Abstract
Purpose of review
Heme biosynthesis requires a series of enzymatic reactions that take place in the cytosol and the mitochondria as well as the proper inter- and intracellular trafficking of iron. Heme can also be acquired by intestinal absorption and intercellular transport. The purpose of this review is to highlight recent work on heme and iron transport with an emphasis on their relevance in erythropoiesis.
Recent findings
While the enzymes responsible for heme biosynthesis have been identified, transport mechanisms for iron, heme, or heme synthesis intermediates are only emerging. Recent studies have shed light into how these molecules are transported among various cellular compartments, as well as tissues. Much of this progress can be attributed to the use of model organisms such as S. cerevisiae, C. elegans, D. rerio, and M. musculus. Genetic studies in these models have led to the identification of several new genes involved in heme metabolism. Although our understanding has greatly improved, it is highly likely that other regulators exist and additional work is required to characterize the pathways by which heme and iron are transported within the erythron.
Summary
The identification of heme and iron transport mechanisms will improve our understanding of blood development and provide new insight into human blood disorders.
Keywords: Iron, anemia, transporters, porphyrins
INTRODUCTION
One of the many important functions of heme is to serve as the oxygen-carrying moiety in haemoglobin (Hb) that is expressed in cells along the erythroid lineage [1**]. Erythropoiesis requires the proper biosynthesis of heme and as erythroblasts mature, their demand for iron and heme increase dramatically [2, 3]. While all the enzymes involved in heme production have been well-characterized, precisely how proto-porphyrin intermediates cross the mitochondrial inner and outer membranes and are shuttled from one enzyme to another remains largely unknown. In addition, the mechanisms of how iron may be subsequently delivered from endosomes to the mitochondria and traverse the mitochondrial outer membrane remain unresolved. A great deal of recent work has, thus, focused on identifying these intracellular heme transport pathways. There is also accumulating evidence that heme can be transported among various tissues in multi-cellular organisms.
HEME SYNTHESIS
Overview
Heme is synthesized through an eight-step enzymatic cascade (referred to as the Shemin pathway) that occurs in the cytosol as well as the mitochondria [4, 5**]. The first step takes place in the mitochondrial matrix and involves the condensation of glycine with succinyl-coenzyme A by δ-aminolevulinic acid synthase 2 (ALAS2) in erythroid cells [4]. The resulting intermediate, δ-aminolevulinic acid (ALA), is subsequently exported to the cytosol where it is converted, through four enzymatic reactions, to coproporphyrinogen III (CPgenIII) [4]. CPgenIII is then transported back into the mitochondrial intermembrane space and converted to protoporphyrin IX (PPIX) by two enzymes [4]. Finally, ferrochelatase (FECH) catalyzes the insertion of ferrous iron (Fe2+) into PPIX to form heme [4].
The biosynthesis of heme, thus, also relies on the intracellular availability of iron. In humans, most cells acquire iron via transferrin receptor-mediated endocytosis of circulating transferrin-iron(III) (Fe3+) complexes. A non-canonical transferrin-independent pathway for iron delivery also exists in higher vertebrates and is nicely summarized by [6]. Once internalized, transferrin-bound Fe3+ is released, reduced to Fe2+ by STEAP3 (Six-Transmembrane Epithelial Antigen of the Prostate 3) [7], exits the endosome via DMT1 (Divalent Metal Transporter-1), and enters the mitochondria. Ferrous iron enters the mitochondrial intermembrane space and is transported across the inner membrane and into the matrix by Mitoferrin1 (MFRN1, SLC25A37) in erythroblasts [1**, 8] and Mitoferrin2 (MFRN2, SLC25A28) in proerythroblasts and non-erythroid cells [8–10].
Given the importance of heme metabolism in erythropoiesis, an improved understanding of heme and iron trafficking will provide insight into human blood disorders. In accordance, several studies have linked genetic mutations in heme metabolism genes with human blood disorders. Mutations in heme synthesis enzymes as well as porphyrin transporters have been associated with several variants of anemias and porphyrias [11]. It has also been recently proposed that single nucleotide polymorphisms of ALAS2 may have a modulatory effect on erythropoietic diseases [12*]. It is highly likely that additional genes may be involved in human blood disorders and a full understanding of iron and porphyrin transport represents the first step in the development of better diagnostic and treatment options.
Transport of porphyrin intermediates
Glycine is required in the first step of porphyrin synthesis and must be transported from the cytosol into the mitochondria [4]. SLC25A38, was recently identified through positional cloning of a gene implicated in nonsyndromic congenital sideroblastic anemia and has been proposed as a mitochondrial glycine importer [13]. Yeast deficient in the SLC25A38 homolog, YDL119c, exhibited lower levels of ALA consistent with the notion that SLC25A38 is required for glycine import. Subcellular localization analysis found that SLC25A38 is expressed in the inner mitochondrial membrane [13], raising the possibility that a second transporter may be responsible for the movement of glycine across the outer membrane.
Using a combination of bioinformatics, genetics, and biochemical approaches, several novel genes potentially involved in porphyrin biogenesis have recently been identified. In particular, one of these genes, SLC25A39, encodes a mitochondrial membrane protein that is required for heme synthesis in yeast, zebrafish, and mammalian erythroid cells [14]. Based on these results, SLC25A39 has been proposed to function in the early steps of heme synthesis or in the regulation of ALAS function [14]. Whether this is, indeed, the case with SLC25A39 requires further investigation. Additional work is also needed to define roles for several other candidate genes that were identified in this screen in blood development.
