Abstract
The genetic mechanisms causing seed development by gametophytic apomixis in plants are predominantly unknown. As apomixis is consistently associated with hybridity and polyploidy, these confounding factors may either A) be the underlying mechanism for the expression of apomixis, or B) obscure the genetic factors which cause apomixis. To distinguish between these hypotheses, we analyzed the population genetic patterns of diploid and triploid apomictic lineages and their sexual progenitors in the genus Boechera (Brassicaceae). We find that while triploid apomixis is associated with hybridization, the majority of diploid apomictic lineages are likely the product of intra-specific crosses. We then show that these diploid apomicts are more likely to sire triploid apomictic lineages than conspecific sexuals. Combined with flow cytometric seed screen phenotyping for male and female components of apomixis, our analyses demonstrate that hybridization is an indirect correlate of apomixis in Boechera.
Keywords: Boechera, Apomixis, Apomeiosis, Hybridization, Polyploidy
Sexual, outcrossing reproduction is the ancestral state of embryo development in flowering plants (Karron et al. 2012). This breeding system has generated much of the biodiversity on earth and provides species with the potential to adapt to changing conditions, thus improving the chance for long term survival. Despite these advantages, many groups of plants have independently evolved asexual methods to produce seed (van Dijk and Vijverberg 2005; Carman 1997). Among these mechanisms, gametophytic apomixis (hereon “apomixis”) is one of the most common (Hörandl and Hojsgaard 2012). The expression of apomixis requires the coordination of several independent phenotypes including the formation of an unreduced embryo sac (female apomeiosis) and embryo development from an unfertilized and unreduced egg cell (parthenogenesis). Other traits, including the production of unreduced pollen (male apomeiosis) and the development of functional endosperm (e.g. pseudogamy) are typical of apomictic genotypes (Mogie 1992; Bicknell and Koltunow 2004). Considering that apomixis evolves from sexuality, the expression of any one of these traits alone would be deleterious. For example, female apomeiosis without parthenogenesis would lead to ploidy increases after each generation (although see Van Dijk & Vijverberg, 2005). The evolutionary mechanism causing the simultaneous establishment of these traits is the foremost debate in the apomixis literature.
Many authors have suggested that the genome-wide affects of hybridization and polyploidy may induce apomixis (Carman 1997; Comai et al. 2003; Madlung et al. 2002; Sharbel et al. 2010; Wang et al. 2004) as evidenced by the nearly uniform pattern of hybridity and polyploidy in apomictic lineages (Pongratz et al. 1997; Bicknell and Koltunow 2004; Mogie 1986; Asker and Jerling 1992; Nelson-Jones et al. 2002). However, this correlation may be indirect: since apomictic lineages are expected to accumulate mutations through time (Hopf et al. 1988; Kondrashov 1985), hybridity and polyploidy may be beneficial as states in which deleterious mutations are hidden by multiple allelic variants of most genes.
To assess the causes of apomixis, it is necessary to separate the effects of ploidy and interspecific hybridization. This is possible in only a handful of taxa that exhibit diploid apomixis. The emerging model genus, Boechera (Brassicaceae) is such a system (Rushworth et al. 2011). Boechera is characterized by diploid sexuals, and diploid and triploid apomicts which are distributed throughout North America (Al-Shehbaz 2003; Böcher 1951; Alexander et al. 2013).
Asexual seed production in Boechera is exclusively through gametophytic apomixis (Böcher 1951). Through population genetic (Roy 1995; Schranz et al. 2005; Beck et al. 2012) and phylogeographic (Dobeš et al. 2004a; Dobeš et al. 2004b; Dobeš et al. 2007; Kiefer et al. 2009a; Kiefer et al. 2009b; Kiefer and Koch 2012) analyses, several authors have concluded that apomictic lineages have arisen recurrently from hybridization. However, this strong association between hybridity, polyploidy and apomixis has not been directly linked. Crossing experiments have shown that newly-formed triploid Boechera may be infertile (Schranz et al. 2005) and interspecific hybridization can produce sexual, diploid individuals (Schranz et al. 2006); therefore, polyploidy and hybridization are not de facto requirements for the phenotypic expression of apomixis in Boechera. Other studies have proposed that apomixis is caused by the inheritance of discrete genetic factors (e.g. Het chromosomes (Kantama et al. 2007) or altered genetic architecture (Carman 1997)); here, we explore this alternate, but not mutually exclusive, mechanism for the cause of apomixis. We first test if apomictic lineages are uniformly hybrids, then analyze the population genetic patterns of triploid hybrid formation. Finally we discuss the patterns of endosperm ploidy in apomictic diploids and present a conceptual model which fits the data presented here.
