|---|
| Geographic differences | |||||||
| Living in Ethiopia | 99 | −0.35 to 0.64 | 169% | 0.12±0.26 | −0.034±0.03 | −0.097 to 0.029 | 0.29 |
| Living in Sweden | 72 | −0.42 to 0.70 | 144% | 0.15±0.22 | |||
| Gender differences | |||||||
| Female | 69 | −0.42 to 0.70 | 173% | 0.12±0.20 | −0.02±0.03 | −0.087 to 0.040 | 0.47 |
| Male | 102 | −0.34 to 0.61 | 148% | 0.14±0.24 | |||
| Smoking habit | |||||||
| Smokers | 19 | −0.42 to 0.70 | 155% | 0.14±0.22 | −0.01±0.05 | −0.11 to 0.09 | 0.78 |
| Non-smoker | 152 | −0.22 to 52 | 158% | 0.13±0.23 | |||
One outlier with very low 17U/17X ratio was excluded. The result was not changed with or without exclusion of this outlier.
The median (interquartile range) of 17U/17X ratio in Ethiopians was 1.35 (0.99–1.84), whereas the respective value that we reported previously for Swedes and Koreans was 0.52 (0.27–1.00) and 0.13 (0.0–0.35), respectively. The median 17U/17X ratio in Ethiopians was 3- and 11-fold higher than Swedes and Koreans, respectively (p<0.001). Comparing the log urinary 17U/17X data in Ethiopian with our previously published data from Swedes and Koreans (Djordjevic et al., 2013), Ethiopians display significantly higher log 17U/17X ratio and hence CYP2A6 activity as compared to Swedes (p<0.0001, mean±SE=−0.44±0.06, N=126, mean difference=, −0.5593±0.05495, 95%CI for the mean difference=−0.667 to −0.4516, r2=0.26) or Koreans (p<0.0001, mean±SE=−1.44±0.13, N=83, mean difference=−1.57±0.10, 95%CI for the mean difference=−1.76 to −1.38, r2=0.51).
The distribution of CYP2A6 variant alleles, haplotypes, and genotypes in all subjects and stratified by country of residence is presented in Table 2. No significant difference was observed in CYP2A6 variant allele and genotype frequency distribution of between Ethiopians living in Ethiopia and Ethiopians living in Sweden (Table 2, p≥0.05). None of subjects were homozygous for the defective variant alleles CYP2A6*2, *4 or *9. CYP2A6*5 was not detected. The frequency of CYP2A6 defective variant alleles is low in Ethiopian population compared to whites, Asian, or black populations as seen from Table 3.
Table 2.
Nucleotide Changes, Haplotypes, and Genotype Frequencies of CYP2A6 in Ethiopians Living in Ethiopia and Ethiopians Living in Sweden
| CYP2A6 allelic variants | All (95% CI) | Living in Ethiopia (95% CI) | Living in Sweden (95% CI) |
|---|---|---|---|
| −1013A>G | 0.576 (0.55–0.60) | 0.572 (0.54–0.61) | 0.581 (0.54–0.62) |
| −48T>G | 0.029 (0.02–0.04) | 0.03 (0.02–0.04) | 0.028 ((0.01–0.04) |
| Gene deletion | 0.009 (0.0–0.01) | 0 | 0.014 (0.01–0.02) |
| Gene conversion in 3′ region | 0.318 (0.29–0.34) | 0.327 (0.29–0.36) | 0.306 (0.27–0.34) |
| Gene duplication | 0.003 (0.0–0.01) | 0 | 0.008 (0.00–0.02) |
| 479T>A | 0.006 (0.0–0.01) | 0.01 (0.0–0.02) | 0 |
| 6582G>T | 0 | 0 | 0 |