Cellular and mitochondrial iron uptake
Two models have been proposed to explain how Fe2+ may reach the mitochondria from endosomes. In the first model, Fe2+ leaves endosomes via DMT1 and becomes bound to chaperone proteins that transport Fe2+ [15**]. Recently, a protein called Poly r(C)-Binding Protein 1 (PCBP1) was identified in a screen for chaperones that may increase the loading of iron onto ferritin. Human PCBP1 bound directly to iron and increased the amount of iron loaded onto ferritin [16]. However, while these results are very interesting, a major caveat is that the vast majority of this work was performed using human proteins in yeast. Further work is required to determine a role for PCBP1 in vertebrate erythropoiesis.
Alternatively, a second model – termed the “kiss and run” hypothesis – argues that Fe2+-containing endosomes home to and directly contacts the mitochondria, allowing Fe2+ to be transferred without exposure to the cytosol [15**]. In support, an elegant study by Zhang and colleagues using radioisotope 59Fe-labelled reticulocytes showed that iron could only be chelated when endosomes were actively trafficking [17]. Also, pharmacological inhibition of myosins, microfilament motors that drive endosome movement, dramatically diminished heme synthesis [17]. Transmission electron microscope analysis found that transferrin-containing endosomes transiently and directly contact the mitochondria [18]. It should be noted, however, that the direct exchange of proteins through microtubule-driven mitochondrial fusion without endosome contact has been reported [19]. Although microtubule inhibitors did not abrogate 59Fe delivery to the mitochondria, it is possible that under those experimental conditions, inter-mitochondrial transfer of Fe2+ was not prevalent [17]. Regardless, these data make a compelling case for the latter model in which a cytosolic chaperone may not be required in the acquisition of mitochondrial iron in developing erythrocytes.
How do endosomes home to mitochondria? One possibility is that endosomes in erythroblasts express specific factors that govern their localization. To date, no direct evidence exists to support this mechanism in mammals and additional work is needed to examine why cells along the erythroid lineage process iron in a distinct fashion. A large part may be explained by the tremendous demand for mitochondrial iron required for a uniquely erythroid function – heme synthesis for hemoglobin production [17].
Regulation of mitochondrial iron import
Loss-of-function studies in zebrafish and mice have shown that MFRN1 is required for Fe2+ insertion into PPIX and red blood cell (RBC) development [8, 20*]. In humans, aberrant expression of MFRN1 is associated with erythropoietic protoporphyria [21], underscoring the importance of MFRN1 in erythropoiesis. Conditional loss of MFRN1 in mouse hepatocytes results in altered heme synthesis, leading to protoporphyria and hepatobiliary injury [20*]. MFRN1 is highly expressed in erythroid cells and is tightly regulated at both the transcriptional [8, 9] and post-translational levels [10, 22**, 23].