Does hybridization cause apomixis in Boechera?
A study focusing on a few species (Dobeš et al. 2004b) and the recent genus-wide study of Beck et al. (2012) showed that apomixis (as defined by unreduced pollen production) is strongly associated with increased heterozygosity. This is taken as evidence that diploid apomicts are interspecific hybrids. In some cases this conclusion is well supported by the presence of apomictic individuals with intermediate positions in genotypic space between two sexual species. However, high heterozygosity alone may not indicate hybridity. To assess the origin (hybrid or intra-specific) of apomictic lineages, we analyzed the ploidy, mating system (via the flow cytometric seed screen: FCSS) (Matzk et al. 2000) and multi-locus SSR genotypes of 231 lines collected from 37 natural populations of four species (B. stricta, B. retrofracta, B. polyantha and B. pendulocarpa) and interspecific hybrids between the parental species (see online supporting material for details on our methods; tables S1–S3). Ploidy analysis of leaf samples revealed that all individuals morphologically described as hybrids are in fact apomictic triploids, while all “true” species are diploid. We then subjected a subset of these lines to FCSS. While all our B. stricta accessions are constitutively sexual, both apomictic and sexual individuals are found in the other three diploid species. We calculated the observed heterozygosity of individuals from the three mating system classes: triploid apomicts (3xApo), diploid sexuals (2xSex) and diploid apomicts (2xApo). Triploid apomictic lines had the highest heterozygosity (F=307.9, df=2, p<.0001), 2xSex were most homozygous (F=287.4, df=2, p<.0001) and 2xApo lineages were intermediate. Since Boechera species are primarily inbreeding (Song and Mitchell-Olds 2007; Schranz et al. 2005), a high level of homozygosity is expected in sexual Boechera individuals.
Due to the non-recombinant nature of apomicts, apomictic populations, which were formed by either hybridization or within-species mating events among sexual lineages, will exhibit similar heterozygosity to an F1 population. Additionally, heterozygosity in apomictic lineages will increase over time as a function of the number of mutations accumulated post divergence (especially at SSR and other highly mutable loci) (Paun and Hörandl 2006); therefore, we expect generally elevated levels of heterozygosity in apomictic individuals relative to the ancestral, sexual lineages.
We simulated F1 populations for crosses within the diploid species B. pendulocarpa (intra-specific crosses) and among B. pendulocarpa and the other diploid species (interspecific hybrid). The diploid simulations were conducted by repeatedly sampling a single random haploid genotype from one individual in each source population, then concatenating these genotypes into a simulated F1 individual. Once an eight-individual F1 population was simulated, we output the population-level observed heterozygosity (#heterozygous loci/total# of loci). We simulated 1000 of these populations for inter-specific hybrids and intra-specific crosses. As most triploid apomictic lineages are formed by a cross between two species (bi-genomic), we then repeated this analysis by sampling the diploid genotype from a random individual in one population and concatenating that with a haploid genotype from another. These simulations were conducted via a custom script in the R environment for statistical computing (R Development Core Team 2009).