| Haplotype | |||
| CYP2A6*1A | 0.348 (0.32–0.37) | 0.349 (0.32–0.38) | 0.347 (0.31–0.39) |
| CYP2A6*1B | 0.313 (0.29–0.34) | 0.323 (0.29–0.36) | 0.298 (0.26–0.34) |
| CYP2A6*1D | 0.294 (0.27–0.32) | 0.292 (0.26–0.32) | 0.298 (0.26–0.34) |
| CYP2A6*2 | 0.006 (0.0–0.01) | 0.01 (0.0–0.02) | 0 |
| CYP2A6*4 | 0.006 (0.0–0.01) | 0 | 0.016 (0.01–0.03) |
| CYP2A6*5 | 0 | 0 | 0 |
| CYP2A6*9 | 0.028 (0.02–0.04) | 0.026 (0.23–0.29) | 0.032 (0.02–0.05) |
| CYP2A6*1x2 | 0.003 (0.0–0.01) | 0 | 0.8% (0.0–0.02) |
| Genotype (n, %) | |||
| CYP2A6*1A/*1A | 15 (9.49%) | 10 (10.4%) | 5 (8.06%) |
| CYP2A6*1A/*1B | 40 (25.3%) | 24 (25.0%) | 16 (25.8%) |
| CYP2A6*1A/*1D | 32 (20.3) | 19 (19.8%) | 13 (21.0%) |
| CYP2A6*1A/*2 | 2 (1.27%) | 2 (2.1%) | |
| CYP2A6*1A/*4 | 1 (0.63%) | 1 (1.6%) | |
| CYP2A6*1A/*9 | 5 (3.16%) | 3 (4.8%) | |
| CYP2A6*1B/*1B | 15 (9.49%) | 10 (10.4%) | 5 (8.1%) |
| CYP2A6*1B/*1D | 26 (16.5%) | 17 (17.7%) | 9 (14.5%) |
| CYP2A6*1B/*4 | 1 (0.632%) | 1 (1.6%) | |
| CYP2A6*1B/*9 | 2 (1.26%) | 1 (1.1%) | 1 (1.6%) |
| CYP2A6*1D/*1D | 16 (10.1%) | 9 (9.4%) | 7 (11.3%) |
| CYP2A6*1D/*9 | 2 (1.27%) | 2 (2.1%) | |
| CYP2A6*1D/1x2 | 1 (0.63%) | 1 (1.6%) | |
CYP2A6*1A (wild type), CYP2A6*1B (gene conversion in 3′ region with or without −1013A>G), CYP2A6*1D (−1013A>G), CYP2A6*1Dx2 (−1013A>G, gene duplication), CYP2A6*1x2 (gene duplication), CYP2A6*2 (479T>A), CYP2A6*4 (gene deletion), CYP2A6*9 (−48T>G).
Table 3.
Distribution of CYP2A6 Variant Alleles in Ethiopians as Compared to Other Populations Described Previously1
| CYP2A6 allele | Ethiopian (n=172) | Swedes (n=190) | Koreans (n=144) | Chinese (n=102) | African American (n=160) | Ghanaians (n=105) | Yoruba, Nigeria* | Kenya* |
|---|---|---|---|---|---|---|---|---|
| CYP2A6*1B | 31.3 | 32.6 | 26.7 | 34.5 | 16.5 | 11.9 | ND | ND |
| CYP2A6*1D | 29.4 | 34.4 | 40.6 | ND | 38.5 | ND | ND | ND |
| CYP2A6*2 | 0.6 | 3.2 | 0 | 0 | 0.3 | 0 | 0 | 0 |
| CYP2A6*4 | 0.6 | 1.1 | 9.4 | 15.1 | 0.9 | 1.9 | ND | ND |
| CYP2A6*5 | 0 | 0 | 0.5 | 1.0 | 0 | 0 | 0 | 0 |
| CYP2A6*9 | 2.8 | 7.9 | 21.9 | 15.7 | 8.5 | 5.7 | 9 | 9 |
| CYP2A6*1x2 | 0.3 | 0.8 | 0.2 | 0.4 | 0 | ND | ND | ND |
Univariate analysis of variance indicated significant effect of CYP2A6 genotype (p=0.045, F=1.73, r2=0.15) on inter-individual variations in log 17U/17X ratio. Only 8 subjects were heterozygous for the defective variant alleles. The mean log 17U/17X ratio was three-fold lower in heterozygous subjects, (mean±SE: 0.05±0.1) than subjects not carrying any of the defective variant alleles (n=150; mean±SE: 0.14±0.02). Subjects homozygous for 3′ gene conversion (2A6*1B/*1B) or CYP2A6*1D/*1D genotype had significantly higher 17U/17X ratio compared to subjects with *1A/*1A genotype. One subject carrying CYP2A6 gene duplication had relatively higher enzyme activity log 17U/17X ratio.
