Abstract
There are currently no validated minimally invasive objective metrics for the classification and evaluation of ocular surface diseases and/or for evaluating treatment efficacy. We thus sought to establish a standardized methodology for determining the relative amount of the inflammatory biomarker HLA-DR on the ocular surface and to evaluate the precision, reliability and repeatability of its use for large multicenter clinical trials and translational research studies of ocular surface disease. Multiple studies were conducted to establish a Standard Operating Procedure (SOP) for utilizing HLA-DR expression as a minimally invasive, objective, ocular surface inflammatory biomarker. The established SOPs provide specific guidelines for HLA-DR collection and analysis, in order to incorporate it reliably into multicenter clinical trials and/or translational research. Duplicate cell samples from impression cytology (IC) samples of both normal and dry eye individuals were collected and split to assess repeatability (between the splits and between the duplicate samples). To determine storage capability, one duplicate was stained immediately and the other after 30 days cold storage. To demonstrate the feasibility of the use of the SOP for a multicenter clinical trial, clinicians out-of-state were trained to collect IC samples, and the samples shipped to our Biomarker Laboratory, logged, processed and analyzed. Demonstration of the ability to incorporate of IC into a randomized double masked clinical trial of dry eye disease (DED) was performed. In all cases, processing and analyses were performed by a masked independent observer. The validity/viability of the SOPs was established by demonstrating that: 1) sufficient numbers of cells can be collected via IC; 2) the precision/repeatability of the relative biomarker expression quantified in samples; 3) personnel at distant sites can be taught to collect, store and ship samples successfully; 4) samples can be stored for up to 30 days (refrigeration) before processing without affecting results; 5) IC can be incorporated into a double blind randomized clinical trial (RCT) of DED; and 6) the Biomarker Laboratory can track a large number of masked samples reliably. In conclusion, our standard operating procedure for impression cytology analysis of HLA-DR expression appears to be repeatable and reproducible for use in multicenter clinical trials, providing a minimally invasive objective biomarker of inflammation of the ocular surface.
Keywords: Impression Cytology, HLA-DR, Dry Eye, Precision, Reliability, Repeatability, Storage, Feasibility
1. Introduction
Ocular surface disease can be defined as disorders that affect components of the ocular surface resulting in changes in the ocular tear film and/or the integrity of the ocular surface. Examples include diseases such as: dry eye disease (DED), allergy, blepharitis, and infections.
DED, a multi-factorial disease of the tears and ocular surface, is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface (Definition and Classification Subcommittee of the International Dry Eye Workshop, 2007a). Although common, diagnosis and clinical evaluation (Definition and Classification Subcommittee of the International Dry Eye Workshop, 2007a), and efficacy of treatment (Definition and Classification Subcommittee of the International Dry Eye Workshop, 2007b) of dry eye typically rely on patient symptoms and clinicians’ subjective grading of biomicroscopic ocular surface staining patterns. In the past, research of ocular surface diseases, such as DED, was limited by the lack of objective metrics for classifying severity and treatment outcomes.
A biomarker is an objectively measured characteristic that is evaluated as an indicator of pathogenic processes or pharmacological/physiological responses to intervention (Biomarkers Definitions Working Group, 2001). In this case, it is meant as a pharmacological or physiological measurement that can: 1) be used to diagnose the increase in inflammation of the ocular surface seen in DED, 2) help in the classification of the severity of DED, 3) assess the response to treatment and 4) shed light on the pathophysiology of ocular surface disease (i.e. correlate with clinical signs and symptoms).
Many authors have searched for a definitive, pathognomonic biomarker (Beuerman et al, 2011; Chaglasian, 2011; Zhao et al, 2009) of DED. Even though the pathogenesis is not fully understood and likely multifactorial, it is recognized that inflammation has a prominent role in its’ development and propagation (Definition and Classification Subcommittee of the International Dry Eye Workshop, 2007b). Inflammatory biomarkers such as human leukocyte antigen (HLA-DR) have been shown to be upregulated in DED (see Table 1) and could serve as a method for the classification of its severity and response to treatment. However, the results are variable, and possibly due to a lack of standardized methodology, making results difficult to interpret among different treatments and clinical trials.
Table 1. Reported Percentage of Cells Highly Expressing HLA-DR in Conjunctival Impression Cytology Samples of Normal or Dry Eye Disease Patients.
Summary of publications reporting on relative percentage of cells which highly express HLA-DR antigen in conjunctival impression cytology samples of normal and/or dry eye disease patients.
| Reference | Brief Description | Normal | Dry Eye | Changes |
|---|---|---|---|---|
| Versura, et al (2011) | 15 normal 25 DE |
7.2 ± 1.1 | 46.2 ± 7.2 | ↑ 6.4× |
| Sanchez, et al (2010) | 24 normal 21 DE |
5.3 ± 3.0 | 4.7 ± 2.8 | ↓ 1.1× |
| Mrugacz, et al (2007) | 25 normal 25 DE |
8.1 ± 1.9 | 16.9 ± 10.3 | ↑ 2.0× |
| Rolando, et al (2005) | 16 normal 16 DE |
Superior: 1.2 ± 0.2 Temporal: 0.8 ± 0.8 Nasal: 1.2 ± 0.2 |
1.3 ± 0.2 (mild); 1.3 ± 0.2 (moderate) 1.5 ± 0.2 (mild); 1.8 ± 0.8 (moderate) 1.7 ± 0.2 (mild); 1.7 ± 0.1 (moderate) |
↑ 1.1× (mild); 1.5× (moderate) ↑ 1.9× (mild); 2.3× (moderate) ↑ 1.4× (mild); 1.4× (moderate) |
| Pisella, et al (2000) | 12 normal 13 DE |
9.9 ± 5.9 | 56.9 ± 24.6 | ↑ 5.7× |
| Brignole, et al (2000) | 50 normal 243 DE |
54 ± 32 | 61 ± 31 | ↑ 1.1× |
Human leukocyte antigen-DR is a biomarker of inflammation, normally expressed on most immune-competent cells (Newman, 1997), and some non-immune epithelial cells (Baudouin et al, 1992). HLA-DR molecules are upregulated in response to signaling (reviewed by Lopin et al, 2009) and are a biomarker for immune activation (Newman, 1997). Increased HLA-DR expression has been associated with many immune disorders/inflammatory processes (Jaraquemada et al, 1986; Ichikawa et al, 1990; Ichikawa et al, 1990; Ishihara et al, 1994; Goronzy and Weyand, 1990; Pisella et al, 2000) and ocular surface diseases such as DED (Baudouin et al, 2000).
Impression cytology (IC) can be utilized to collect and harvest cells from the ocular surface to quantify HLA-DR expression using immunocytochemical staining followed by flow cytometry (Fernandez et al, 2001; Calonge et al, 2004). Since its introduction (Egbert et al, 1977), IC has been found to be useful in assessing the ocular surface in various ocular disorders such as dry eye/keratoconjunctivitis sicca (KCS; Wittpenn et al, 1986; Brignole et al, 2001), cicatricial ocular pemphigoid (Rivas et al, 2004), and vitamin A deficiency (McKelvie, 2003; Natadisastra et al, 1988) and has been used in clinical trials to evaluate HLA-DR expression in ocular surface diseases (Barabino et al, 2010; Mrugacz et al, 2007; Rolando et al, 2005; Baudouin et al, 2002; Brignole et al, 2000; Pisella et al, 2000; Tsubota et al, 1999; Baudouin et al, 1997; Baudouin et al, 1992). However, the reliability, reproducibility and storage limitations for the use of relative HLA-DR expression on IC-collected cells as a biomarker of inflammation for clinical trials has not yet been clearly established although there are several articles highlighting the uses of IC (Barabino et al, 2010; Baudouin et al, 1992; Baudouin et al, 1997; Baudouin et al, 2000; Brignole et al, 1998; Brignole et al, 2000; Brignole et al, 2001; Brignole-Baudouin et al, 2004a; Brignole-Baudouin et al, 2004b; Calonge et al, 2004; Lopin et al, 2009; Fernandez et al, 2001; McKelvie, 2003; Natadisastra et al, 1987; Natadisastra et al, 1988; Nelson et al, 1983; Puangsricharern and Tseng, 1995; Rolando et al, 2005; Roy et al, 2011; Singh et al, 2005; Stern et al, 2002; Stevenson et al, 2000; Thiel et al, 1997; Tole et al, 2001; Tseng, 1985. Tsubota et al, 1999; Wittpenn et al, 1986).