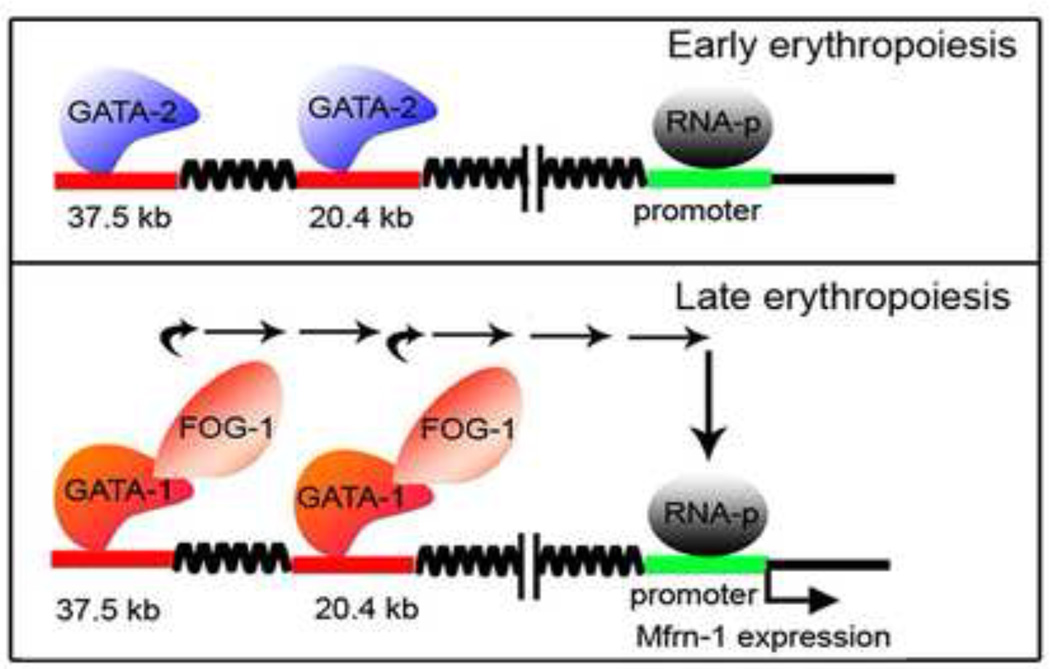
MFRN1 transcripts are upregulated between the second and third stages of erythroblast differentiation coincidental with the induction of the FOG-1 and GATA-1 transcription factors (unpublished data and [24*, 25*, 26]). GATA DNA-binding sites have been identified in the murine regulatory transcriptional enhancers of MFRN1, and a model has been proposed, whereby GATA-2 occupies these DNA elements early in erythroid progenitor development (Fig. 1). The induction of GATA-1 and FOG-1 expression during erythroblast maturation displaces GATA-2, and GATA-1/FOG-1-mediated transcription of MFRN1 leads to an increase in MFRN1 transcription [9] (Fig. 1). MFRN1 is also subject to unique post-translational regulatory mechanisms in erythroid cells. Pulse-chase labelling experiments found that MFRN1 has a half-life of 7 hours in immature erythroblasts that increases to over 24 hours after differentiation of Friend murine erythroleukemia (MEL) cells [10]. A subsequent study found that ABCB10, a member of the ATP-binding cassette transporter family that is also highly induced by GATA-1 [27], formed a molecular complex with MFRN1 in the mitochondrial inner membrane (Fig. 2). In the absence of ABCB10, MFRN1 protein half-life was unchanged in maturing MEL cells, suggesting that ABCB10 is a critical factor that mediates the increase in MFRN1 protein stability, thereby allowing more Fe2+ to be imported into the mitochondria [23]. The mechanisms underlying the changes in MFRN1 protein stability remain unknown. Thus, it appears that GATA-1 is a master regulator of mitochondrial Fe2+ influx in RBCs by direct induction of MFRN1 transcription and ABCB10-mediated increase in MFRN1 post-translational steady state levels.
Figure 1. Transcriptional regulation of MFRN1.
In early erythropoiesis, GATA-2 binds to two GATA-sites (−37.5 kb and −20.4 kb) in the MFRN1 distal transcriptional enhancer, but does not activate gene transcription (top panel). As erythroblasts mature, GATA-1 becomes expressed and the GATA-1/FOG-1 complex displaces GATA-2 from both DNA-binding sites in the MFRN1 distal enhancer (bottom panel) and triggers MFRN1 transcription. Reprinted with permission from J.D. Amigo et al. (ref. 9) and The American Society of Microbiology.
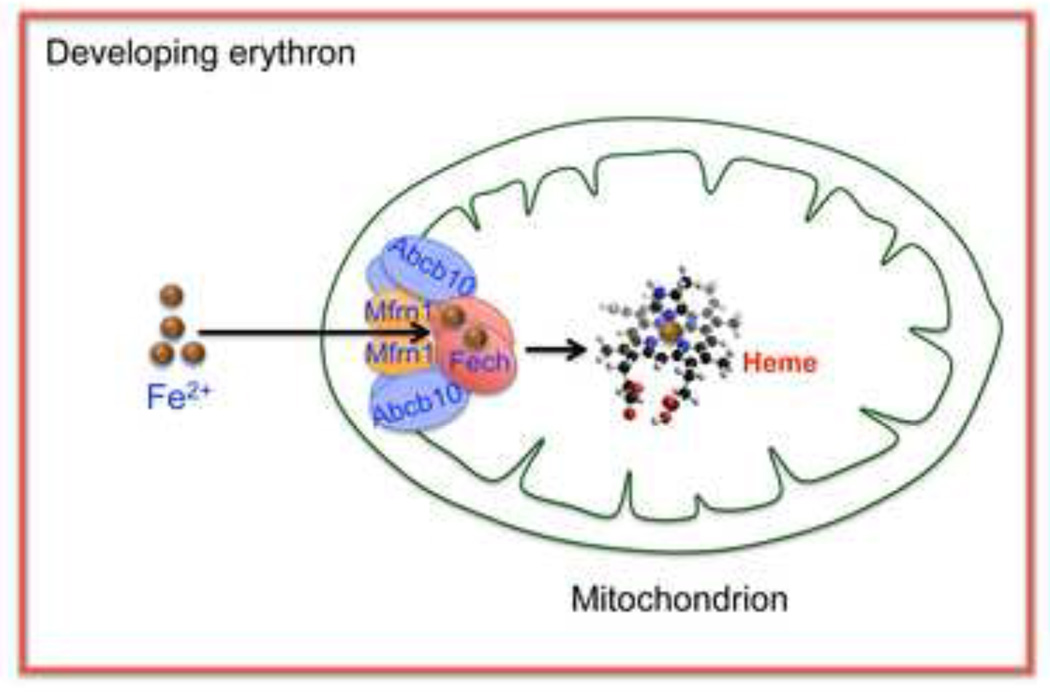
Figure 2. FECH, ABCB10, and MFRN1 form an oligomeric complex to import mitochondrial Fe2+ and its utilization for heme biogenesis.
FECH, ABCB10, and MFRN1 form a macromolecular complex at the inner mitochondrial membrane to facilitate the import of Fe2+. ABCB10 increases the protein half-life of MFRN1 in differentiating erythroblasts to accommodate the increase demand for Fe2+ for heme biosynthesis. Binding of MFRN1 to FECH suggests that the import of Fe2+ may be coupled to Fe2+ incorporation into porphyrins. Reprinted with permission from W. Chen et al. (ref. 22) and The American Society of Hematology.