Discussion
The present study investigated CYP2A6 genotype–phenotype relationship and effect of gender or smoking on enzyme activity in Ethiopians using caffeine as a probe. Controlling for effect of genotype, we further evaluated any possible effect of living in different geographic region or environment on CYP2A6 enzyme activity by comparing Ethiopians living in Ethiopia and Ethiopians living in Sweden. Our main finding is the lack of significant difference in CYP2A6 enzyme activity, in contrast to previous findings regarding CYP2D6 and xanthine oxidase, between the two study groups residing in Europe or Africa. This indicates that inherited rather than acquired elements depending on geography are major determinant of CYP2A6 enzyme activity. In line with this, we found that the CYP2A6 genotype as the main significant factor to influence between subject variability in CYP2A6 enzyme activity but not gender or cigarette smoking. As to our best knowledge, this is the first CYP2A6 genotype–phenotype study in African populations and to investigate any impact of geographic differences on CYP2A6 enzyme activity.
It is known that CYP2A6 genetic variation determines inter-individual variability in CYP2A6 enzyme activity (Oscarson et al., 1999). Ethiopian subjects were genotyped for several CYP2A6 variant alleles, leading to either none (CYP2A6*2 and CYP2A6*4), decreased (CYP2A6*9), or increased (CYP2A6*1B and CYP2A6*1x2) enzyme activity. The observed frequencies of CYP2A6*2 and CYP2A6*4 and CYP2A6*1x2 in Ethiopians corresponded well with the previous reports from whites and blacks, including Americans of African descent and Ghanaians (Gyamfi et al., 2005; Mwenifumbo et al., 2007; Nakajima et al., 2006; Paschke et al., 2001). However, the CYP2A6*4 variant allele frequency in Ethiopians is much lower than in Asians. On the contrary, CYP2A6*1B (31.3%) in Ethiopians seemed to be noticeably higher than in other black populations, where the reported frequency was up to 11 to 18% for CYP2A6*1B (Gyamfi et al., 2005; Mwenifumbo et al., 2007; Nakajima et al., 2006). Association of CYP2A6*1B (Mwenifumbo et al., 2008; Wang et al., 2006) with higher enzyme activity has been reported. Interestingly, the prevalence of defective variant allele CYP2A6*9 (2.8%) in Ethiopians is much lower than any other population described so far. The reported CYP2A6*9 allele frequency range from 7% to 9% in whites and other black populations including Ugandans, Ghanaians, African Americans, Kenyan, and Yoruba from 1000 genome project (Gyamfi et al., 2005; Mukonzo et al., 2013; Nakajima et al., 2006), adding to apparent CYP2A6 genetic diversity of black population. The frequency of CYP2A6*9 allele is even much higher in Asian population (22% in Koreans, 15% in Chinese,) (Djordjevic et al., 2012; Pitarque et al., 2001). The observed effect of CYP2A6 genotype on enzyme activity is in agreement with the previously published reports related to both black and other populations.
In the present study, Ethiopians living in Ethiopia and Ethiopians living in Sweden were similar regarding both CYP2A6 genetic profile and allele frequency distribution and other possible influencing factors, such as cigarette smoking habit and gender. This enabled us to investigate the effect of environment on CYP2A6 activity by comparing the two groups. For CYP2A6 phenotyping we chose caffeine, due to its favorable pharmacokinetics properties and proven safety. Comparison between the two study groups showed no significant difference in terms of CYP2A6 capacity and consequently no effect of geographically-related environment on CYP2A6 enzyme activity.