Results from various recent studies have suggested HLA-DR to be increased in DED (see Table 1), but indicate a large variability, likely because of considerable technique and/or biologic variability, lack of standard operating procedures (SOPs) and variation in populations with small sample size (typically, single site with small cohorts).
Lack of objective metrics has made data from clinical trials in DED very difficult to interpret, since current endpoints have so much variability. The purpose of the present study was to establish a standardized methodology for determining HLA-DR expression as a minimally invasive objective biomarker and metric of inflammation of the ocular surface and evaluate the precision, reliability and repeatability of its use.
2. Methods
2.1 Materials and Supplies
2.1.1 Impression Cytology Sample Collection
Ophthalmic Jewelers’ Forceps #5 Tapered, Straight (Robbins Instruments, Inc., Chatham, NJ)
Neutral Buffered Formalin Solution (10% paraformaldehyde (PF), Sigma-Aldrich Co. LLC., St. Louis, MO)
Phosphate Buffered Saline 1× (PBS, Mediatech, Inc., Manassas, VA)
Proparacaine Hydrochloride (0.5%) Ophthalmic Solution (Bausch & Lomb, Rochester, NY)
Sterile 0.20µm 13mm polyether sulfone filter membranes (Supor -6-Membranes, Pall Life Sciences, Ann Arbor, MI)
Prophylactic Antibiotics (Moxifloxacin Hydrochloride 0.5%)
Temperature resistant labels (VWR International, LLC., Radnor, PA)
Test Tubes 5ml, 12×75mm (BD Biosciences, Bedford, MA)
Westcott scissors (Orthomed, Inc., Rochester, NY)
2.1.2 Storage of Samples
Welbilt Freestanding Lockable Refrigerator (Welbilt Corporation, W565E, Stamford, CT)
Storage Boxes (Fisher Scientific, NC9525439, Pittsburg, PA)
2.1.3 Shipping of Samples
Biohazardous Exempt Human Sample Shipping Container: Infecon 3000 (VWR 11217-660, Radnor, PA)
Cold Pack (Fisher Scientific, 03-530-110, Pittsburg, PA)
Parafilm (Fisher Scientific, 13-374-16, Pittsburg, PA)
2.1.4 Processing/Immunostaining of Samples
Bovine Serum Albumin 0.5% (Sigma Aldrich, St. Louis, Mo)
Phosphate Buffered Saline 1× (Mediatech, Inc., Manassas, VA)
Vortex Genie (Scientific Industries, Inc., Bohemia, NY)
Beckman GS 6R Refrigerated Centrifuge (Beckman Coulter, Inc, Fullerton, CA)
Fluoroscein Isothiocyanate (FITC) Conjugated Monoclonal Mouse Anti-Human HLA-DR, Alpha Chain Clone Antibody (BD Biosciences, TAL.1B5, Bedford, MA)
Fluoroscein Isothiocyanate Conjugated Mouse Antihuman IgG2b Antibody (BD Biosciences, Bedford, MA)
2.1.5 Flow Cytometry Calibration and Data Collection
Accuri C6 Argon Ion Laser Flow Cytometer Emitting at 488nm (Accuri Cytometers Inc, Ann Arbor, MI)
Cyto-trol Control Cells (Beckman Coulter, Inc., Indianapolis, IN)
Immuno-trol Process Controls (Diagnostic Solutions, Inc., Victoria, Australia)
Rainbow Calibration Particles, Six Peak (Spherotech Inc, Lake Forest, IL)
Rainbow Calibration Particles, Eight Peak (Spherotech Inc, Lake Forest, IL)
Immortalized Conjunctival Epithelium [clone 1-5c-4 (Wong-Kilbourne derivative of Chang conjunctiva), ATCC, Manassas, VA]
HTK-H cells (human corneal fibroblast cell line; ATCC, Manassas, VA)
2.1.6 Analysis of Flow Cytometric Data
FlowJo Flow Cytometry Analysis Software (Ashland, OR)
2.1.7 Statistical Analyses
SAS Statistical Software (SAS Institute Inc., Cary, NC, U.S.A.)
SPSS Statistical Software (SPSS Inc., Chicago, IL, U.S.A.)
NCSS Statistical Analysis Software (NCSS, Kaysville, Utah)
2.2 Detailed Methods
2.2.1 Study Design
Multiple studies were conducted to establish standardized operating procedures [SOP(s) for determining HLA-DR expression as a minimally invasive objective biomarker of inflammation of the ocular surface. The SOPs provide specific guidelines for performing each of the steps involved in HLA-DR collection and analysis, in order to reliably incorporate it into multi-center clinical trial and/or translational research. These include collection of cells in tubes labeled without personal identifying information, storage, shipping and tracking of the samples prior to analysis, separation of the cells from the filter membranes, staining of the cells for the biomarker (in this case, HLA-DR), the running of these isolated, stained cells through the previously calibrated flow cytometer, analysis of the resulting raw data by a dedicated flow cytometry analyzer and obtaining the resulting percentages of cells highly expressing HLA-DR antigen. These results are then imported into a secure electronic relational database tailored for high throughput to track the data, then sent to the Coordinating Center for unmasking and analysis.
The validity/viability of the SOPs for IC collection and analysis were established through experiments to demonstrate the following: 1) that sufficient numbers of cells can be collected via IC; 2) the precision/repeatability of the relative biomarker expression quantified in samples; 3) the ability to teach personnel at distant sites to collect samples, store and ship to Biomarker Laboratory; 4) the ability to store samples without affecting results; 5) IC can be incorporated into a double blind randomized clinical trial (RCT) of DED and 6) the Biomarker Laboratory can track a large number of masked samples reliably.
2.2.2 Patient Participation
The informed consent form and protocol were approved by the Mount Sinai Program for Protection of Human Subjects, New York, NY. Subjects included patients with DED and individuals with normal anterior segment examinations. The studies were conducted in accordance with the good clinical practice guidelines for the evaluation of medical products and followed the tenets of the Declaration of Helsinki.
2.2.3 Impression Cytology (IC)
2.2.3.1 Sample Collection Methodology
SOP
Cells are collected via the technique of Baudouin et al (Brignole et al, 1998; Brignole-Baudouin et al, 2004a; Brignole-Baudouin et al, 2004b) as modified by Massingale (Massingale et al, 2009). IC is performed at least 15 minutes after all other exams and ophthalmic procedure(s) are completed. Patients should be asked to gaze downwards for superior temporal collection and asked to gaze nasally for temporal collection. Sterile scissors can be used to cut each piece of filter membrane into two semicircles. After administration of a single drop of topical anesthetic (0.5% proparacaine hydrochloride ophthalmic solution), up to four (although in most studies only 2) sterile 0.20µm 13mm polyether sulfone filter membranes are applied to each eye, in a sterile fashion, onto the bulbar conjunctiva (see Figure 1) without applying pressure, and are then gently lifted off after a few seconds. Examination of the filter will demonstrate a “sheen” that correlates with cells sticking to the filter paper. The filters are then placed into tubes containing 2mL of 0.05% PF in PBS and labeled with numeric and alphabetic codes that contain no personal identifiers, so as to link to other information on the patient labeled with the same codes (see section 2.2.11.1).
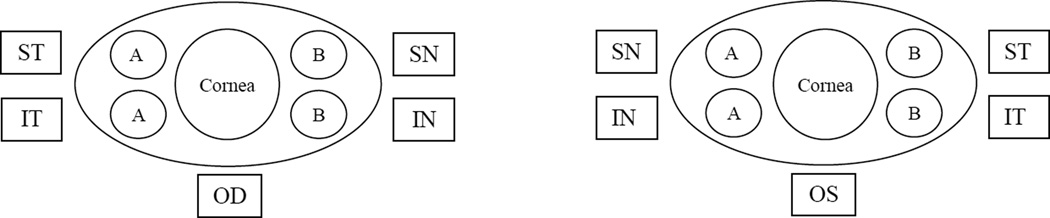
Figure 1.