Using a proteomics approach Chen and colleagues found that MFRN1 and ABCB10 actually exist as a macromolecular complex together with FECH [22**] (Fig. 2). This result is interesting because it suggests that Fe2+ import via MFRN1 is coupled to its incorporation into PPIX by FECH to form heme. Consistent with this notion, loss of either MFRN1 [20*], ABCB10 [28], or FECH [29] in mice is associated with similar defects in erythropoiesis. If Fe2+ import and incorporation are coupled, then a reasonable prediction is that there would be a stoichiometric relationship between PPIX consumption and Fe2+ incorporation. To date, such direct evidence linking these processes is lacking; however, the zebrafish mutant, shiraz, whose defect in a Fe-S cluster biosynthesis mitochondrial enzyme, Glutaredoxin 5 (GRX5), shows the tight inter-relationship between heme synthesis and Fe-S biogenesis [30]. This relationship has been reinforced by the identification of a patient with sideroblastic anemia due to a GRX5 defect [31, 32]. Another outstanding issue is the role of ABCB10. Although ABCB10 influences MFRN1 expression and function, whether ABCB10 directly contacts Fe2+PPIX, or heme intermediates has not been examined. Other members of the ATP-binding cassette transporter family participate in heme metabolism [4]. Notably, ABCB6 has been implicated in the direct binding and uptake of CPgenIII into the mitochondria and its expression profile mimics that of other heme biogenesis genes [33]. Currently, however, an association between ABCB6 function and blood defects in mice or humans have not been documented [34]. A related ABCB7 is associated with the functional biogenesis of cytosolic Fe-S cluster from the mitochondria [35], and its deficiency is associated with sideroblastic anemia and ataxia [36, 37]. Thus, it is possible that ABCB10 may play an active role in the transport of heme intermediates in addition to its effects on MFRN1.
HEME DEGRADATION AND IRON RECYCLE
Senescent RBCs are constantly removed from the circulation by macrophages that also play a principle role in the break-down of heme and the recycling of iron. Heme is released from lysed RBCs within phagolysosomes and is degraded by heme oxygenase-1 (HO-1) in the endoplasmic reticulum [1**]. Germline deletion of HO-1 leads to anemia in mice [38] and recent work suggests that HO-1 may play a cell-autonomous role in erythroblast differentiation. Irradiated, wild-type recipient mice transplanted with HO-1+/− bone marrow cells have significantly fewer splenic erythroid precursors [39], indicating that HO-1 may be required for erythropoiesis both directly by promoting RBC maturation as well as indirectly by regulating systemic iron recycling.
The degradation of heme in macrophages releases iron that is either stored as ferritin-bound iron or exported out of macrophages by the iron exporter, ferroportin-1 (FPN1, SLC40A1) [1**]. Circulating iron is quickly bound by the sequestration protein transferrin and the transferrin-iron complex can be internalized by the ubiquitously expressed transferrin receptor where iron can then be recycled for various functions [1**, 5**]. The importance of heme degradation and iron recycling in erythropoiesis is underscored by findings that inactivation of heme degradation machinery in mice is associated with anemias [38, 40**, 41]. In particular, specific ablation of FPN1 in macrophages is also associated with the development of anemia, indicating that the erythropoietic defects are not intrinsic to erythroblasts but rather iron recycling [40**]. Further investigations into the mechanisms of heme turnover may help in the treatment of blood disorders as evidenced by the use of the peptide hepcidin to fine tune FPN1-mediated iron export as a means to ameliorate symptoms of thalassemias and anemias [42, 43].
HEME TRANSPORT
Heme uptake – possible roles of HRG-1 and HCP1
Dietary uptake of heme constitutes a major source of iron [4]. Heme carrier protein 1 (HCP1) is a membrane protein expressed by enterocytes in the duodenum implicated in the absorption of heme in the intestine as well as Xenopus oocytes [44]. Subsequent studies, however, have cast doubt on the physiological significance of HCP1 as a heme importer. HCP1 has much higher affinity for folic acid and loss-of-function missense mutations in HCP1 in humans are associated with hereditary folate malabsorption with no apparent defects in erythropoiesis [45]. Interestingly, recent examination of HCP1-knockout mice found that while these animals do exhibit defects in folate absorption similar to human patients, they also develop anemia. HCP1−/− erythroblasts are more prone to apoptosis and fail to differentiate [46]. Currently, it is unclear whether the erythropoietic defects observed in HCP1−/− animals are the direct result of inadequate dietary heme uptake or secondary to folic acid deficiency that per se is a major risk factor for developing megaloblastic anemia in humans [47].
A substantial obstacle in the study of intercellular heme transport in vertebrate models is that cells synthesize heme de novo and is intricately linked with iron metabolism. The C. elegans model has proven to be particularly useful in this regard since it is a heme auxotroph and requires uptake of heme from the external milieu and dissemination throughout the organism for viability [4]. In fact, the first heme importer – HRG-1 – was identified in C. elegans and knockdown of the HRG-1 homolog in zebrafish embryos resulted in severe anemia [48]. Recent expression analysis performed in C. elegans has identified two additional genes – MRP-5 and F22B5.4 – that are required for proper intestinal heme transport in adult worms [49]. Both genes encode membrane proteins and may play a role in heme transport following dietary intake though their roles in blood development are unknown.