The CYP2A6 enzyme activity is influenced by ethnicity partly due to a genetic basis. We were unable to compare CYP2A6 enzyme activity in Ethiopians with other black populations due to the lack of published caffeine-based studies of CYP2A6 activity in blacks. Nevertheless, comparison using the same phenotyping strategy revealed significantly higher CYP2A6 activity in Ethiopians than Swedes or Koreans (Djordjevic et al., 2013). We observed remarkably higher CYP2A6 enzyme activity in Ethiopians than Swedes or Koreans, as measured by a caffeine phenotyping assay. The CYP2A6 enzyme activity, as measured by caffeine test, in Ethiopians was 3-fold and 11-fold higher than Swedes and Koreans, respectively. Using 0.01 as the common antimode cut off for log 17U/17X ratio, 3.16% of Swedes and 18.75% of Koreans are classified as slow metabolizers (Djordjevic et al., 2013), whereas only one subject (0.5%) of the Ethiopians is classified as CYP2A6 slow metabolizers. This is in contrast to earlier reports of lower CYP2A6 activity in blacks compared to either Caucasians or Asians (Benowitz et al., 1999; Kandel et al., 2007; Nakajima et al., 2006), implying the possible heterogeneity within the black population in terms of CYP2A6 metabolic capacity. None of the Ethiopian subjects were homozygous for the defective variant alleles. Apparently the higher CYP2A6 enzyme activity in Ethiopian population might partly be due to very low or absence of defective variant alleles (CYP2A6*2, *4, *5, *9) and higher occurrence of variant alleles coding for normal (CYP2A6*1A, *1D) or increased enzyme activity (CYP2A6*1B) accounting together for >95% allele frequency.
Our study may have clinical relevance in African population, particularly in the treatment of HIV/AIDS and malaria because of CYP2A6 involvement in efavirenz and artesunate disposition, respectively. CYP2A6-mediated 7-hydroxylation accounts about 23% of total efavirenz metabolism (Ogburn et al., 2010). Efavirenz is the cornerstone and first line recommended antiretroviral drug particularly in TB-HIV co-infected patients and for prevention of mother to child transmission with the core targets of the Global Plan—providing ARV medicines to 90% of pregnant women living with HIV globally by the end of 2015. Efavirenz disposition display wide between population differences, partly due to CYP2B6 pharmacogenetics (Habtewold et al., 2011; Mukonzo et al., 2009; Ngaimisi et al., 2010, 2011). We recently reported that among individuals having the same CYP2B6 genotype, higher efavirenz plasma concentration was observed in Ethiopian HIV patients than in Tanzanian (Ngaimisi et al., 2013). The importance of CYP2A6 (Arab-Alameddine et al., 2009; di Iulio et al., 2009; Kwara et al., 2009) and CYP3A5 metabolic pathways (Habtewold et al., 2011) for efavirenz disposition, particularly in CYP2B6 slow metabolizers, has been reported. Thus, we here found higher CYP2A6 enzyme activity in Ethiopians and have previously reported higher total CYP3A activity and unique distribution of CYP3A5 genotype in Ethiopians than in Tanzanians (Gebeyehu et al., 2011; Habtewold et al., 2013). Apparently the high CYP2A6 and CYP3A enzyme activity in Ethiopians may salvage the CYP2B6-mediated slow metabolism among patients carrying defective CYP2B6 variant alleles. High CYP2A6 enzyme activity may result in accelerated metabolism and elimination of clinically used drugs and this may result in treatment failure particularly for those drugs with narrow therapeutic range. On the other hand, high enzyme activity may result in accumulation of reactive intermediate metabolites causing pronounced adverse event. In fact, association between CYP2A6*1B responsible for ultra-rapid metabolism of artesunate with significantly higher incidence of adverse drug reaction is recently reported (Yusof and Hua, 2012). Accordingly, higher CYP2A6 enzyme activity in Ethiopians may predispose patients to risk of artesunate induced adverse events.
Expression and activity of drug metabolizing enzymes is governed by complex traits involving allele-specific genetic and epigenetic variations involving multiple genes (Ghotbi et al., 2009), as well as environmental factors such as dietary constituents (Aklillu et al., 2002). Therefore, future studies involving advanced models to characterize effect of interaction between multiple gene polymorphisms and environmental factors on the expression and activity of CYP enzymes are needed.
Conclusion
We report no major contribution of geographic differences, gender, or smoking habit on CYP2A6 enzyme activity. We note the importance of ethnicity and CYP2A6 genotype for population variations in CYP2A6 enzyme activity. The finding of a unique distribution of CYP2A6 genetic variants, and most importantly, the high total CYP2A6 activity as measured by a caffeine phenotyping assay in Ethiopians warrant further investigations to evaluate the clinical relevance of these factors. As genomics and other biomarker research accelerate in Africa, CYP2A6 pharmacogenetics will have greater importance for rational therapeutics and long-term clinical epidemiology studies in Africa.