Impression Cytology: Diagram of placement of filter membrane collection sites to collect cells from conjunctiva of each eye. The superotemperal (ST), inferotemporal (IT) filter membranes from the right eye (OD) and the superonasal (SN) and inferonasal (IN) filter membranes from the left eye (OS) were designated Area “A”, while the superonasal (SN) and inferonasal (IN) filter membranes from the right eye (OD) and the superotemperal (ST), inferotemporal (IT) filter membranes from the left (OS) were designated Area “B”.
Samples are kept at room temperature for 90 minutes and then placed in refrigeration (4°–8°C) until transport to the Biomarker Laboratory (see section 2.2.10). One (1) to 2 drops of prophylactic antibiotic (such as: 0.5% moxifloxacin hydrochloride, polymyxin B and trimethoprim or ofloxacin) are administered to both eyes post collection.
2.2.3.2 Determination of Number of Cells per IC Sample (Sufficient for Analysis) Sample Evaluation
Other than a general stipulation of using greater than 1,000 cells for analysis (Baudouin, et al, 1997) and of collecting “adequate” numbers of cells (Athmanathan et al, 2001; Nolan et al, 1994), little has been published regarding the cell yields from IC to date (Barabino et al, 2010; Baudouin, et al, 1997). In our studies, overall, 366 samples (individual tubes with 2–4 filter membranes/tube) yielded an average of 6,677 cells/filter ± 6,302 (normal: 8,511 ± 7,743, n = 114, range: 1,389 – 101,031; DED: 5,414 ± 5,128, n = 252, range: 1,032 – 58,892). Only 4/366 samples collected (1.1%) were below the 1,000 threshold and 2 others between 1,000 – 2,000 cells (<0.6%). Thus, sufficient numbers of cells could be collected from 99% of the samples received and therefore IC is an effective method to collect sufficient numbers of cells for flow cytometric analysis.
While some authors advocate brush histology (Guglielminetti et al, 2002; Pauly et al, 2007; Tsubota et al, 1990), in our hands, impression cytology yielded higher numbers of cells in the samples. Future innovations, such as a standardized sampling technique (OPIA Technologies, Paris, France) which uses a single use device to obtain IC conjunctival impressions of the living eye [presently under development (Roy, 2012)], could be adopted as soon as it is validated and becomes commercially available.
2.2.4 Processing/Immunostaining of IC Samples
SOP
Labeled stored samples (still attached to the filter membranes) are removed from refrigeration (4°–8°C), diluted with 2mL (4°–8°C) 0.5% bovine serum albumin (BSA) in PBS and the cells mechanically separated from the filters by agitation (vortex; 15sec), then divided into two (2) equivalents (“1+” and “1−”) for staining and background/control. The cells are concentrated by centrifugation at 1600 rpms for 5minutes (4°C), the filters removed and the cells resuspended in 200 µL 0.5% BSA in PBS. The mate tube (“1+”) is then stained for HLA-DR using a direct immunofluorescent technique [fluoroscein isothiocyanate (FITC) conjugated monoclonal mouse anti-human HLA-DR antibody in a 1:50 dilution in 0.5% BSA in PBS at 4°C in the dark for 90 minutes], while the other (“1−”) is stained with fluoroscein isothiocyanate conjugated mouse antihuman IgG2b (also in a 1:50 dilution in 0.5% BSA in PBS at 4°C in the dark for 90 minutes) which serves as a negative isotypic control. All cells are then collected by centrifugation (1600rpm×5min, 4°C), washed twice with 0.5% BSA in PBS and resuspended in 400uL BSA (0.5%) in PBS at room temperature (approximately 27°C) in preparation for flow cytometry. New lots of all antibodies are regularly compared and standardized to preexisting lots before use.
2.2.5 Collection of Flow Cytometric Data
SOP
After immunostaining, the cells, diluted in 400µL 0.5% BSA in PBS, are inserted into the sample input port (SIP) of the flow cytometer (argon ion laser emitting at 488nm; appropriately set to collect data) and analyzed by flow cytometry. The fluidics are backflushed and washed for 2 minutes with 0.5% BSA in PBS in between each sample.
2.2.6 Quality Control
2.2.6.1 Flow Cytometer Controls
SOP
The calibration of the instrument is confirmed each day before use with the manufacturer recommended standardization/calibration procedures and “quality control” methodologies including the use of Flow-Check™ (fluidics standards), Flow-Set™ (laser output standards), Alignflow™ [laser alignment (performance standards)], BD™ Compbeads (compensation), and six and eight peaked Rainbow Calibration Particles [“beads” serving dual (cytometer and technique control) functions]. New lots of all standards, controls, antibodies, etc. should be compared and standardized to preexisting lots before being used to confirm calibration of the instrument.
Although compensation is not necessary if using only one fluorochrome/fluorescent channel, it is performed as a means to further verify the proper functioning and calibration of the cytometer. Following proper staining procedures, compensation is performed either prior to (i.e. immediately prior to data collection) or during analysis (i.e. after data collection).
2.2.6.2 Technique Controls
SOP
In addition to the calibration of the instrument, antibody adherence and fluorescence is confirmed each day with Immuno-Trol FACS Control Kits (cell-like particles whose light scatter, population distribution, fluorescence intensity, and antigen density mimic those of whole cells), and Cyto-Trol FACS Control Kits (lyophilized human lymphocytes, isolated from peripheral blood, to confirm/assess the activity of monoclonal antibodies), as well as immortalized conjunctival epithelium (De Saint Jean et al, 2004) as the negative control (Jester et al, 2003), and HTK-H cells (human corneal fibroblast cell line; De Saint Jean et al, 2004) as the positive control for the HLA-DR staining. In addition to the FACS Control Kits, buffy coat blood cell fractions, previously collected, standardized, aliquoted and frozen (Pietersz et al, 1987) are also utilized to ensure standardization throughout the entire study/clinical trial. New lots of all standards, controls, antibodies, etc. should be compared and standardized to preexisting lots before being used to confirm proper staining and standardization of results.
2.2.7 Analysis of Flow Cytometric Data
SOP
For analysis, dot plots for cell size (forward scatter) vs. cellular granularity (side scatter) and fluorescence channel (FL-)1 vs. cellular granularity as well as a histogram [fluorescence channel (FL-)1 vs. event count] are plotted by the flow cytometry analysis software (CFlow: BD Accuri, BD Biosciences, Ann Arbor, MI; FACSSuite, FACSDiva, FCS Express: BD Biosciences, San Jose, CA; FlowJo Flow Cytometry Analysis Software, Tree Star, Inc, Ashland, OR), demonstrating a single homogenous cell population (as defined/described by a well delimited single clump of cellular events with size, granularity and cellular HLA-DR/FITC fluorescence characteristics consistent with those of the ocular conjunctival cells normally collected by impression cytology). Sequential analytic gates are set around the area of interest (labeled Area of Interest “P2” by the analysis software) in order to exclude cellular debris and aggregates. The number of highly positive cells (as well as the percentage of the total) is thus obtained from the cytogram resulting from the FL-1 vs. cellular granularity (side scatter) dot plot’s statistics of Area of Interest “P2”, yielding the mean intensity of cell fluorescein which is proportional to the relative HLA-DR expression.
The relative number of highly positive cells as a percentage of the total number of cells is the best method of quantifying inflammatory stimulus. Purely quantifying “HLA-DR+ (“HLADR-high”) cell numbers or HLA-DR expression per cell could be “skewed” by variabilities originating from the different amounts of cells collected by different filters/patients and/or environmental impact. By reporting on the percentage of cells collected which highly express HLA-DR antigen within the entire cell population collected, the possibility of such variability affecting the results is avoided. In addition, although it is not entirely clear whether the observed relative increase in HLA-DR antigen is a result of the “activation” of conjunctival epithelial cells, or due to the migration of antigen presenting cells which highly express HLA-DR antigen, the results report on the increased number of HLA-DR+ cells observed in dry eye patients regardless of the etiology of the increase. Additional antibody testing may provide further information on the type of cells that express HLA-DR and this too can then be correlated with clinical findings.
In each sample, 1,000 – 10,000 cells are routinely analyzed; samples containing less than 1,000 cells are discarded. Typically, users of flow cytometry feel at least 1,000 cells are needed to ensure representative sampling (Baudouin, et al, 1997).