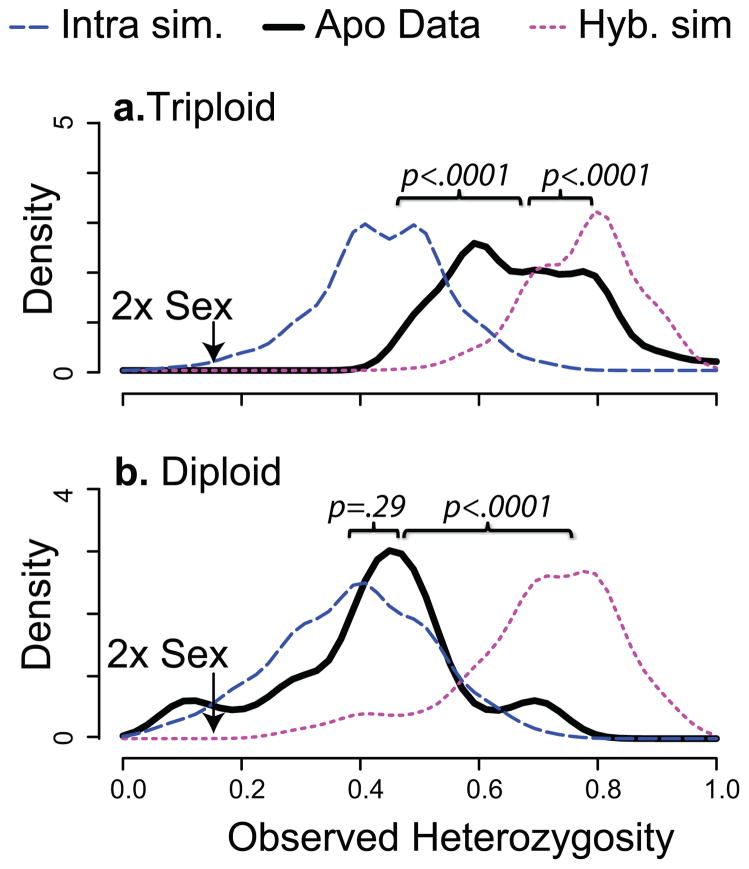
We compared the heterozygosity observed in our data to that of the simulated offspring. Triploid apomicts exhibit significantly different levels of heterozygosity than either the simulated hybrids (t=-5.7, df=49, p<0.0001) or the intra-specific crosses (t=13.3, df=50, p<0.0001; fig 1A). However, diploid apomicts exhibit slightly elevated (but statistically insignificant) heterozygosity relative to the simulated intra-specific crosses (t=1.06, df=23, p=0.30) but much lower heterozygosity than the simulated diploid hybrid (t=−9.19, df=23, p<0.0001; fig 1B). This result is consistent with diploid apomictic lineages that are formed by intra-specific crosses, followed by mutation accumulation. Although some 2xApos are potential hybrids, the majority exhibit heterozygosity indicative of within-species mating. Alternatively, the 3xApos exhibit heterozygosity that more closely resembles that of the simulated hybrids. While some 3xApos may be formed by intra-specific crosses, the majority are of hybrid origin. These results demonstrate that, in Boechera, 3xApos are likely hybrids (this is confirmed by intermediate phenotypes of many 3x Boechera species), but diploid apomixis is not constitutively associated with hybridization. However, we cannot rule out the possibility that all diploid apomicts are the product of crosses between divergent genotypes.
Fig 1.
Patterns of heterozygosity indicate differential origins of 2x and 3x apomixis. Genotypes for eight offspring were calculated for each of 1000 simulated crosses among diploid populations of B. pendulocarpa (Intra sim.) and between B. pendulocarpa and the other diploid species (Hyb. sim). Observed heterozygosity was calculated as the proportion of heterozygous markers in each simulated population (intra=blue line, hybrid= pink line) and for the actual genotypes in the populations (black line). The kernel density distribution is plotted against the observed heterozygosity for both the 3xApo accessions (a) and the 2xApo accessions (b). The mean heterozygosity of sexual diploids is labeled in each plot. P-values from pairwise t-test report the significance of the difference between the mean simulated and real data.
Non-random hybridization generates triploid apomixis
Our morphological (see supporting online material) and genetic analyses classified the 2xApo accessions as true species but 3xApo as primarily interspecific hybrids. Therefore, it is possible that triploid apomictic lineages are derived from hybridization between two 2xSex species. Alternatively, if a genetic factor induces apomixis, the causal allelic variant would be passed from one generation to the next. As such, hybrid apomictic lineages would likely have at least one apomictic parent, and hybridization between two sexual accessions would not produce an apomictic offspring unless the genetic factor(s) interact epistatically or are recessive (which is unlikely in inbred genotypes).