Acknowledgment
We would like to thank all volunteers who participated in the study. The study was financially supported by the Swedish Research Council, Medicine (VR 3902 and VR 521-2011-3437) and grants GR10122 from Consejería de Empleo, Empresa e Innovación, Gobierno de Extremadura, Mérida, Spain.
Author Disclosure Statement
The authors declare that there are no conflicting financial interests.
References
- Aklillu E, Carrillo JA, Makonnen E, Bertilsson L, and Ingelman-Sundberg M. (2003). Xanthine oxidase activity is influenced by environmental factors in Ethiopians. Eur J Clin Pharmacol 59, 533–536 [DOI] [PubMed] [Google Scholar]
- Aklillu E, Carrillo JA, Makonnen E, et al. (2003). Genetic polymorphism of CYP1A2 in Ethiopians affecting induction and expression: Characterization of novel haplotypes with single-nucleotide polymorphisms in intron 1. Mol Pharmacol 64, 659–669 [DOI] [PubMed] [Google Scholar]
- Aklillu E, Dandara C, and Bertilsson LCM. (2007). Pharmacogenetics of cytochrome P450s in African populations: Clinical and molecular evolutionary implications. In: Suarez-Kurtz G, ed. Pharmacogenomics in Admixed Populations. Austin, TX: Landes Bioscience, 99–119 [Google Scholar]
- Aklillu E, Herrlin K, Gustafsson LL, Bertilsson L, and Ingelman-Sundberg M. (2002). Evidence for environmental influence on CYP2D6-catalysed debrisoquine hydroxylation as demonstrated by phenotyping and genotyping of Ethiopians living in Ethiopia or in Sweden. Pharmacogenetics 12, 375–383 [DOI] [PubMed] [Google Scholar]
- Arab-Alameddine M, Di Iulio J, Buclin T, et al. (2009). Pharmacogenetics-based population pharmacokinetic analysis of efavirenz in HIV-1-infected individuals. Clin Pharmacol Ther 85, 485–494 [DOI] [PubMed] [Google Scholar]
- Begas E, Kouvaras E, Tsakalof A, Papakosta S, and Asprodini EK. (2007). In vivo evaluation of CYP1A2, CYP2A6, NAT-2 and xanthine oxidase activities in a Greek population sample by the RP-HPLC monitoring of caffeine metabolic ratios. Biomed Chromatogr 21, 190–200 [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Lessov-Schlaggar CN, Swan GE, and Jacob P., 3rd (2006). Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther 79, 480–488 [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Perez-Stable EJ, Fong I, et al. (1999). Ethnic differences in N-glucuronidation of nicotine and cotinine. J Pharmacol Exp Ther 291, 1196–1203 [PubMed] [Google Scholar]
- Carrillo JA, Christensen M, Ramos SI, et al. (2000). Evaluation of caffeine as an in vivo probe for CYP1A2 using measurements in plasma, saliva, and urine. Ther Drug Monit 22, 409–417 [DOI] [PubMed] [Google Scholar]
- di Iulio J, Fayet A, Arab-Alameddine M, et al. (2009). In vivo analysis of efavirenz metabolism in individuals with impaired CYP2A6 function. Pharmacogenet Genomics 19, 300–309 [DOI] [PubMed] [Google Scholar]
- Djordjevic N, Carrillo JA, Gervasini G, Jankovic S, and Aklillu E. (2010) In vivo evaluation of CYP2A6 and xanthine oxidase enzyme activities in the Serbian population. Eur J Clin Pharmacol 66, 571–578 [DOI] [PubMed] [Google Scholar]
- Djordjevic N, Carrillo JA, van den Broek MP, et al. (2013). Comparisons of CYP2A6 genotype and enzyme activity between Swedes and Koreans. Drug Metab Pharmacokinet 28, 93–97 [DOI] [PubMed] [Google Scholar]
- Gebeyehu E, Engidawork E, Bijnsdorp A, et al. (2011). Sex and CYP3A5 genotype influence total CYP3A activity: High CYP3A activity and a unique distribution of CYP3A5 variant alleles in Ethiopians. Pharmacogenomics J 11, 130–137 [DOI] [PubMed] [Google Scholar]
- Ghotbi R, Gomez A, Milani L, et al. (2009). Allele-specific expression and gene methylation in the control of CYP1A2 mRNA level in human livers. Pharmacogenomics J 9, 208–217 [DOI] [PubMed] [Google Scholar]