2.2.8 Precision Validation of Flow Cytometry Analysis of IC Samples Test
To evaluate the precision (reliability/reproducibility) of the SOPs for quantifying the relative HLA-DR expression as a minimally invasive objective biomarker of the ocular surface, IC samples were collected (see section 2.2.3.1) and processed (see section 2.2.4) per the described SOPs, however once cells were eluted off the filter membranes each sample was split into 2 sample tubes (for separate processing/analysis) and relabeled in a masked fashion (see section 2.2.10.1). Analysis was then continued per the SOPs, starting with taking each split sample and again dividing it into “1+” and “1−” (for positive staining and negative isotypic background/control).
Two (2) samples each from 17 normal individuals (n = 34, range: 2,153 – 58,296 cells) and 15 presenting with established DED (n = 30, range: 1,055 – 32,240 cells) were analyzed for HLA-DR expression. For these split samples of normal individuals, the mean difference between these split samples was −0.14%, p = 0.54; the 95% limits of agreement (confidence interval) between the two splits (value of “Split 1” subtracted from that of “Split 2”) was −2.01%, +1.74%; for those with established DED it was 0.19%, p = 0.63 and the 95% limits of agreement were −3.37%, +3.75%. There was no statistically significant difference in the percentage of cells highly expressing HLA-DR when comparing such split samples (demonstrating good statistical similarity and valid SOPs).
Both cell size [mean geographic forward scatter scores (FSC)] and granularity [mean geographic side scatter scores (SSC)] for either normal healthy individuals, or those with established DED, were not significantly different (normal: FSC: range: 413,783.40 – 891,438.30, P > 0.89, SSC: range: 161,586.30 – 985,372.40, P > 0.45; DED: FSC: range: 18,865.69 – 198,907.77, P > 0.92, SSC: range: 170,601.00 – 654,264.20, P > 0.54, respectively). These studies demonstrate the precision of the SOPs to determine the relative percentage of cells highly expressing HLA-DR antigen.
2.2.9 Sample Storage: Demonstration of Ability to Store IC Samples Before Analysis
For biomarkers, such as HLA-DR, to be useful for multicenter clinical trials, samples need to be collected and then stored prior to analysis. While some studies have been published looking at HLA-DR antigen expression over time, all involved the collection and immediate analysis of sequential samples: that is, the samples were not stored (Barabino et al, 2010; Brignole-Baudouin et al, 2011) and the storage limitations and reliability/reproducibility of stored IC samples has not been clearly established.
IC samples cannot be divided and stored once processing is initiated (i.e. cells are eluted from filter membranes). Therefore, in order to validate SOP for storage of IC samples, the similarity of 2 samples from the same subject (Areas “A” and “B”, see Figure 1) first had to be established. Area “A” consisted of the superotemperal (ST), inferotemporal (IT) from the right (OD) and the superonasal (SN) and inferonasal (IN) filter membranes from the left eye (OS)., While Area “B” consisted of the superonasal (SN) and inferonasal (IN) filter membranes from the right eye (OD) and the superotemperal (ST), inferotemporal (IT) from the left (OS)(see Figure 1). For the normal individuals (n = 12, range: 2,160 – 58,296 cells), the mean difference between the “A” and “B” areas was 0.13%, p = 0.73, the 95% limits of agreement (confidence interval) between the two was −1.01%, +1.26%; while that for those with DED (n = 6, range: 6,812 – 47,546 cells) was −0.59%, p = 0.89, and the 95% limits of agreement (confidence interval) between the two areas was −3.00%, +1.82% The percent difference between areas “A” and “B” in normal versus DED is likely due to greater biological variability in DED (σ = 4.54) and/or the sample size. Evaluation of normal and DED demonstrated the variability between areas was not greater than the variability between split samples (see sections 2.2.8 & 2.2.9; F1,19 = 0.21, p = 0.65). These studies demonstrated that Area “A” is similar to Area “B” for both normal and DED patients.
Both cell size (mean geographic FSC) and granularity (mean geographic SSC) of areas “A” and “B” for normal healthy individuals or individuals with established DED were similarly not significantly different from each other (normal: FSC: range: 413,783.40 – 891,438.30, P > 0.89, SSC: range: 161,586.30 – 985,372.40, P > 0.45; DED: FSC: range: 593,208.15 – 960,201.50, P > 0.93; SSC: range: 220,402.66 – 403,785.51, P > 0.90, respectively).
Having validated that Area “A” is similar to Area “B” for each subject, we then compared Area “A” samples processed within 1 day of collection with Area “B” samples stored for 21 to 30 days at 4°–8°C until processing/immunostaining. This will allow for 1 week’s storage at a satellite site prior to shipment, overnight delivery, an additional week’s storage at the Coordinating Center/Biomarker Laboratory prior to/during processing with an additional 2 week “safety window”.
The samples were stored in the dark in an alarmed refrigerator outfitted with emergency power generator backup to maintain consistent, adequate storage conditions in the event of a power failure. For the normal group (21 day storage: n = 30; 30 day storage: n = 11), the mean differences for HLA-DR% between the “A” and “B” areas was 0.24%, 0.30% (i.e., Day 21 values were on average higher from those of Day 1 by 0.24%, p = 0.56 and Day 30 values were on average higher by 0.30%, p = 0.21) and the 95% limits of agreement were (Day 21: −2.52%, +2.99%; Day 30: −0.95%, +1.55%). For the DED group (21 day storage: n = 30; 30 day storage: n = 30), the mean differences for HLA-DR% between these “A” and “B” areas was −0.02%, – 1.46% (i.e., Day 21 values were on average lower than those of Day 1 by 0.02 %, p = 0.94 and Day 30 values were on average lower by 1.46%; p=0.15) and the 95% limits of agreement were (Day 21: −2.93%, +2.89%; Day 30: −7.48%, +4. 57%).
Although these studies demonstrated that samples can be safely stored for as long as 30 days, we suggest limiting storage to 21 days to ensure antigen stability and sample precision.
2.2.10 Labeling, Masking, and Tracking Samples
2.2.10.1 Labeling and Masking of Sample Tubes
SOP
Tubes are labeled with only a masked, unique code number and the date of collection (does not reveal information about subject) and then stored at 4° to 8°C in the dark until staining. Labeling is done with temperature resistant labels and markers to ensure sample labels remain legible --- even with storage and shipping. Labels should be covered with scotch tape wrapped twice around to prevent water damage. Parafilm should be used to seal the tubes for shipping. All specimens are examined by a masked observer. The Coordinating Center keeps the “code break” (the information about each particular patient that relates to each unique code number) and all related personal patient identifying information; no personal identifying information is stored with the samples and the Biomarker Laboratory is kept unaware of any identifying information to ensure analysis is performed in a masked fashion to provide objective metrics.
2.2.10.2 Sample Tracking
SOP
All samples are received by the Biomarker Laboratory masked, then logged and entered into an electronic relational database tailored for high throughput. The relational database is backed up daily and the backup is stored encrypted in a location separate from the samples and analyzer. Prior to deployment and use the electronic systems are subjected to extensive testing to validate the proper functioning of the system.
Using this technique, we reliably tracked a large number of samples (1,038 samples; over 73,000,000 data points) while maintaining their masked state, establishing the ability to process samples for a multicenter clinical trial.
2.2.11 Ability to Obtain Samples Collected at a Distant Site for a Multicenter Clinical Trial
To establish our ability to utilize HLA-DR expression in a multicenter clinical trial, Clinicians at a distant site were trained to collect IC samples, store and then ship to the Biomarker Laboratory for logging and processing.
2.2.11.1 Instruction of Personnel for Multicenter Clinical Trial IC Sample Collection Technique
SOP (Training)
Training is an essential part. Collaborating ophthalmologists, optometrists and ophthalmic technicians at distant sites are taught the collection, storage and shipping techniques via an automated, internet based, video with subsequent conference call question and answer sessions. These sessions are recorded for use in the education and certification program of new staff. Also, a “Help Desk” provides telephone and email support services for the clinical center staff and service providers.
2.2.11.2 Shipping of Samples
SOP
Samples are collected at distant sites stored at 4°–8°C and then shipped weekly to the Biomarker Laboratory in the dark at 4°–8°C (cold pack) utilizing IATA Packing Instruction 602-certified materials as per IATA liquid regulations and assembled as per manufacturer’s instructions. Upon receipt, samples are logged in (de-identified), then stored as described previously until processing.