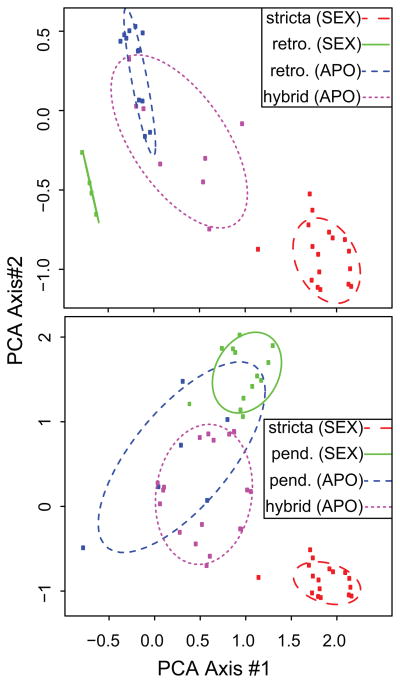
To differentiate between the two types of hybridization (2xSex: 2xSex and 2xApo: 2xSex), we analyzed two groups of triploid apomictic hybrids that have the obligate sexual species, B. stricta as one parent, while the second parental species (B. retrofracta or B. pendulocarpa) consists of both 2xApo and 2xSex individuals. By comparing the relative relatedness of apomictic and sexual lineages of B. retrofracta and B. pendulocarpa to their hybrids, we can assess whether the apomictic lineage is more likely to sire the hybrid or if hybridization occurs randomly with respect to the reproductive mode of the parental species. We conducted a principal components analysis (PCA) calculated via a scaled distance matrix which corrects for ploidy (Bruvo et al. 2004) and found that apomictic lineages of both B. retrofracta and B. pendulocarpa are more closely related to the hybrid than conspecific sexual (ncomparisons= 750, f=15.85, p<.0001). Furthermore, the PCA position of the apomictic lineages in these two comparisons is consistent with 2xApos, not sexuals as the parents (fig 2). This provides evidence that hybridization that generates triploid apomixis does not occur randomly with respect to parental reproductive mode, but instead involves an apomictic diploid.
Fig 2.
The position of apomictic triploid hybrids relative to parental species (split by reproductive mode) in genetic space. Principle components reveal that apomictic diploid lineages are more likely to sire triploid lineages than diploid sexual conspecific lineages in two comparisons: B. stricta X B. retrofracta (upper panel), B. stricta X B. pendulocarpa (lower panel) PCA scores for the 1st two axes and 95% confidence ellipses are plotted. As in fig 1, triploids are pink and diploid apomicts are blue.
Heritable variation of male and female apomixis
The separation of male and female components of apomixis has been anecdotally observed in Boechera (Aliyu et al. 2010). While most diploid apomictic lineages produce seeds with 2C:6C embryo to endosperm ratio (an apomeiotically derived embryo and endosperm from the two unreduced polar nuclei fertilized by unreduced pollen), some apomictic diploid lineages produce seed with an “unbalanced” 2C:5C ratio. Unbalanced endosperm is typically thought of as the result of rare outcrossing via pollen from sexual neighbors. However, it is also possible that some genotypes produce apomeiotically derived embryo sacs, but reduced pollen. If the presence of unbalanced endosperm is a heritable trait, the later scenario is more likely.
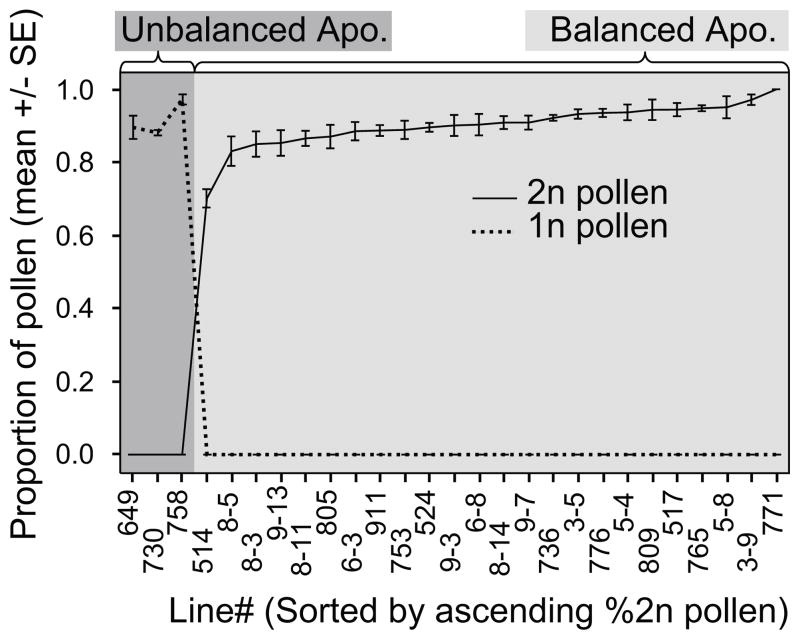
To assess whether endosperm ploidy variation within diploid apomicts is due to reduced pollen production from “female apomeiosis-only” (unbalanced) plants, we re-analyzed within-seed family seed screen data collected by Aliyu et al. (2010). In this study, FCSS data were collected on 384 individual seeds from 27 diploid apomictic seed families. We observed balanced apomixis in 24 lines, but three seed families deviated from this pattern and generated seed with endosperm derived nearly exclusively from female apomeiosis and reduced pollen (unbalanced apomixis, fig 3). Unbalanced apomictic lineages produced seed with little evidence of male apomeiosis while the opposite is also true; balanced apomictic families show no evidence of reduced pollen fertilization (fig 3). As within seed family variation is extremely low, but families differ greatly in the proportion of seeds with unbalanced endosperm, male apomeiosis is likely an intrinsic trait of some genotypes and evidently is separable from female apomeiosis.