- Gyamfi MA, Fujieda M, Kiyotani K, Yamazaki H, and Kamataki T. (2005). High prevalence of cytochrome P450 2A6*1A alleles in a black African population of Ghana. Eur J Clin Pharmacol 60, 855–857 [DOI] [PubMed] [Google Scholar]
- Habtewold A, Amogne W, Makonnen E, et al. (2011). Long-term effect of efavirenz autoinduction on plasma/peripheral blood mononuclear cell drug exposure and CD4 count is influenced by UGT2B7 and CYP2B6 genotypes among HIV patients. J Antimicrob Chemother 66, 2350–2361 [DOI] [PubMed] [Google Scholar]
- Habtewold A, Amogne W, Makonnen E, et al. (2013). Pharmacogenetic and pharmacokinetic aspects of CYP3A induction by efavirenz in HIV patients. Pharmacogenomics J 13, 484–489 [DOI] [PubMed] [Google Scholar]
- Johnstone E, Benowitz N, Cargill A, et al. (2006). Determinants of the rate of nicotine metabolism and effects on smoking behavior. Clin Pharmacol Ther 80, 319–330 [DOI] [PubMed] [Google Scholar]
- Kadlubar S, Anderson JP, Sweeney C, et al. (2009). Phenotypic CYP2A6 variation and the risk of pancreatic cancer. JOP 10, 263–270 [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Hu MC, Schaffran C, Udry JR, and Benowitz NL. (2007). Urine nicotine metabolites and smoking behavior in a multiracial/multiethnic national sample of young adults. Am J Epidemiol 165, 901–910 [DOI] [PubMed] [Google Scholar]
- Kwara A, Lartey M, Sagoe KW, Kenu E, and Court MH. (2009). CYP2B6, CYP2A6 and UGT2B7 genetic polymorphisms are predictors of efavirenz mid-dose concentration in HIV-infected patients. AIDS 23, 2101–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahavorasirikul W, Tassaneeyakul W, Satarug S, et al. (2009). CYP2A6 genotypes and coumarin-oxidation phenotypes in a Thai population and their relationship to tobacco smoking. Eur J Clin Pharmacol 65, 377–384 [DOI] [PubMed] [Google Scholar]
- Mukonzo JK, Okwera A, Nakasujja N, et al. (2013). Influence of efavirenz pharmacokinetics and pharmacogenetics on neuropsychological disorders in Ugandan HIV-positive patients with or without tuberculosis: A prospective cohort study. BMC Infect Dis 13, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukonzo JK, Roshammar D, Waako P, et al. (2009). A novel polymorphism in ABCB1 gene, CYP2B6*6 and sex predict single-dose efavirenz population pharmacokinetics in Ugandans. Br J Clin Pharmacol 68, 690–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwenifumbo JC, Lessov-Schlaggar CN, Zhou Q, et al. (2008). Identification of novel CYP2A6*1B variants: The CYP2A6*1B allele is associated with faster in vivo nicotine metabolism. Clin Pharmacol Ther 83, 115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwenifumbo JC, Sellers EM, and Tyndale RF. (2007). Nicotine metabolism and CYP2A6 activity in a population of black African descent: Impact of gender and light smoking. Drug Alcohol Depend 89, 24–33 [DOI] [PubMed] [Google Scholar]
- Nakajima M, Fukami T, Yamanaka H, et al. (2006). Comprehensive evaluation of variability in nicotine metabolism and CYP2A6 polymorphic alleles in four ethnic populations. Clin Pharmacol Ther 80, 282–297 [DOI] [PubMed] [Google Scholar]
- Newton P, Suputtamongkol Y, Teja-Isavadharm P, et al. (2000). Antimalarial bioavailability and disposition of artesunate in acute falciparum malaria. Antimicrob Agents Chemother 44, 972–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngaimisi E, Habtewold A, Minzi O, et al. (2013). Importance of ethnicity, CYP2B6 and ABCB1 genotype for efavirenz pharmacokinetics and treatment outcomes: A parallel-group prospective cohort study in two sub-Saharan Africa populations. PLoS One 8, e67946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngaimisi E, Mugusi S, Minzi O, et al. (2011). Effect of rifampicin and CYP2B6 genotype on long-term efavirenz autoinduction and plasma exposure in HIV patients with or without tuberculosis. Clin Pharmacol Ther 90, 406–413 [DOI] [PubMed] [Google Scholar]
