Abstract
Prenatal exposures to polybrominated diphenyl ethers (PBDEs) can harm neurodevelopment in humans and animals. In 2003–2004, PentaBDE and OctaBDE were banned in California and phased-out of US production; resulting impacts on human exposures are unknown. We previously reported that median serum concentrations of PBDEs and their metabolites (OH-PBDEs) among second trimester pregnant women recruited from San Francisco General Hospital (2008–2009; n=25) were the highest among pregnant women worldwide. We recruited another cohort from the same clinic in 2011–2012 (n=36) and now compare serum concentrations of PBDEs, OH-PBDEs, polychlorinated biphenyl ethers (PCBs) (structurally similar compounds banned in 1979), and OH-PCBs between two demographically similar cohorts. Between 2008–2009 and 2011–2012, adjusted least square geometric mean (LSGM) concentrations of ΣPBDEs decreased 65% (95% CI: 18, 130) from 90.0 ng/g lipid (95% CI: 64.7,125.2) to 54.6 ng/g lipid (95% CI: 39.2, 76.2) (p=0.004); Σ OH-PBDEs decreased six-fold (p<0.0001); and BDE-47, -99, and -100 declined more than BDE-153. There was a modest, non-significant (p=0.13) decline in LSGM concentrations of ΣPCBs and minimal differences in ΣOH-PCBs between 2008–2009 and 2011–2012. PBDE exposures are likely declining due to regulatory action, but the relative stability in PCB exposures suggests PBDE exposures may eventually plateau and persist for decades.
INTRODUCTION
Polybrominated diphenyl ethers (PBDEs) are persistent organic pollutants (POPs) that have widely been used as flame retardants in consumer products since the 1970s. Historically, three different mixtures known as PentaBDE, OctaBDE, and DecaBDE were commercially available. The predominant use of PentaBDE was in polyurethane foam within furniture, while OctaBDE and DecaBDE were used in electronics and other plastic products.
Regulatory and scientific communities are concerned about the public health consequences of PBDEs in part because both animal and human studies find that prenatal exposures can contribute to endocrine disruption and adverse neurodevelopmental outcomes. Higher PBDE exposures during pregnancy are associated with thyroid hormone disruption in both pregnant women1–3 and their newborns.4 Prenatal exposures are associated with neurodevelopmental harm in children including poorer concentration, attention, and reduced IQ.5, 6 These observations are consistent with animal studies in which PBDE exposures can reduce circulating thyroid hormone levels7–9 and affect nervous system development.10, 11
House dust has been identified as a primary route of human exposure to PBDEs, and diet is also a contributing factor as PBDEs are found in fish, meat, and dairy products.12–14 Sources and routes of exposure can vary by life stage and by individual PBDE congeners.14, 15 For example, serum concentrations of BDE-47, -99, and -100 (characteristic of PentaBDE)16 are highly correlated with dust exposures.15, 17 In contrast, BDE-153 (a minor component of PentaBDE and OctaBDE16) shows strong correlations with dietary exposures (including breast milk) and less consistent relationships with dust exposures.17–19
Exposures in North America are an order of magnitude higher than in Europe and Asia.20 Residents of California historically have the world’s highest non-occupational exposures to PentaBDE congeners because of the state’s unique flammability standard for foam furniture.21 Higher concentrations of PentaBDE congeners are also found among low-income communities and communities of color.22 We previously reported that median concentrations of PentaBDE congeners and hydroxylated PBDE metabolites (OH-PBDEs) in a small sample of second trimester pregnant women obtaining care between 2008–2009 at San Francisco General Hospital (SFGH) in San Francisco, California are the highest among pregnant women worldwide.1
PentaBDE and OctaBDE were banned in California and phased-out of US production in 2003–2004.23 These regulatory actions may have the effect of reducing human exposures to these PBDEs over time, but there are few data available to confirm or refute this idea. A recent study of California homes found that PBDE concentrations in household dust decreased between 2006 and 2011.24 However, no study to our knowledge, has examined the impact of the PBDE phase-out on population body burdens in the US. Harrad and Diamond25 hypothesize that PBDE exposure profiles will follow the example of other legacy contaminants such as polychlorinated biphenyl ethers (PCBs), a class of POPs structurally similar to PBDEs that were banned from U.S. production in 1979. Regulatory actions restricting new emissions were only partially successful in reducing human exposures to PCBs. There was a rapid decline in the 1970s following restrictions but the rate of decline has slowed in recent years due to continued releases from existing reservoirs, and a shift in human exposure routes.25–27 Historically, dust and indoor air were important exposure routes for PCBs, however, in contemporary populations, the major exposure route is diet.25 Similarly, PBDEs have long elimination half lives in the body,14, 28 may persist in the indoor environment,29 and can biomagnify in the food web.30 Additionally, there may be a slow replacement time for PBDE-containing consumer products in the home. Therefore, human exposure to PBDEs can continue through ingestion of contaminated house dust and food.