Fig 3.
The relative presence of male apomeiosis in 27 diploid female apomictic Boechera lines. The male and female components of apomixis are usually both present in an individual that produces ovules via apomeiosis. Production of reduced pollen and unreduced ovules is limited to three individuals. Mean (+/−SE) %pollen type for each individual is presented (384 seeds/individual) as the solid (unreduced) and dashed (reduced) lines. Individuals are classified as balanced or unbalanced apomicts by the background color. Note: these families are not represented in the population genetic analysis. See Aliyu et al. (2010) for information on these lines.
This provides insight into the conclusions drawn in the recent large-scale apomixis study in Boechera. In concert with our study, Beck et al. (2012) demonstrated that individuals that produce diploid apomictic pollen are characterized by increased heterozygosity. However the heterozygosity distribution of individuals with sexual diploid pollen is bi-modal in their analysis: many “sexual” individuals exhibit heterozygosity similar to that of apomicts. Here we demonstrate that female-only apomixis is relatively common. These would be identified as sexual if pollen was used to assess reproductive mode. Thus, high heterozygosity diploids that produce reduced pollen may exhibit unbalanced apomixis.
A conceptual model for the genetic control of apomixis in Boechera
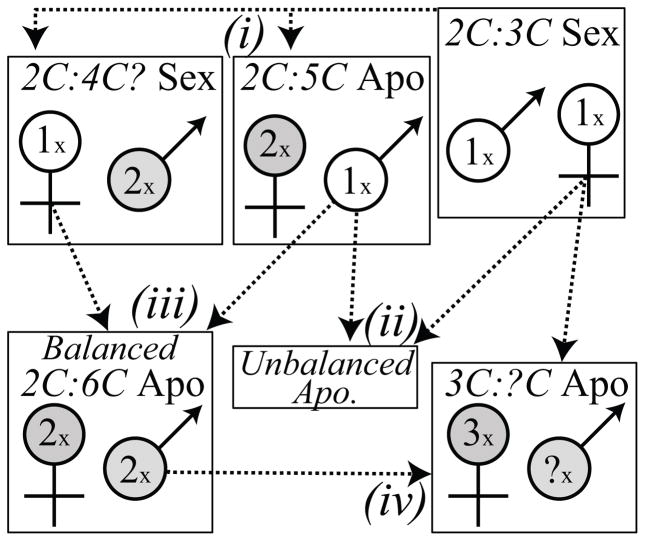
Many studies have shown that apomixis is a product of recurrent hybridization (or in some cases auto-polyploidization, e.g. Cosendai et al. 2011). The diploids analyzed here contradict these findings: apomictic diploids are not purely the result of recurrent interspecific hybridization; however, triploid apomictic lineages may be recurrently derived from 2xApo-2xSex hybridization. In this scenario, apomictic diploids produce unreduced pollen that fertilizes reduced ovules, repeatedly generating triploid apomictic lineages. We also found that the male and female apomeiosis are highly correlated within seed families, but differ between genotypes (fig 3). This pattern holds at the population level (J.T. Lovell, unpublished data). These results are consistent with several studies that demonstrate that genetic factor(s) (e.g. Kantama et al. 2007, Carman 1997) that control apomixis may best explain these patterns. Thus, we hypothesize a four step process that may produce the patterns of reproductive mode and ploidy variation observed across Boechera (fig 4).
Fig 4.