2.2.11.3 Validation of Methodology of Samples Collected at a Distant Site Evaluation
To validate the precision of the HLA-DR antigen quantification obtained from samples collected at a distant site, samples were collected from the right (OD) and left (OS) eyes of 20 subjects (10 normal individuals and 10 individuals who wear contact lenses) by web-based trained clinicians in Ohio, then shipped to our facility on ice by overnight air. Upon receipt, these samples were logged in and stored in the dark at 4°–8°C as described above (see section 2.2.10), then immunocytochemically stained and analyzed (flow cytometry). Of the 40 samples (20 individuals×2 eyes), 100% arrived intact, were analyzed without difficulty, and yielded a sufficient number of cells (6,180 cells/filter ± 4,275), similar to the amount collected at our clinical site (6,667 cells/filter ± 6,302).
Comparing the respective HLA-DR expression levels from the IC collected conjunctival cells of the right (OD) and left (OS) eyes of each subject reported to be clinically similar (ocular exams), demonstrated no statistically significant difference in the percentage of cells highly expressing HLA-DR antigen. The mean difference between OD and OS was −0.15% [i.e., the left eye (OS) values were on average lower than those of the right eye (OD) by 0.15 %]; p = 0.10; the 95% limits of agreement (confidence interval) between the two was −0.98%, +0.68%.
Both cell size (mean geographic FSC) and granularity (mean geographic SSC) between the right (OD) and left (OS) eye samples of subjects collected at a distant site, then shipped to, and processed and analyzed at our facility were similarly not significantly different from each other (FSC: range: 568,889.91 – 826,924.95, 95% limits of agreement: −984.45, +44,446.62, p = 0.94; SSC: range: 119,996.19 – 262,544.14, 95% limits of agreement: −8,556.51, +28,000.26, p = 0.38, respectively; data not shown) and were not significantly different from samples collected at our facility (FSC: range: 413,783.40 – 891,438.30, 95% limits of agreement: −43,934.86, +176,740.70, p = 0.42; SSC: range: 161,586.30 – 985,372.40, 95% limits of agreement: −104,380.60, +7,004.37, p = 0.18).
2.2.12 Validation of SOPs for HLA-DR Analysis from IC Samples for Use in a Double Blind Randomized Clinical Trial
IC sampling and analysis was incorporated into a double blind randomized clinical trial of Omega 3 supplementation of DED subjects. Eighteen (18) subjects were enrolled and IC sampling on the right and left eye was done at baseline and month 3. Sixty-seven (67) samples were collected (2 filters/eye/sample), 1 subject lost to follow up had IC done only at baseline and in 3 others one eye sample yielded low cell numbers (743, 546 and 338 cells/sample, respectively), likely due to a newly trained coordinator. Therefore we achieved a 96% collection rate of samples that could be analyzed reliably.
A total of 6,381 cells/filter membrane ± 6,528 (n = 67, range: 1,463 – 58,632) were collected which is not significantly different from the 5,431 ± 5,129 cells/filter membrane collected from individuals with established DED (n = 299, range: 1,032 – 58,892) or from the 6,677 cells/filter membrane ± 6,302 collected overall (n = 604, range: 1,032 – 58,892). HLA-DR pretreatment expression was similar to earlier DE patients [mean difference for HLA-DR expression was 0.20%; p=0.84]. Sample size in this RCT was too small to reach statistical significance: a larger study powered to determine treatment efficacy will be needed, but the ability to incorporate IC sampling and analysis into RCT was confirmed.
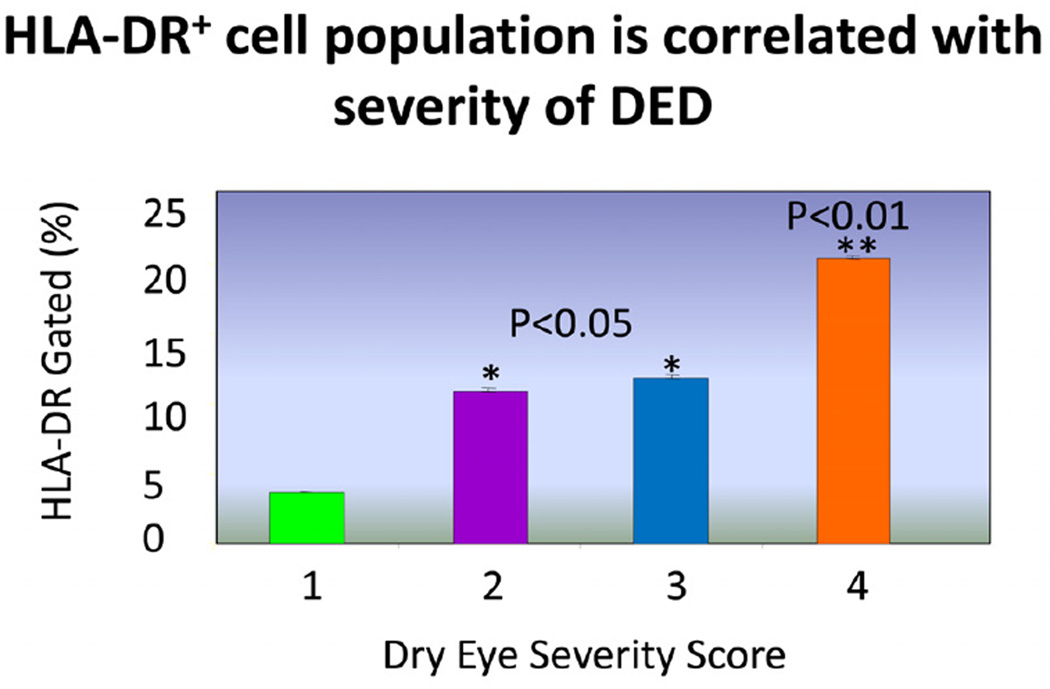
In order to study the changes in HLA-DR expression with severity of DED, an observational study of 80 DED subjects was conducted. The severity of DED was categorized using the Dry Eye Work Shop (DEWS) categorization system. (Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007b). Co-relating the subjects’ DEWS scores(DEWS category 1: n=13, DEWS category 2: n=29, DEWS category 3: n=31, DEWS category 4: n=7) to their HLA-DR expression, results showed a trend indicating increasing expression of antigen directly correlates with increasing severity of DED (see Figure 2), supporting the use of HLA-DR expression as a biomarker for the severity of DED.
Figure 2.
The relative expression of HLA-DR antigen (%) ± standard deviation (σ) in conjunctival cells collected by impression cytology against an independently ranked dry eye severity per the DEWS classification system. Asterisks denote statistical significance (* p < 0.05, ** p < 0.01).
2.2.13 Quality Control Collection Metrics
SOP
Performance metrics on the Clinical Sites and Biomarker Laboratory (antibody performance and flow cytometer lasers and fluidics) must be regularly collected and reported to the Coordinating Center and/or Principal Investigator, so corrective action can be taken, when necessary, to counsel/repair the sub-standardly performing individuals, sites, and/or equipment so as to maintain performance quality control. The following reports are generated by the Biomarker Laboratory and forwarded to the Coordinating Center and Principal Investigator monthly for distribution to the individual Clinical Sites:
2.2.13.1 Clinical Sites’ Collection Metrics
SOP
A monthly report compiled by the Biomarker Laboratory details each clinical site’s performance on a site-by-site basis. This report contains: the number of samples received, the number of samples processed and successfully analyzed with the number and percentage (compared to the total) of those not able to be processed and the reason(s) behind the processing/analysis failure, the time from sample collection to its’ receipt by the Biomarker Laboratory (for each sample), the number of cells collected (on a per sample basis), cell size [mean geographic forward scatter scores (FSC), on a per sample basis] and granularity [mean geographic side scatter scores (SSC), on a per sample basis].
An additional compilation of the performance metrics of all of the clinical sites is also assembled. This monthly report contains: the number of samples received on a per site basis, the number of samples processed and successfully analyzed with the number and percentage (compared to the total) of those not able to be processed (also on a per site basis), the average number of cells, the average cell size and the average granularity of the samples collected by each site (on a per site basis) and their corresponding standard deviations.