The objective of our current study is to compare serum concentrations of PBDEs, OH-PBDEs, PCBs, and hydroxylated PCB metabolites (OH-PCBs) between second trimester pregnant women seeking medical care at SFGH in 2008–2009 to those seeking care at the same clinic in 2011–2012. We hypothesize that 1) PBDE exposures will be lower in the recent cohort and 2) there will be a more pronounced difference between cohorts for chemicals that are driven by indoor sources of exposure (e.g. PentaBDE congeners) compared to those driven by dietary sources of exposures (e.g. PCBs and BDE-153).
MATERIALS AND METHODS
Study Population
In both 2008–2009 and 2011–2012, we recruited and consented English- and Spanish-speaking patients between 15 and 24 weeks of pregnancy seeking medical care from the University of California, San Francisco (UCSF) Women’s Options Center at San Francisco General Hospital in San Francisco, California. The Women’s Options Center is an outpatient clinic providing pregnancy terminations and serving an ethnically diverse and predominantly lower income population from the San Francisco Bay area and other parts of Northern and Central California. Eligible study participants were identified by reviewing the patient’s medical record only after they had 1) consulted with a trained counselor for an elective second trimester termination procedure and 2) consented to the procedure as documentation of intent to proceed with the elective pregnancy termination. The 2008–2009 cohort consists of 25 non-smoking women and the 2011–2012 cohort consists of 36 (smoking and non-smoking) women. Smokers were excluded from the first cohort because the primary aim of that study was to evaluate effects of PBDE exposure on thyroid function and cigarette smoking can alter thyroid function.1 For both cohorts, we excluded participants if they were using street drugs or seeking a termination because of fetal anomalies. All study protocols were approved by the UCSF Committee on Human Research.
Data Collection
Blood was collected from each participant prior to medical procedures in red top Vacutainer tubes. The serum was separated and stored in aliquots of 2 ml in either polypropylene storage tubes (first cohort) or amber glass vials (second cohort) at −80°C until analysis. Information on pregnancy and demographic characteristics was abstracted from medical records or collected via interview-administered questionnaire.
Analytical Methods
We analyzed PBDE and PCBs congeners in serum at the Department of Toxic Substances Control (DTSC) (Berkeley, CA, USA) within its clean laboratory facility, where only human specimens are processed. The blood extraction method and analysis were adopted from our earlier methods to separate phenolic compounds from maternal serum31, 32 and are described in detail elsewhere.1 For both the first and second cohorts, PBDEs and PCBs were measured simultaneously using gas chromatography/high resolution double-focusing sector mass spectrometry (GC-HRMS, DFS, ThermoFisher, Bremen, Germany). Two separate columns were used: a DB-5 MS column (15 m × 0.25 mm I.D., 0.10 μm film thickness, J & W Scientific, Folsom, CA) for PBDE congener analyses and an HT8-PCB column (60 m × 0.25 mm I.D., 0.25 μm film thickness, SGE International Pty Ltd. Australia & Pacific Region) for PCB congener analyses. Hydroxylated compounds (OH-PBDEs and OH-PCBs) were measured simultaneously after derivatization with diazomethane using GC-HRMS for the first cohort1 and GC-NCI/MS (Varian 1200 GC/MS operated in negative chemical ionization, Walnut