A conceptual model for the generation of apomixis in Boechera. The five genotypes that have led to the current patterns of apomixis are portrayed in the boxes. The text at the top of the boxes describes 1) the stable seed ploidy following a generation of self pollinating and 2) the female reproductive mode. The male and female symbols highlight the expected ploidy of pollen and embryo sacs respectively. Unreduced gametes have grey fill. Arrows connecting gametes to boxes indicate the contribution of that gamete for each formative mating. The four steps, which are described in the text, are highlighted (i–iv). For example, in step (iii), fertilization of a reduced ovule (from a line which only produces unreduced pollen) by reduced pollen (from the female apomeiosis-only individual) would produce a seed with a 2:3C embryo:endosperm ratio, but the genetic factors which cause the production of unreduced gametes are also transferred. Therefore, the offspring will produce seed with 2:6C ratio when the endosperm is self pollinated.
(i) Discrete genetic factors that control production of unreduced pollen or embryo sacs evolve independently. After self pollination of the endosperm, the female apomeiosis-only (unbalanced) apomict would likely exhibit a stable seed embryo-endosperm ratio of 2:5C. The lineage that produces meiotically derived embryo sacs, but unreduced pollen, would be unstable with several seed ploidy possibilities. (ii) Reduced, fertile pollen from unbalanced apomicts allows for recurrent crossing with purely sexual individuals. Transmission of the genetic factor that causes unbalanced apomixis may only occur in a subset of the crosses, however, over a long enough temporal scale, these crosses would permit the phylogenetic dispersion of a factor that induces female apomeiosis. (iii) A balanced diploid apomict is formed: reduced gametes from the independently derived genotypes, which undergo male or female apomeiosis, come into contact. In this lineage, both pollen and embryo sacs are generated through apomeiosis. A balanced endosperm-embryo ratio of 2:6C is generated following self pollination of the endosperm from unreduced pollen grains. (iv) Fertile, but unreduced, pollen is produced from balanced apomictic diploids. This pollen contains the apomixis factor(s) and fertilizes sexual diploid, reduced ovules, generating triploid embryos. Although aneuploid offspring are often generated by this type of cross (Schranz et al. 2006), apomictic triploid lineages may also be generated recurrently or formed in more advanced generations.
Conclusions
We conduct several preliminary analyses which provide evidence for the hypothesis that gametophytic apomixis in Boechera is caused by genetic factors and is correlated with but not caused by hybridization and polyploidy. The primary goal of these analyses is to encourage more detailed experimental studies of the molecular biology and population genetic patterns associated with apomixis. As genomic tools are rapidly evolving in Boechera (Rushworth et al. 2011), the ability to test the hypotheses presented here will improve in the near future. Genome-wide population genetics in particular has great potential to uncover the processes that cause apomixis and are responsible for the vast amount of diversity observed in Boechera.
Supplementary Material
Acknowledgments
We thank J.M. Corral and J. Beck for insightful discussions. This work was partially funded by the Apomixis Research Group, using basic level funding provided by the IPK. Additional funding comes from DFG grant #SH337/7-1 to TFS, and a μMorph training grant to JTL. TMO was supported by NIH grant R01-GM086496.
References
- Al-Shehbaz IA. Transfer of most North American species of Arabis to Boechera (Brassicaceae) Novon. 2003;13 (4):381–391. [Google Scholar]
- Alexander PJ, Windham MD, Beck JB, Al-Shehbaz IA, Allphin L, Bailey CD. Molecular Phylogenetics and Taxonomy of the Genus Boechera and Related Genera (Brassicaceae: Boechereae) Syst Bot. 2013;38 (1):192–209. [Google Scholar]
- Aliyu OM, Schranz ME, Sharbel TF. Quantitative variation for apomictic reproduction in the genus Boechera (Brassicaceae) Am J Bot. 2010;97(10):1719–1731. doi: 10.3732/ajb.1000188. [DOI] [PubMed] [Google Scholar]