2.2.13.2 Biomarker Laboratory Metrics
SOP
A third monthly report compiled by the Biomarker Laboratory details the performance of the lasers and fluidics of the flow cytometer as well as the performance statistics of the antibodies and immunohistochemical staining. As mentioned previously in section 2.2.6, the calibration of the flow cytometer and antibody staining efficacy is confirmed each day before use. The output (i.e. “results") obtained from these manufacturer recommended standardization/calibration procedures and “quality control” methodologies are logged and reported. This includes the instruments performance relative to the specification gating limits (i.e. whether or not the results from the Flow Check, Flow Set, Alignment, Compensation, and six and eight peaked Rainbow Calibration Particles were each within the manufacturer specified ranges of allowable values, outside these values or “borderline”), as well as the antibody adherence and fluorescence performance specifics relative to the baselines from the previously mentioned Immuno-Trol and Cyto-Trol FACS Control Kits, and the individual cell counts and staining specifics (i.e. the total number of cells within the gates and the locations of the gates in relation to the total number of cells) of the negative and positive controls and the previously collected, standardized and aliquoted buffy coat blood cell fractions.
SOP
An additional monthly report outlines the performance of the Biomarker Laboratory itself. This report contains: the number of samples received, the number of samples processed and successfully analyzed with the number and percentage (compared to the total) of those not able to be processed and the reason(s) behind the processing/analysis failure, the time from sample receipt by the Biomarker Laboratory to the processing/analysis (for each sample), the number of cells collected per sample, cell size [mean geographic forward scatter scores (FSC), on a per sample basis] and granularity [mean geographic side scatter scores (SSC), on a per sample basis].
The logging of all generated data results into an electronic, high throughput relational database (backed up daily) facilitates the generation of reports as a single template that can be designed for each report and then “run” monthly.
3. Potential Pitfalls and Troubleshooting
3.1 Impression Cytology Sample Collection
Sampling location may add variability to the results. Up to four (4) samples can be collected from each eye, using one (1) half of a filter membrane for each of the four (4) areas of the bulbar conjunctiva (superonasal, superotemperal, inferotemporal, inferonasal; see Figure 1) so as to maximize yield and limit variability secondary to microanatomic variation. Repeating sampling from the same location within the eye is also of concern if taken frequently, however, in our clinical trials samples are taken every 2 to 3 months, so this is not likely to be an issue. Also, IC must occur at least 15 minutes later than any ocular surface staining procedures to allow time for any dyes that have been used to be flushed from the eye. Failure to do so will result in transfer of dye to the filter membranes and low yields of cells adherent to the membrane.
Many users of flow cytometry specify at least 1,000 cells are needed to ensure representative sampling (Baudouin, et al, 1997) and the number of cells collected by IC is somewhat limited and can be an issue so care should be taken to collect in a manner so as to maximize yield. Filters should be visually inspected to ensure >80% confluency. Samples with <80% cells should have the collection procedure repeated.
3.2 Processing/Immunostaining of IC Samples
Overly aggressive mechanical separation of the cells from the filters by agitation (vortex; must be gentle), or for too long (greater than 60 seconds), results in the lysis of the cell membranes with subsequent release of the internal cellular organelles/components. Overly aggressive decanting of the supernatant postcentrifugation results in the loss of cells from the sample (if the decanted supernatants are collected and not discarded, the cells can be later recovered and stained).
Also, failing to incubate the samples with the antibodies in the dark will result in the bleaching of the fluorochrome [fluoroscein isothiocyanate (FITC); rendering it not observable by the flow cytometer’s sensors]. Such cellular or antibody fluorochrome destruction constitutes a non-recoverable error and necessitates the collection of new samples. Incubating the samples with inadequate antibody titers (greater than 1:50 dilution) or inadequate time duration (less than 60 mins), will similarly result in low/poor immunofluorescence and using an incorrect negative isotypic control, or one which is not the “matched” control for the “positive experimental” antibody [fluoroscein isothiocyanate (FITC) conjugated monoclonal mouse anti-human HLA-DR], will result in background fluorescence which does not correlate with the observed experimental.
Insufficient washing during the immunostaining procedure can result in overly high backgrounds and/or overwhelming amounts of debris picked up by the flow cytometer. Such can even lead to the clogging of the instrument if care is not taken.
3.3 Collection of Flow Cytometric Data
Accidental aspiration of air (i.e. “running out” of sample during data collection) will result in the clogging of the flow cytometer. If this occurs, immediately backflush the instrument, run as many “unclogging” cycles as is necessary to clear the instrument, and if necessary, replace the filters and fluidics’ tubing. Overconcentration of the cells (i.e. inadequate volume of sheath/PBS) will likewise result in the clogging of the flow cytometer. If this occurs, follow the identical “unclogging” procedures and further dilute the sample with sheath.
3.4 Analysis of Flow Cytometric Data
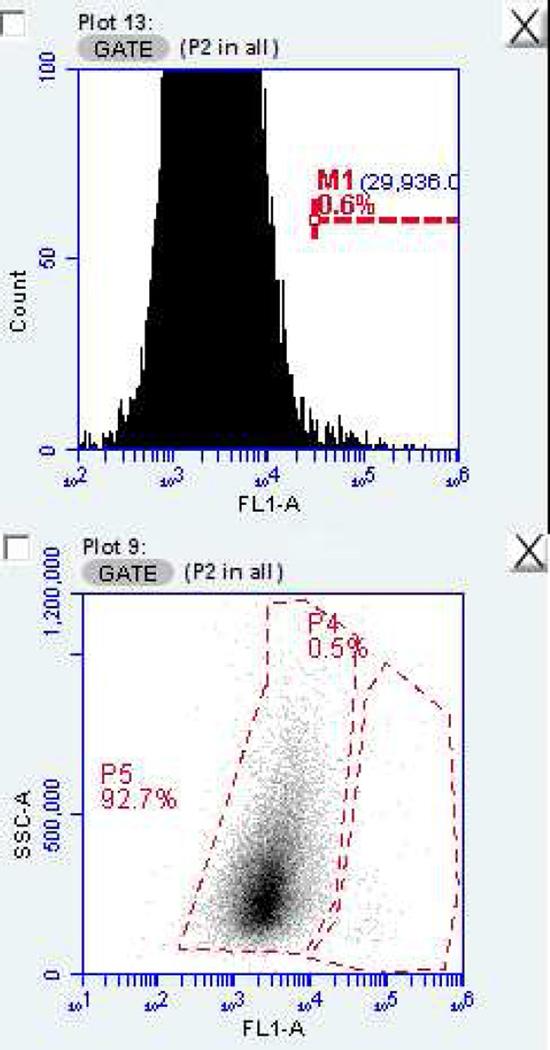
With histogram presentation of the data, the slightly slanting nature of the fluorescence channel (FL-1) of cytograms of cell populations evaluated by flow cytometry leads to overlap of those cells which highly express HLA-DR with non-highly expressing cells (see Figure 3) in the histogram. However, these are clearly differentiated when a dot plot is used. For this reason, dot plots displaying fluorescence channel (FL-)1 vs. cellular granularity (side scatter) are used in place of histograms [fluorescence channel (FL-)1 vs. event count (see Figure 3)]. Variations in gating can lead to significant differences in the determined percentage of cells expressing high levels of any immunolabeled target(s) such as biomarkers; therefore, consistent methodology needs to be established and the analyst, should be well trained and be capable of demonstrating repeatability in gating techniques.
Figure 3.
Cell cytology histogram (upper panel) and dot plot (lower panel) of data from a representative impression cytology sample demonstrating the slanting nature of the fluorescence channel (FL-1) and the resulting overlap of cells which highly express HLA-DR with the non-highly expressing cells which is present in the histogram, but clearly differentiated in the dot plot.
3.5 Quality Control: Flow Cytometer Controls
Improper calibration of the flow cytometer will result in either exceptionally high or low relative fluences (depending upon the type of calibration error) which can surpass the receptor sensitivity of the instrument and lead to values outside the limits of the cytometers’ sensors. Standard calibration techniques (see section 2.2.6) before each use will ensure proper calibration.
3.6 Sample Storage
For maximum yield, samples collected via impression cytology should be left at room temperature for 90 minutes to allow stabilization (“cure”) of the antigen prior to refrigeration. Failure to leave at room temperature for 90 minutes will reduce the number of cells, but not otherwise compromise the sample. Leaving the samples at room temperature for too long will result in destabilization of the antigen with subsequently poor staining.
Once the samples are “processed” (eluted from the filters, stained, and run through the flow cytometer), the raw data can be analyzed without concerns regarding degradation of the antibody target (HLA-DR).