Creek, CA, USA) for the second cohort. With either instrument, a DB-5MS column (30 m x 0.25 mm I.D. x 0.25 μm film thickness, J & W Scientific, Folsom, CA) was used for the simultaneous analysis of OH-PCBs and OH-PBDEs. Some of the ultra-trace levels of OH-PCBs measured by GC-NCI/MS were further confirmed by GC-Electron Capture Detection (Varian, Inc.). The GC conditions and parameters were described in our previous studies.1, 31, 32 The instrument detection threshold (IDT) was defined according to the peak height/area. The method detection limit (MDL) was calculated as three times the standard deviation of the blank concentrations (MDLs by sampling round are provided in Table S1). Precision and accuracy of PBDEs from surrogate spikes, reference material (SRM 1957, National Institute of Standards and Technology), and in-house control samples were within reasonable analytical error ranges. The DTSC laboratory participates in the Artic Monitoring and Assessment Programme (also called AMAP Ring Test of Persistent Organic Pollutants in Human Serum) for the PCB and PBDE analyses and has consistently shown excellent performance test results in all compounds with a Z score < 1. For the analysis of samples from the second cohort, the average recoveries (± standard deviation (SD)) of all isotopic surrogate compounds (nine 13C12 PCBs and nine 13C12 PBDEs) used in HRMS/isotope dilution ranged from 72 ± 24% to 123 ± 23%. The average recoveries (± SD) of hydroxylated surrogate standards, 2-OH-BDE28 and 4′-OH-CB159, used in GC/NCI/MS analysis were 78 ± 8%, and 66 ± 11%, respectively. Chemical concentrations in both cohorts were corrected by the surrogate recoveries. Total cholesterol and triglyceride levels were measured by Quest Diagnostics in the first cohort and the Boston Children’s Hospital in the second cohort. Total lipid content was calculated from measurements of total cholesterol and triglycerides using Phillip’s formula.33 Mean lipid content did not statistically differ between cohorts (Table 1).
Table 1.
Comparison of demographic characteristics between the first and second cohorts of second trimester pregnant women recruited from San Francisco General Hospital, San Francisco, Californiaa.
| 2008–2009 (n = 25) N (%) |
2011–2012 (n =36) N (%) |
pb | |
|---|---|---|---|
| Age, years | 0.06 | ||
| 16 – 19 | 9 (36%) | 4 (11%) | |
| 20 – 29 | 12 (48%) | 25 (69%) | |
| 30 – 42 | 4 (16%) | 7 (19%) | |
| Ethnicity | 0.22 | ||
| Latina | 9 (36%) | 8 (23%) | |
| African-American | 10 (40%) | 10 (29%) | |
| White | 3 (12%) | 12 (34%) | |
| Asian/Other | 3 (12%) | 5 (14%) | |
| Insurance | |||
| Medi-Cal | 20 (80%) | 28 (80%) | 0.99 |
| Private/Self-Pay | 5 (20%) | 7 (20%) | |
| Marital status | |||
| Single | 22 (92%) | 28 (93%) | 0.97 |
| Married | 1 (4%) | 1 (3%) | |
| Separated | 1 (4%) | 1 (3%) | |
| Country of origin | 0.69 | ||
| U.S. | 22 (88%) | 33 (94%) | |
| non-U.S. | 3 (12%) | 2 (6%) | |
| Parity | 0.27 | ||
| nulliparous | 8 (32%) | 11 (31%) | |
| parity ≥ 1 | 17 (68%) | 25 (69%) | |
| Current smokersc | <0.0001 | ||
| No | 25 (100%) | 23 (64%) | |
| Yes | 0 (0%) | 13 (36%) | |
|
| |||
| Mean (± SD) | Mean (± SD) | ||
| Age, years | 23.5 ± 7.3 | 24.9 ± 5.5 | 0.41 |
| Gestational age, weeks | 20.5 ± 1.2 | 20.0 ± 2.1 | 0.23 |
| Lipids, mg/ml | 6.9 ± 1.0 | 6.5 ± 0.8 | 0.11 |
Abbreviations: SD = standard deviation
Data were missing for marital status (n=1) in the 2008–2009 cohort; and race/ethnicity (n=1), insurance (n=1), marital status (n=6), and country of origin (n=1) in the 2011–2012 cohort.