- Asker S, Jerling L. Apomixis in Plants. CRC Press; Boca Raton, FL: 1992. [Google Scholar]
- Beck JB, Alexander PJ, Allphin L, Al-Shehbaz IA, Rushworth C, Bailey CD, Windham MD. Does hybridization drive the transition to asexuality in diploid Boechera? Evolution. 2012;66 (4):985–995. doi: 10.1111/j.1558-5646.2011.01507.x. [DOI] [PubMed] [Google Scholar]
- Bicknell RA, Koltunow AM. Understanding apomixis: recent advances and remaining conundrums. Plant Cell. 2004;16(Suppl):S228–245. doi: 10.1105/tpc.017921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böcher TW. Cytological and embryological studies in the amphi-apomictic Arabis holboellii complex. Det Kongelige Danske Videnskabernes Selskab. 1951;6 (7):1–59. [Google Scholar]
- Bruvo R, Michiels NK, D’Souza TG, Schulenburg H. A simple method for the calculation of microsatellite genotype distances irrespective of ploidy level. Molecular Ecology. 2004;13 (7):2101–2106. doi: 10.1111/j.1365-294X.2004.02209.x. [DOI] [PubMed] [Google Scholar]
- Carman JG. Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembryony. Biol J Linnean Soc. 1997;61 (1):51–94. [Google Scholar]
- Comai L, Madlung A, Josefsson C, Tyagi A. Do the different parental ‘heteromes’ cause genomic shock in newly formed allopolyploids? Philos Trans R Soc Lond B Biol Sci. 2003;358 (1434):1149–1155. doi: 10.1098/rstb.2003.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosendai A-C, Rodewald J, randl E. Origin and distribution of autopolyploids via apomixis in the alpine species Ranunculus kuepferi (Ranunculaceae) Taxon. 2011;60 (2):355–364. [Google Scholar]
- Dobeš C, Mitchell-Olds T, Koch M. Extensive chloroplast haplotype variation indicates Pleistocene hybridization and radiation of North American Arabis drummondii, A. xdivericarpa, and A. holboellii (Brassicaceae) Molecular Ecology. 2004a doi: 10.1046/j.1365-294x.2003.02064.x. [DOI] [PubMed] [Google Scholar]
- Dobeš C, Mitchell-Olds T, Koch M. Intraspecific diversification in North American Boechera stricta (= Arabis drummondii), Boechera x divaricarpa, and Boechera holboellii (Brassicaceae) inferred from nuclear and chloroplast molecular markers--an integrative approach. Am J Bot. 2004b;91 (12):2087–2101. doi: 10.3732/ajb.91.12.2087. [DOI] [PubMed] [Google Scholar]
- Dobeš C, Sharbel TF, Koch M. Towards understanding the dynamics of hybridization and apomixis in the evolution of the genus Boechera (Brassicaceae) Syst Biodiversity. 2007;5 (3):321–331. [Google Scholar]
- Hopf FA, Michod RE, Sanderson MJ. The effect of the reproductive system on mutation load. Theor Popul Biol. 1988;33 (3):243–265. doi: 10.1016/0040-5809(88)90015-9. [DOI] [PubMed] [Google Scholar]
- Horandl E, Hojsgaard D. The evolution of apomixis in angiosperms: A reappraisal. Plant Biosystems. 2012;146(3):681–693. [Google Scholar]
- Kantama L, Sharbel TF, Schranz ME, Mitchell-Olds T, de Vries S, de Jong H. Diploid apomicts of the Boechera holboellii complex display large-scale chromosome substitutions and aberrant chromosomes. Proc Natl Acad Sci USA. 2007;104 (35):14026–14031. doi: 10.1073/pnas.0706647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karron JD, Ivey CT, Mitchell RJ, Whitehead MR, Peakall R, Case AL. New perspectives on the evolution of plant mating systems. Ann Bot. 2012;109 (3):493–503. doi: 10.1093/aob/mcr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer C, Dobes C, Koch MA. Boechera or not? Phylogeny and phylogeography of eastern North American Boechera species (Brassicaceae) Taxon. 2009a;58 (4):1109–1121. [Google Scholar]
- Kiefer C, Dobes C, Sharbel TF, Koch MA. Phylogeographic structure of the chloroplast DNA gene pool in North American Boechera - A genus and continental-wide perspective. Molecular Phylogenetics and Evolution. 2009b;52 (2):303–311. doi: 10.1016/j.ympev.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Kiefer C, Koch MA. A Continental-Wide Perspective: The genepool of nuclear encoded ribosomal DNA and single-copy gene sequences in North American Boechera (Brassicaceae) Plos One. 2012;7 (5):e36491. doi: 10.1371/journal.pone.0036491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondrashov AS. Deleterious mutations as an evolutionary factor. II. Facultative apomixis and selfing. Genetics. 1985;111:635–653. doi: 10.1093/genetics/111.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madlung A, Masuelli RW, Watson B, Reynolds SH, Davison J, Comai L. Remodeling of DNA methylation and phenotypic and transcriptional changes in synthetic Arabidopsis allotetraploids. Plant Physiol. 2002;129 (2):733–746. doi: 10.1104/pp.003095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzk F, Meister A, Schubert I. An efficient screen for reproductive pathways using mature seeds of monocots and dicots. The Plant Journal. 2000;21 (1):97–108. doi: 10.1046/j.1365-313x.2000.00647.x. [DOI] [PubMed] [Google Scholar]