- Ngaimisi E, Mugusi S, Minzi OM, et al. (2010). Long-term efavirenz autoinduction and its effect on plasma exposure in HIV patients. Clin Pharmacol Ther 88, 676–684 [DOI] [PubMed] [Google Scholar]
- Nowell S, Sweeney C, Hammons G, Kadlubar FF, and Lang NP. (2002). CYP2A6 activity determined by caffeine phenotyping: Association with colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 11, 377–383 [PubMed] [Google Scholar]
- Ogburn ET, Jones DR, Masters AR, et al. (2010). Efavirenz primary and secondary metabolism in vitro and in vivo: Identification of novel metabolic pathways and cytochrome P450 2A6 as the principal catalyst of efavirenz 7-hydroxylation. Drug Metab Dispos 38, 1218–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarson M, McLellan RA, Gullsten H, et al. (1999). Identification and characterisation of novel polymorphisms in the CYP2A locus: Implications for nicotine metabolism. FEBS Lett 460, 321–327 [DOI] [PubMed] [Google Scholar]
- Oscarson M, McLellan RA, Gullsten H, et al. (1999). Characterisation and PCR-based detection of a CYP2A6 gene deletion found at a high frequency in a Chinese population. FEBS Lett 448, 105–110 [DOI] [PubMed] [Google Scholar]
- Paschke T, Riefler M, Schuler-Metz A, et al. (2001). Comparison of cytochrome P450 2A6 polymorphism frequencies in Caucasians and African-Americans using a new one-step PCR-RFLP genotyping method. Toxicology 168, 259–268 [DOI] [PubMed] [Google Scholar]
- Pelkonen O, Rautio A, Raunio H, and Pasanen M. (2000). CYP2A6: A human coumarin 7-hydroxylase. Toxicology 144, 139–147 [DOI] [PubMed] [Google Scholar]
- Pitarque M, von Richter O, Oke B, et al. (2001). Identification of a single nucleotide polymorphism in the TATA box of the CYP2A6 gene: Impairment of its promoter activity. Biochem Biophys Res Commun 284, 455–460 [DOI] [PubMed] [Google Scholar]
- Pitarque M, von Richter O, Rodriguez-Antona C, et al. (2004). A nicotine C-oxidase gene (CYP2A6) polymorphism important for promoter activity. Hum Mutat 23, 258–266 [DOI] [PubMed] [Google Scholar]
- Rao Y, Hoffmann E, Zia M, et al. (2000). Duplications and defects in the CYP2A6 gene: Identification, genotyping, and in vivo effects on smoking. Mol Pharmacol 58, 747–755 [DOI] [PubMed] [Google Scholar]
- Sinues B, Fanlo A, Mayayo E, et al. (2008). CYP2A6 activity in a healthy Spanish population: Effect of age, sex, smoking, and oral contraceptives. Hum Exp Toxicol 27, 367–372 [DOI] [PubMed] [Google Scholar]
- Tomaszewski P, Kubiak-Tomaszewska G, and Pachecka J. (2008). Cytochrome P450 polymorphism–molecular, metabolic, and pharmacogenetic aspects. II. Participation of CYP isoenzymes in the metabolism of endogenous substances and drugs. Acta Pol Pharm 65, 307–318 [PubMed] [Google Scholar]
- Wang J, Pitarque M, and Ingelman-Sundberg M. (2006). 3′-UTR polymorphism in the human CYP2A6 gene affects mRNA stability and enzyme expression. Biochem Biophys Res Commun 340, 491–497 [DOI] [PubMed] [Google Scholar]
- Yusof W, and Hua GS. (2012). Gene, ethnic and gender influences predisposition of adverse drug reactions to artesunate among Malaysians. Toxicol Mech Methods 22, 184–192 [DOI] [PubMed] [Google Scholar]
- Zhu AZ, Binnington MJ, Renner CC, et al. (2013). Alaska Native smokers and smokeless tobacco users with slower CYP2A6 activity have lower tobacco consumption, lower tobacco-specific nitrosamine exposure and lower tobacco-specific nitrosamine bioactivation. Carcinogenesis 34, 93–101 [DOI] [PMC free article] [PubMed] [Google Scholar]