Heme export
In mammals, FLVCR1 (feline leukemia virus, subgroup C receptor 1) was recently shown to be a cell-surface heme exporter and FLVCR1−/− mice develop severe anemia due to the inability to export excess cytotoxic heme from macrophages and erythroblasts [50, 51]. Mutations in FLVCR1 cause posterior column ataxia and retinitis pigmentosa due to dysregulation of heme homeostasis in a subpopulation of neurons in the retina and posterior columns of the spinal cord [52]. More recently, the anopheline orthologs of FLVCR1 have been proposed as potential targets to inhibit Plasmodium-mediated malaria transmission [53]. A closely related homolog, FLVCR2, is reported to be a membrane-attached heme importer and may play a role in the extracellular endocytosis of heme. Knockdown of FLVCR2 in human cells resulted in a significant decline in zinc mesoporphyrin (a fluorescent heme analog) import. Quantitative real-time PCR analysis found that FLVCR2 transcripts can be detected in a wide range of human tissues including the fetal liver [54]. However, a physiological role for FLVCR2 in erythropoiesis is lacking as many of the experiments in this study were performed on human cancer cell lines.
A second protein, ABCG2, has been implicated in heme export in humans. Similar to ABCB10, ABCG2 is a member of the ATP-binding cassette transporter family that is expressed by hematopoietic progenitors [55, 56]. Recent evidence indicates that ABCG2 binds directly to heme via an extracellular domain and delivers heme to extracellular chaperone proteins such as albumin [55]. Currently, the importance of ABCG2 in normal blood development is unknown. However, it is upregulated under hypoxic conditions and it has been proposed that ABCG2 may function analogous to FLVCR1 to protect against the accumulation of excess heme under hypoxia [55, 56].
Intercellular heme trafficking: worm HRG-3
Even in the complete absence of heme synthesis, murine and zebrafish embryos remain viable until E3.5 or 10–25 days post-fertilization, respectively [57, 58]. One explanation for these observations is that some heme may be transported intercellularly to the developing embryo from a maternal source. Accordingly, work performed on cell culture models have demonstrated that heme can be released extracellularly [59, 60].
Recently, HRG-3 was identified as a secreted protein in C. elegans that is required for delivering heme from maternal intestine to developing embryos. While deletion of HRG-3 had no overt effect on adult worms, ~30% eggs from HRG-3 mutant mothers failed to hatch and all the hatched progeny were growth arrested at the first larval stage. These growth phenotypes were only observed when the mutant worms were grown under heme-limited conditions. Remarkably, only ectopic expression of HRG-3 in maternal intestinal tissues and not in the embryo rescued heme-restricted growth arrest of HRG-3 mutant worms [61**]. Collectively, this work provides strong evidence that HRG-3 binds to and delivers heme to the embryo from adult tissues.
What relevance does this have on human erythropoiesis? To date, no such intercellular heme transport protein has been identified in vertebrates that contribute to blood development. However, it is important to note that HRG-3 is induced under heme-restricted conditions and neither HRG-3 mutant adults nor larvae exhibited any growth defects when cultured in normal media [61**]. This suggests that HRG-3 is an adaptive mechanism and analogous pathways in vertebrates may only become evident in the absence of endogenous heme synthesis. It should also be noted that vertebrate intercellular trafficking pathways do exist. Free, circulating heme binds to and is sequestered by a plasma protein called hemopexin that delivers it to various cells types where heme is degraded [4]. Hemopexin may also be responsible for the systemic delivery of intravenously administered heme to human patients suffering from blood disorders [62, 63]. Another intercellular transport mechanism in vertebrates involves haptoglobin-mediated delivery of circulating Hb to monocytes and macrophages, leading to heme degradation and the recycling of iron [4]. Knockout studies in mice have revealed that while hemopexin and haptoglobin are mechanisms that guard against haemolytic stresses, these mice do not exhibit signs of defective erythropoiesis [64]. Thus, while intercellular heme trafficking regulators have been found in vertebrates, so far none have roles in normal blood development.
CONCLUSION
While the enzymes involved in heme metabolism have long been identified, the transport of iron and heme intermediates in erythroid cells remains one of the most enigmatic processes in biology. There is an invariable connection between erythropoiesis and heme metabolism and the identification of trafficking regulators has direct implications for human disease. Genetic aberrations in genes encoding enzymes in the Shemin pathway as well as iron transporters have been associated with porphyrias and anemias [11–13, 21, 65]. Characterizing these novel mechanisms will greatly enhance our understanding of human blood disorders.
KEY POINTS.
Coordinated transport of iron, heme, porphyrins, and its intermediates are essential for erythropoiesis.
Recent work using a vast array of molecular approaches has identified several new transport regulators and unravelled the underlying mechanisms through which they function.
Unidentified regulators still likely exist and further studies are required to not only identified these proteins but also to dissect their functions.
Characterizing novel pathways is clinically important as it is the first step in designing better diagnostic tests and treatments for human blood disorders.
Footnotes
Conflicts of interest
This work was supported in part by grants from the Canadian Institutes of Health Research (J.C.), the Cooley’s Anemia Foundation (C.C.), the March of Dimes Foundation (B.H.P.), and the National Institutes of Health grants R01 DK070838 and P01 HL032262 (B.H.P.).