3.7 Labeling, Masking and Tracking Samples
Proper analysis and HLA-DR quantification of the samples is to no avail if absolute confidence in the exact identification (both labeling and location) of samples is not strictly followed. Extreme care must be taken in the proper training of individuals and their adherence to the standard operating procedures regarding labeling, storage and tracking of all samples.
3.8 Shipping of Samples
Failure to maintain temperature control in shipping/storage will adversely affect the yield of cells within the sample and reliability of the subsequent quantification of relative HLA-DR expression.
Highlights.
In this study, the ability to use HLA-DR expression via the use of cell cytometry analysis of impression cytology (IC) samples was successfully demonstrated. Thus, HLA-DR expression could serve as a reliable, minimally invasive objective biomarker of inflammation of the ocular surface for use in multicenter clinical trial studies. We validated the SOPs for biomarker analysis as follows:
SOP valid. Similar areas of subject yields similar results. Use for other markers.
Storing for up to 30 days does not have significant effect on HLA-DR detection.
Other sites taught to collect samples. Successful incorporation in clinical trial.
Data supports HLA-DR as biomarker for DED severity and degree of inflammation.
Our SOPs demonstrate technique reliable, usable in multicenter clinical trials.
Acknowledgements
The authors would like to acknowledge the kind and invaluable assistance of Brittlyn Pearlman, Peter G. Dentone, Padmabriya Ranamoorthy, Jason Nichols, Jillian Meadows and Kelly Nichols.
Supported in part by the National Eye Institute/National Institutes of Health (R34-EY017626) as well as by The Martin and Toni Sosnoff Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the 2010 annual meeting of the Association for Research in Vision and Ophthalmology.
References
- Athmanathan S, Bandlapally SR, Gullapalli NR. Collection of corneal impression cytology directly on a sterile glass slide for the detection of viral antigen: An inexpensive and simple technique for the diagnosis of HSV epithelial keratitis – A pilot study. BMC Ophthalmol. 2001;1(3):1471–1476. doi: 10.1186/1471-2415-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabino S, Montaldo E, Solignani F, Valente C, Mingari MC, Rolando M. Immune response in the conjunctival epithelium of patients with dry eye. Exp. Eye Res. 2010;91(4):524–529. doi: 10.1016/j.exer.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Baudouin C, Haouat N, Brignole F, et al. Immunopathological findings in conjunctival cells using immunofluorescence staining of impression cytology specimens. Br. J. Ophthalmol. 1992;76:545–549. doi: 10.1136/bjo.76.9.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudouin C, Brignole F, Becquet F, Pisella PJ, Goguel A. Flow cytometry in impression cytology specimens. A new method for evaluation of conjunctival inflammation. Invest. Ophthalmol. Vis. Sci. 1997;38(7):1458–1464. [PubMed] [Google Scholar]
- Baudouin C, Bourcier T, Brignole F, et al. Correlation between tear IgE levels and HLA-DR expression by conjunctival cells in allergic and nonallergic chronic conjunctivitis. Graefes Arch. Clin. Exp. Ophthalmol. 2000;238(11):900–904. doi: 10.1007/s004170000179. [DOI] [PubMed] [Google Scholar]
- Baudouin C, Brignole F, Pisella PJ, De Jean MS, Goguel A. Flow cytometric analysis of the inflammatory marker HLA DR in dry eye syndrome: results from 12 months of randomized treatment with topical cyclosporin A. Adv. Exp. Med. Biol. 2002;506(B):761–769. doi: 10.1007/978-1-4615-0717-8_107. [DOI] [PubMed] [Google Scholar]
- Beuerman RW, Zhou L, Weon SR, Chew J, Zhou L, Zhu H, Riau AK. Biomarkers and Proteomics in Corneal Cell Biology. Acta Ophthalmol. 2011;89:s248. [Google Scholar]
- Biomarkers Definitions Working Group. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- Brignole F, De Saint-Jean M, Goldschild M, Becqet F, Goguel A, Baudouin C. Expression of Fas-Fas ligand antigens and apoptotic marker APO2-7 by the human conjunctival epithelium. Positive correlation with class II HLA DR expression in inflammatory ocular surface disorders. Exp. Eye Res. 1998;67:687–697. doi: 10.1006/exer.1998.0566. [DOI] [PubMed] [Google Scholar]
- Brignole F, Pisella PJ, Goldschild M, De Saint Jean M, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Invest. Ophthalmol. Vis. Sci. 2000;41(6):1356–1363. [PubMed] [Google Scholar]
- Brignole F, Pisella PJ, De Saint Jean M, Goldschild M, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in KCS 6-month treatment with topical cyclosporine A. Inv. Ophthalmol. Vis. Sci. 2001;42(1):90–95. [PubMed] [Google Scholar]
- Brignole-Baudouin F, Ott AC, Warnet JM, Baudouin C. Flow cytometry in conjunctival impression cytology: a new tool for exploring ocular surface pathologies. Exp Eye Res. 2004;78:473–481. doi: 10.1016/j.exer.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Brignole-Baudouin F, Pisella PJ, Dupas B, Baeyens V, Baudouin C. Efficacy and safety of 0.18% sodium hyaluronate in patients with moderate dry eye syndrome and superficial keratitis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004;243:531–538. doi: 10.1007/s00417-004-1040-6. [DOI] [PubMed] [Google Scholar]
- Brignole-Baudouin F, Baudouin C, Aragona P, Rolando M, Labetoulle M, Pisella PJ, Barabino S, Siou-Mermet R, Creuzot-Garcher C. A multicentre, double-masked, randomized, controlled trial assessing the effect of oral supplementation of omega-3 and omega- 6 fatty acids on a conjunctival inflammatory marker in dry eye patients. Acta Ophthalmol. 2011;89(7):591–597. doi: 10.1111/j.1755-3768.2011.02196.x. Epub 2011 Aug 11. [DOI] [PubMed] [Google Scholar]
- Calonge M, Diebold Y, Saez V, et al. Impression cytology of the ocular surface: a review. Exp. Eye Res. 2004;78:457–72. doi: 10.1016/j.exer.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Chaglasian EL. The Evolving Role of Biomarkers. Rev. Cornea Contact Lens. 2011;11:18. http://www.reviewofcontactlenses.com/content/c/31008/ [Google Scholar]
- De Saint Jean M, Baudouin C, Di Nolfo M, et al. Comparison of morphological and functional characteristics of primary-cultured human conjunctival epithelium and of Wong-Kilbourne derivative of Chang conjunctival cell line. Exp. Eye Res. 2004;78(2):257–274. doi: 10.1016/j.exer.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Definition and Classification Subcommittee of the International Dry Eye Workshop. The definition and classification of Dry Eye Disease: Report of the definition and classification subcommittee of the International Dry Eye Workshop. Ocul. Surf. 2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- Definition and Classification Subcommittee of the International Dry Eye Workshop. The epidemiology of dry eye disease: Report of the definition and classification subcommittee of the International Dry Eye Workshop. Ocul. Surf. 2007;5:93–107. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- Egbert PR, Lauber S, Maurice DM. A simple conjunctival biopsy. Am. J. Ophthalmol. 1977;84:798–801. doi: 10.1016/0002-9394(77)90499-8. [DOI] [PubMed] [Google Scholar]
- Fernandez K, Raynor GS, Sheyman AT, Epstein SP, Massingale ML, Dentone PG, Asbell PA. Modulation of HLA-DR in Dry Eye Subjects Following 30 days of Treatment with a Novel Artificial Tear Product. Invest Ophthalmol Vis Sci. 2001 doi: 10.2147/OPTH.S81355. (submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy JJ, Weyand CM. Long-term immunomodulatory effects of T lymphocyte depletion in patients with systemic sclerosis. Arthritis Rheum. 1990;33(4):511–519. doi: 10.1002/art.1780330408. [DOI] [PubMed] [Google Scholar]