- Mogie M. On the relationship between asexual reproduction and polyploidy. J theor Biol. 1986;122:493–498. [Google Scholar]
- Mogie M. The Evolution of Asexual Reproduction in Plants. Chapman & Hall; London: 1992. [Google Scholar]
- Nelson-Jones B, Briggs D, Smith G. The origin of intermediate species of the genus Sorbus. Theor Appl Genet. 2002;105 (6–7):953–963. doi: 10.1007/s00122-002-0957-6. [DOI] [PubMed] [Google Scholar]
- Paun O, Hörandl E. Evolution of hypervariable microsatellites in apomictic polyploid lineages of Ranunculus carpaticola: Directional bias at dinucleotide loci. Genetics. 2006;174(1):387–398. doi: 10.1534/genetics.105.052761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongratz N, Sharbel TF, Beukeboom LW, Michiels NK. Allozyme variability in sexual and parthenogenetic freshwater planarians: evidence for polyphyletic origin of parthenogenetic lineages through hybridization with coexisting sexuals. Heredity. 1997;81:38–47. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing. Vienna, Austria: 2009. ( http://www.R-project.org) [Google Scholar]
- Roy BA. The breeding systems of six species of Arabis (Brassicaceae) American Journal of Botany. 1995;82 (7):869–877. [Google Scholar]
- Rushworth CA, Song BH, Lee CR, Mitchell-Olds T. Boechera, a model system for ecological genomics. Mol Ecol. 2011;20 (23):4843–4857. doi: 10.1111/j.1365-294X.2011.05340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schranz ME, Dobeš C, Koch MA, Mitchell-Olds T. Sexual reproduction, hybridization, apomixis, and polyploidization in the genus Boechera (Brassicaceae) Am J Bot. 2005;92 (11):1797–1810. doi: 10.3732/ajb.92.11.1797. [DOI] [PubMed] [Google Scholar]
- Schranz ME, Kantama L, de Jong H, Mitchell-Olds T. Asexual reproduction in a close relative of Arabidopsis: a genetic investigation of apomixis in Boechera (Brassicaceae) New Phytol. 2006;171 (2):425–438. doi: 10.1111/j.1469-8137.2006.01765.x. [DOI] [PubMed] [Google Scholar]
- Sharbel TF, Mitchell-Olds TM, Dobes C, Kantama L, de Jong H. Biogeographic distribution of polyploidy and B chromosomes in the apomictic Boechera holboellii complex. Cytogenetic and Genome Research. 2005;109 (1–3):283–292. doi: 10.1159/000082411. [DOI] [PubMed] [Google Scholar]
- Sharbel TF, Voigt ML, Corral JM, Galla G, Kumlehn J, Klukas C, Schreiber F, Vogel H, Rotter B. Apomictic and sexual ovules of Boechera display heterochronic global gene expression patterns. Plant Cell. 2010;22 (3):655–671. doi: 10.1105/tpc.109.072223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BH, Mitchell-Olds T. High genetic diversity and population differentiation in Boechera fecunda, a rare relative of Arabidopsis. Molecular Ecology. 2007;16 (19):4079–4088. doi: 10.1111/j.1365-294X.2007.03500.x. [DOI] [PubMed] [Google Scholar]
- van Dijk PJ, Vijverberg K. The significance of apomixis in the evolution of the angiosperms: a reappraisal. In: Bakker FT, Chatrou LW, Gravendeel B, Pelser PB, editors. Plant Species-Level Systematics: New Perspectives on Pattern & Process. Vol. 143. A R G Gantner Verlag K G; Koenigstein: 2005. pp. 101–116. Regnum Vegetabile. [Google Scholar]
- Wang J, Tian L, Madlung A, Lee HS, Chen M, Lee JJ, Watson B, Kagochi T, Comai L, Chen ZJ. Stochastic and epigenetic changes of gene expression in Arabidopsis polyploids. Genetics. 2004;167 (4):1961–1973. doi: 10.1534/genetics.104.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham MD, Al-Shehbaz IA. New and noteworthy species of Boechera (Brassicaceae) II: apomictic hybrids. Harvard Papers in Botany. 2007;11 (2):257–274. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.