For consistency purposes, the human convention was used in reference to all genes and proteins noted in this review regardless of species.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the period of review, have been highlighted as:
* Of special interest
** Of outstanding interest
- 1. Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: Regulation of mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. This is an excellent review that describes iron metabolism and transport.
- 2.Ponka P. Tissue-specific regulation of iron metabolism and heme synthesis: Distinct control mechanisms in erythroid cells. Blood. 1997;89:1–25. [PubMed] [Google Scholar]
- 3.Shafizadeh E, Paw BH. Zebrafish as a model of human hematologic disorders. Curr Opin Hematol. 2004;11:255–261. doi: 10.1097/01.moh.0000138686.15806.71. [DOI] [PubMed] [Google Scholar]
- 4.Severance S, Hamza I. Trafficking of heme and porphyrins in metazoa. Chem Rev. 2009;109:4596–4616. doi: 10.1021/cr9001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schultz IJ, Chen C, Paw BH, Hamza I. Iron and porphyrin trafficking in heme biogenesis. J Biol Chem. 2010;285:26753–26759. doi: 10.1074/jbc.R110.119503. This is a comprehensive review of heme biosynthesis and trafficking.
- 6.Chen C, Paw BH. Cellular and mitochondrial iron homeostasis in vertebrates. Biochim Biophys Acta Mol Cell Res. 2011 doi: 10.1016/j.bbamcr.2012.01.003. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohgami RS, Campagna DR, Greer EL, et al. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat Genet. 2005;37:1264–1269. doi: 10.1038/ng1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw GC, Cope JJ, Li L, et al. Mitoferrin is essential for erythroid iron assimilation. Nature. 2006;440:96–100. doi: 10.1038/nature04512. [DOI] [PubMed] [Google Scholar]
- 9.Amigo JD, Yu M, Troadec MB, et al. Identification of distal cis-regulatory elements at mouse mitoferrin loci using zebrafish transgenesis. Mol Cell Biol. 2011;31:1344–1356. doi: 10.1128/MCB.01010-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paradkar PN, Zumbrennen KB, Paw BH, et al. Regulation of mitochondrial iron import through differential turnover of mitoferrin 1 and mitoferrin 2. Mol Cell Biol. 2009;29:1007–1016. doi: 10.1128/MCB.01685-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iolascon A, De Falco L, Beaumont C. Molecular basis of inherited microcytic anemia due to defects in iron acquisition or heme synthesis. Haematologica. 2009;94:395–408. doi: 10.3324/haematol.13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. To-Figueras J, Ducamp S, Clayton J, et al. Alas2 acts as a modifier gene in patients with congenital erythropoietic porphyria. Blood. 2011;118:1443–1451. doi: 10.1182/blood-2011-03-342873. This work is the first to demonstrate the impact of polymorphisms in heme biosynthesis genes in human blood disorders.
- 13.Guernsey DL, Jiang H, Campagna DR, et al. Mutations in mitochondrial carrier family gene slc25a38 cause nonsyndromic autosomal recessive congenital sideroblastic anemia. Nat Genet. 2009;41:651–653. doi: 10.1038/ng.359. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson R, Schultz IJ, Pierce EL, et al. Discovery of genes essential for heme biosynthesis through large-scale gene expression analysis. Cell Metab. 2009;10:119–130. doi: 10.1016/j.cmet.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richardson DR, Lane DJ, Becker EM, et al. Mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol. Proc Natl Acad Sci U S A. 2010;107:10775–10782. doi: 10.1073/pnas.0912925107. This paper is an excellent review that discusses the intracellular mechanisms underlying iron trafficking.
- 16.Shi H, Bencze KZ, Stemmler TL, Philpott CC. A cytosolic iron chaperone that delivers iron to ferritin. Science. 2008;320:1207–1210. doi: 10.1126/science.1157643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang AS, Sheftel AD, Ponka P. Intracellular kinetics of iron in reticulocytes: Evidence for endosome involvement in iron targeting to mitochondria. Blood. 2005;105:368–375. doi: 10.1182/blood-2004-06-2226. [DOI] [PubMed] [Google Scholar]
- 18.Sheftel AD, Zhang AS, Brown C, et al. Direct interorganellar transfer of iron from endosome to mitochondrion. Blood. 2007;110:125–132. doi: 10.1182/blood-2007-01-068148. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Weaver D, Shirihai O, Hajnoczky G. Mitochondrial 'kiss-and-run': Interplay between mitochondrial motility and fusion-fission dynamics. EMBO J. 2009;28:3074–3089. doi: 10.1038/emboj.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Troadec MB, Warner D, Wallace J, et al. Targeted deletion of the mouse mitoferrin1 gene: From anemia to protoporphyria. Blood. 2010;117:5494–5502. doi: 10.1182/blood-2010-11-319483. This work describes the importance of MFRN1 in murine blood development.
- 21.Wang Y, Langer NB, Shaw GC, et al. Abnormal mitoferrin-1 expression in patients with erythropoietic protoporphyria. Exp Hematol. 2011;39:784–794. doi: 10.1016/j.exphem.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen W, Dailey HA, Paw BH. Ferrochelatase forms an oligomeric complex with mitoferrin-1 and abcb10 for erythroid heme biosynthesis. Blood. 2010;116:628–630. doi: 10.1182/blood-2009-12-259614. This is an important study describing the identification of a macromolecular complex between MFRN1, ABCB10, and FECH.