- Guglielminetti E, Barabino S, Monaco M, Mantero S, Rolando M. HLA-DR Expression in Conjunctival Cells After Latanoprost. Journal of Ocular Pharmacology and Therapeutics. 2002;18(1):1–9. doi: 10.1089/108076802317233162. [DOI] [PubMed] [Google Scholar]
- Ichikawa Y, Shimizu H, Yoshida M, Arimori S. Activation antigens expressed on T-cells of the peripheral blood in Sjogren's syndrome and rheumatoid arthritis. Clin. Exp. Rheumatol. 1990;8(3):243–249. [PubMed] [Google Scholar]
- Ishihara K, Yoshimura M, Nakao H, Kanakura Y, Kanayama Y, Matsuzawa Y. T cell abnormalities in mixed connective tissue disease complicated with Klinefelter's syndrome. Intern. Med. 1994;33(11):714–717. doi: 10.2169/internalmedicine.33.714. [DOI] [PubMed] [Google Scholar]
- Jaraquemada D, Ollier W, Awad J, Young A, Festenstein H. HLA and rheumatoid arthritis: susceptibility or severity? Dis. Markers. 1986;4(1–2):43–53. [PubMed] [Google Scholar]
- Jester JV, Ho-Chang J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp. Eye Res. 2003;77(5):581–592. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- Lopin E, Deveney T, Asbell PA. Impression Cytology: Recent advances and applications in Dry Eye Disease. Ocular Surface J. 2009;7(2):93–110. doi: 10.1016/s1542-0124(12)70301-4. [DOI] [PubMed] [Google Scholar]
- Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009;28(9):1023–1027. doi: 10.1097/ICO.0b013e3181a16578. [DOI] [PubMed] [Google Scholar]
- McKelvie P. Ocular surface impression cytology. Adv. Anal. Pathol. 2003;10(6):328–337. doi: 10.1097/00125480-200311000-00003. [DOI] [PubMed] [Google Scholar]
- Mrugacz M, Zak J, Bakunowicz-Lazarczyk A, Wysocka J, Minarowska A. Flow cytometric analysis of HLA-DR antigen in conjunctival epithelial cells of patients with cystic fibrosis. Eye (Lond) 2007;21(8):1062–1066. doi: 10.1038/sj.eye.6702435. [DOI] [PubMed] [Google Scholar]
- Natadisastra G, Wittpenn JR, West KP, et al. Impression cytology for detection of vitamin A deficiency. Arch. Ophthalmol. 1987;105:1224–1228. doi: 10.1001/archopht.1987.01060090082033. [DOI] [PubMed] [Google Scholar]
- Natadisastra G, Wittpenn JR, Muhilal, West KP, Jr, Mele L, Sommer A. Impression cytology: a practical index of vitamin A status. Am. J. Clin. Nutr. 1988;48(3):695–701. doi: 10.1093/ajcn/48.3.695. [DOI] [PubMed] [Google Scholar]
- Nelson DJ, Havener VR, Cameron JD. Cellulose acetate impressions of the ocular surface: dry eye states. Arch. Ophthalmol. 1983;101:1869–1872. doi: 10.1001/archopht.1983.01040020871007. [DOI] [PubMed] [Google Scholar]
- Newman PJ. The biology of PECAM-1. J. Clin. Invest. 1997;100:S25–S29. [PubMed] [Google Scholar]
- Nolan GR, Hirst LW, Wright RG, et al. Application of impression cytology to the diagnosis of conjunctival neoplasms. Diagnostic Cytopathology. 1994;11:246–249. doi: 10.1002/dc.2840110310. [DOI] [PubMed] [Google Scholar]
- Pauly A, Brignole-Baudouin F, Labbé A, Liang H, Warnet JM, Baudouin C. New tools for the evaluation of toxic ocular surface changes in the rat. Invest. Ophthalmol. Vis. Sci. 2007;48(12):5473–5483. doi: 10.1167/iovs.06-0728. [DOI] [PubMed] [Google Scholar]
- Pietersz RN, Loos JA, Reesink HW. Survival in vivo of platelets stored for 48 hours in the buffycoat at 4 degrees C compared to platelet rich plasma stored at 22 degrees C. Blut. 1987;54(4):201–206. doi: 10.1007/BF00594194. [DOI] [PubMed] [Google Scholar]
- Pisella PJ, Brignole F, Debbasch C, Lozato PA, Creuzot-Garcher C, Bara J, et al. Flow cytometric analysis of conjunctival epithelium in ocular rosacea and keratoconjunctivitis sicca. Ophthalmology. 2000;107(10):1841–1849. doi: 10.1016/s0161-6420(00)00347-x. [DOI] [PubMed] [Google Scholar]
- Puangsricharern V, Tseng SC. Cytological evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995;102:1476–1485. doi: 10.1016/s0161-6420(95)30842-1. [DOI] [PubMed] [Google Scholar]
- Rivas L, Murube J, Rivas A, Shalaby O. [The contribution of impression cytology towards the diagnosis of cicatricial ocular pemphigoid in its primary stages]. Contribución de la citología de impresión al diagnóstico del penfigoide cicatricial ocular en sus primeras fases. Arch. Soc. Esp. Oftalmol. 2004;79(2):67–74. doi: 10.4321/s0365-66912004000200006. ISSN 0365-6691. [DOI] [PubMed] [Google Scholar]
- Rolando M, Barabino S, Mingari C, Moretti S, Giuffrida S, Calabria G. Distribution of Conjunctival HLA-DR Expression and the Pathogenesis of Damage in Early Dry Eyes. Cornea. 2005;24(8):951–954. doi: 10.1097/01.ico.0000157421.93522.00. [DOI] [PubMed] [Google Scholar]
- Roy P, Cimbolini N, Feraille L, Elena PP. Evaluation of membranes material for impression cytology. Inv. Ophthalmol. Vis. Sci. 2011;52(4):S1937. [Google Scholar]
- Roy P, Cimbolini N, Feraille L, Elena PP, Baudouin C. Evaluation of membranes material for impression cytology. Invest Ophthalmol & Vis Sci. 2012;53(4):S1868. [Google Scholar]
- Sanchez MA, Arriola-Villalobos P, Torralbo-Jimmenez P, et al. The effect of preservative-free HP-Guar on dry eye after phacoemulsification: a flow cytometric study. Eye (Lond) 2010;24:1331–1337. doi: 10.1038/eye.2010.24. [DOI] [PubMed] [Google Scholar]
- Singh R, Joseph A, Umapathy T, et al. Impression cytology of the ocular surface. Br. J. Ophthalmol. 2005;89:1655–1659. doi: 10.1136/bjo.2005.073916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjogren's and non-Sjogren's patients with dry eye. Invest. Ophthalmol. Vis. Sci. 2002;43(8):2609–2614. [PubMed] [Google Scholar]
- Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: A dose-ranging, randomized trial: The Cyclosporine Phase 2 Study Group. Ophthalmology. 2000;107(5):967–974. doi: 10.1016/s0161-6420(00)00035-x. [DOI] [PubMed] [Google Scholar]
- Thiel MA, Bossart W, Bernauer W. Improved impression cytology techniques for the immunopathological diagnosis of superficial viral infections. Br. J. Ophthalmol. 1997;81:984–988. doi: 10.1136/bjo.81.11.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tole DM, McKelvie PA, Daniell M. Reliability of impression cytology for the diagnosis of ocular surface squamous neoplasia employing the Biopore membrane. Br. J. Ophthalmol. 2001;85:154–158. doi: 10.1136/bjo.85.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng SCG. Staging of conjunctival metaplasia by impression cytology. Ophthalmology. 1985;92:728–733. doi: 10.1016/s0161-6420(85)33967-2. [DOI] [PubMed] [Google Scholar]
- Tsubota K, Fujihara T, Saito K, Takeuchi T. Conjunctival Epithelium Expression of HLA-DR in Dry Eye Patients. Ophthalmologica. 1999;213(1):16–19. doi: 10.1159/000027387. [DOI] [PubMed] [Google Scholar]
- Tsubota K, Kajiwara K, Ugajin S, Hasegawa T. Conjunctival brush cytology. Acta Cytol. 1990;34(2):233–235. [PubMed] [Google Scholar]
- Versura P, Profazio V, Schiavi C, Campos EC. Hyperosmolar stress upregulates HLA-DR expression in human conjunctival epithelium in dry eye patients and in vitro models. Invest Ophthalmol. Vis. Sci. 2011;52(8):5488–5496. doi: 10.1167/iovs.11-7215. [DOI] [PubMed] [Google Scholar]
- Wittpenn JR, Tseng SC, Sommer A. Detection of early xerophthalmia by impression cytology. Arch. Ophthalmol. 1986;104(2):237–239. doi: 10.1001/archopht.1986.01050140091027. [DOI] [PubMed] [Google Scholar]