- 23.Chen W, Paradkar PN, Li L, et al. Abcb10 physically interacts with mitoferrin-1 (slc25a37) to enhance its stability and function in the erythroid mitochondria. Proc Natl Acad Sci U S A. 2009;106:16263–16268. doi: 10.1073/pnas.0904519106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hattangadi SM, Burke KA, Lodish HF. Homeodomain-interacting protein kinase 2 plays an important role in normal terminal erythroid differentiation. Blood. 2010;115:4853–4861. doi: 10.1182/blood-2009-07-235093. This article describes the stages of fetal liver erythroblast differentiation and its relation to the expression of erythroid markers and heme synthesis genes.
- 25. Amigo JD, Ackermann GE, Cope JJ, et al. The role and regulation of friend of gata-1 (fog-1) during blood development in the zebrafish. Blood. 2009;114:4654–4663. doi: 10.1182/blood-2008-12-189910. This elegant study uses genetic approaches in zebrafish to dissect the transcriptional regulation of murine MFRN1 and identifies GATA-1/FOG-1 as important regulators of MFRN1 expression.
- 26.Gregory T, Yu C, Ma A, et al. Gata-1 and erythropoietin cooperate to promote erythroid cell survival by regulating bcl-xl expression. Blood. 1999;94:87–96. [PubMed] [Google Scholar]
- 27.Shirihai OS, Gregory T, Yu C, et al. Abc-me: A novel mitochondrial transporter induced by gata-1 during erythroid differentiation. EMBO J. 2000;19:2492–2502. doi: 10.1093/emboj/19.11.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hyde BB, Elorza AA, Mikkola HK, et al. ABC-Me (ABCB10) is required for erythroid development in the mouse embryo and is protective against mitochondrial oxidative stress [abstract] Blood. 2008 [Google Scholar]
- 29.Tutois S, Montagutelli X, Da Silva V, et al. Erythropoietic protoporphyria in the house mouse. A recessive inherited ferrochelatase deficiency with anemia, photosensitivity, and liver disease. J Clin Invest. 1991;88:1730–1736. doi: 10.1172/JCI115491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wingert RA, Galloway JL, Barut B, et al. Deficiency of glutaredoxin 5 reveals fe-s clusters are required for vertebrate haem synthesis. Nature. 2005;436:1035–1039. doi: 10.1038/nature03887. [DOI] [PubMed] [Google Scholar]
- 31.Camaschella C, Campanella A, De Falco L, et al. The human counterpart of zebrafish shiraz shows sideroblastic-like microcytic anemia and iron overload. Blood. 2007;110:1353–1358. doi: 10.1182/blood-2007-02-072520. [DOI] [PubMed] [Google Scholar]
- 32.Ye H, Jeong SY, Ghosh MC, et al. Glutaredoxin 5 deficiency causes sideroblastic anemia by specifically impairing heme biosynthesis and depleting cytosolic iron in human erythroblasts. J Clin Invest. 120:1749–1761. doi: 10.1172/JCI40372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krishnamurthy PC, Du G, Fukuda Y, et al. Identification of a mammalian mitochondrial porphyrin transporter. Nature. 2006;443:586–589. doi: 10.1038/nature05125. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, He F, Bu J, et al. ABCB6 mutations cause ocular coloboma. Am J Hum Genet. 2011 doi: 10.1016/j.ajhg.2011.11.026. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lill R. Function and biogenesis of iron-sulphur proteins. Nature. 2009;460:831–838. doi: 10.1038/nature08301. [DOI] [PubMed] [Google Scholar]
- 36.Sheftel AD, Lill R. The power plant of the cell is also a smithy: The emerging role of mitochondria in cellular iron homeostasis. Ann Med. 2009;41:82–99. doi: 10.1080/07853890802322229. [DOI] [PubMed] [Google Scholar]
- 37.Ye H, Rouault TA. Human iron-sulfur cluster assembly, cellular iron homeostasis, and disease. Biochemistry. 2010;49:4945–4956. doi: 10.1021/bi1004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci U S A. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao YA, Kusy S, Luong R, et al. Heme oxygenase-1 deletion affects stress erythropoiesis. PLoS One. 2011;6:e20634. doi: 10.1371/journal.pone.0020634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang Z, Zhang F, An P, et al. Ferroportin1 deficiency in mouse macrophages impairs iron homeostasis and inflammatory responses. Blood. 2011;118:1912–1922. doi: 10.1182/blood-2011-01-330324. This elegant work demonstrates that loss of iron export in macrophages can impact erythroblast development.
- 41.Levy JE, Jin O, Fujiwara Y, et al. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat Genet. 1999;21:396–399. doi: 10.1038/7727. [DOI] [PubMed] [Google Scholar]
- 42.Gardenghi S, Ramos P, Marongiu MF, et al. Hepcidin as a therapeutic tool to limit iron overload and improve anemia in beta-thalassemic mice. J Clin Invest. 2010;120:4466–4477. doi: 10.1172/JCI41717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartnikas TB, Fleming MD. A tincture of hepcidin cures all: The potential for hepcidin therapeutics. J Clin Invest. 2010;120:4187–4190. doi: 10.1172/JCI45043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shayeghi M, Latunde-Dada GO, Oakhill JS, et al. Identification of an intestinal heme transporter. Cell. 2005;122:789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 45.Qiu A, Jansen M, Sakaris A, et al. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127:917–928. doi: 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 46.Salojin KV, Cabrera RM, Sun W, et al. A mouse model of hereditary folate malabsorption: Deletion of the pcft gene leads to systemic folate deficiency. Blood. 2011;117:4895–4904. doi: 10.1182/blood-2010-04-279653. [DOI] [PubMed] [Google Scholar]
- 47.Dary O. Nutritional interpretation of folic acid interventions. Nutr Rev. 2009;67:235–244. doi: 10.1111/j.1753-4887.2009.00193.x. [DOI] [PubMed] [Google Scholar]
- 48.Rajagopal A, Rao AU, Amigo J, et al. Haem homeostasis is regulated by the conserved and concerted functions of hrg-1 proteins. Nature. 2008;453:1127–1131. doi: 10.1038/nature06934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Severance S, Rajagopal A, Rao AU, et al. Genome-wide analysis reveals novel genes essential for heme homeostasis in caenorhabditis elegans. PLoS Genet. 2010;6:e1001044. doi: 10.1371/journal.pgen.1001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quigley JG, Yang Z, Worthington MT, et al. Identification of a human heme exporter that is essential for erythropoiesis. Cell. 2004;118:757–766. doi: 10.1016/j.cell.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 51.Keel SB, Doty RT, Yang Z, et al. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science. 2008;319:825–828. doi: 10.1126/science.1151133. [DOI] [PubMed] [Google Scholar]
- 52.Rajadhyaksha AM, Elemento O, Puffenberger EG, et al. Mutations in flvcr1 cause posterior column ataxia and retinitis pigmentosa. Am J Hum Genet. 2010;87:643–654. doi: 10.1016/j.ajhg.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeong JJ, Khan AA, Lamm M, et al. Anopheline orthologs of the human erythroid heme exporter, FLVCR, export heme: potential targets to inhibit Plasmodium transmission [abstract] Blood. 2010 [Google Scholar]
- 54.Duffy SP, Shing J, Saraon P, et al. The fowler syndrome-associated protein flvcr2 is an importer of heme. Mol Cell Biol. 2010;30:5318–5324. doi: 10.1128/MCB.00690-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Desuzinges-Mandon E, Arnaud O, Martinez L, et al. Abcg2 transports and transfers heme to albumin through its large extracellular loop. J Biol Chem. 2011;285:33123–33133. doi: 10.1074/jbc.M110.139170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krishnamurthy P, Ross DD, Nakanishi T, et al. The stem cell marker bcrp/abcg2 enhances hypoxic cell survival through interactions with heme. J Biol Chem. 2004;279:24218–24225. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- 57.Okano S, Zhou L, Kusaka T, et al. Indispensable function for embryogenesis, expression and regulation of the nonspecific form of the 5-aminolevulinate synthase gene in mouse. Genes Cells. 2010;15:77–89. doi: 10.1111/j.1365-2443.2009.01366.x. [DOI] [PubMed] [Google Scholar]
- 58.Dooley KA, Fraenkel PG, Langer NB, et al. Montalcino, a zebrafish model for variegate porphyria. Exp Hematol. 2008;36:1132–1142. doi: 10.1016/j.exphem.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knutson MD, Oukka M, Koss LM, et al. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci U S A. 2005;102:1324–1328. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uc A, Stokes JB, Britigan BE. Heme transport exhibits polarity in caco-2 cells: Evidence for an active and membrane protein-mediated process. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1150–G1157. doi: 10.1152/ajpgi.00157.2004. [DOI] [PubMed] [Google Scholar]
- 61. Chen C, Samuel TK, Sinclair J, et al. An intercellular heme-trafficking protein delivers maternal heme to the embryo during development in C. elegans. Cell. 2011;145:720–731. doi: 10.1016/j.cell.2011.04.025. This elegant work demonstrates the importance of the C. elegans model in the study of heme metabolism and describes the identification of a novel intercellular maternal heme transport pathway.
- 62.Bonkovsky HL, Healey JF, Lourie AN, Gerron GG. Intravenous heme-albumin in acute intermittent porphyria: Evidence for repletion of hepatic hemoproteins and regulatory heme pools. Am J Gastroenterol. 1991;86:1050–1056. [PubMed] [Google Scholar]
- 63.Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375:924–937. doi: 10.1016/S0140-6736(09)61925-5. [DOI] [PubMed] [Google Scholar]
- 64.Tolosano E, Fagoonee S, Hirsch E, et al. Enhanced splenomegaly and severe liver inflammation in haptoglobin/hemopexin double-null mice after acute hemolysis. Blood. 2002;100:4201–4208. doi: 10.1182/blood-2002-04-1270. [DOI] [PubMed] [Google Scholar]
- 65.Petkau TL. Same pathway, different gene: A second gene in the heme biosynthesis pathway causes inherited sideroblastic anemia. Clin Genet. 2010;77:112–113. doi: 10.1111/j.1399-0004.2009.01302.x. [DOI] [PubMed] [Google Scholar]