Differences across cohorts were evaluated by chi-square for categorical variables and ANOVA for continuous variables.
Current smokers were not recruited in the 2008–2009 cohort as part of the study design.
Data Analysis
We calculated the following statistics for all chemicals with greater than 50% detection frequencies: geometric mean (GM), geometric standard deviation (GSD), median, and 95th percentile estimates. For concentrations below the MDL, we used estimated values provided by the GC-HRMS when available. If values were below the IDT (e.g. less than the threshold) and estimated values were not available, then undetected values were imputed based on a log-normal probability distribution whose parameters were calculated through maximum likelihood estimation.34, 35 The percent of samples for each congener below the IDT that were imputed is provided in Table S1.
Concentrations for PBDE and PCB congeners are normalized for lipid content (ng/g lipid), and OH-PBDEs and OH-PCBs are expressed as wet weight (ng/mL). We examined chemical analytes individually and also constructed the following four summary measures: the sum of five major PBDEs (BDE- 28, -47, -99, -100, -153) (ΣPBDEs); the sum of five major PCBs (PCB -74, -118, -138, -153, -180) (ΣPCBs); the sum of three major OH-PBDEs (6-OH-BDE-47, 5-OH-BDE-47, 4′-OH-BDE-49) (ΣOH-PBDEs); and the sum of three major OH-PCBs (4′-OH-CB107, 4′-OH-CB146 (ΣOH-PCBs). We used natural log-transformations of the chemical analytes prior to correlation and regression analysis to account for their non-normal distributions.
We used Pearson correlation coefficients (r) to assess correlations between log-transformed chemical concentrations. We used chi-square and Wilcoxon Rank Sum tests to evaluate differences in demographics and chemical concentrations, respectively, between the first and second cohorts. Next, we ran multiple linear regressions to examine differences in chemical concentrations by cohort after adjustment for covariates. Separate models were constructed for individual congeners, as well as for groups of chemicals (ΣPBDEs, ΣOH-PBDEs, ΣPCBs, and ΣOH-PCBs).
The following covariates were considered for inclusion in our regression models: age (yrs; continuous), gestational age (weeks; continuous), race/ethnicity (Latina, Black, White, or Asian/other), parity (nulliparous versus one or more live births), and type of health insurance (public insurance versus private insurance or self-pay), which is considered a proxy for socioeconomic status (SES) in the US. Marital status and country of origin were not considered because not all categories had cell sizes greater than five. Model selection was completed using backward stepwise regression. We retained covariates in the final model that were significant at p < 0.20 or that changed the estimate of our primary exposure variable (e.g. cohort) by more than 10%. To facilitate comparability across chemical analytes, all final regression models were adjusted for the same set of covariates. From the output of these multivariate regression models, we calculated: 1) percent difference in chemical concentrations between cohorts equal to one minus the exponentiated beta coefficient multiplied by 100, and 2) least square geometric mean (LSGM) estimates or the geometric mean of chemical concentrations by cohort after controlling for covariates.
We conducted two sensitivity analyses to further evaluate whether the observed differences in chemical concentrations by cohort may be attributed to differences in study design. First, we limited the analysis to non-smokers (n = 48) since the earlier cohort did not include smokers. Second, because different laboratories measured total cholesterol and triglyceride for the first and second cohort, we modeled the PBDE and PCB congeners as wet weight concentrations (ng/ml) and included lipid content as a separate covariate.
We examined residual diagnostics on our final models to: evaluate assumptions of normality and equal variances; and identify extreme observations that may unduly influence statistical results. Outliers were defined as observations with absolute studentized residual greater than three. There were no statistical outliers identified in the final models presented in the main text and the majority of model residuals were normally distributed and homoscedastic. All analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC) or R version 2.15. A p-value < 0.05 was considered significant and p < 0.10 as marginally significant on two-sided tests.
RESULTS
The 2008–2009 and 2011–2012 cohort populations were demographically similar except for smoking status, which was due to our study design (Table 1). Both cohorts consisted of predominately low-income, U.S.-born, non-white women in their second trimester of pregnancy. The mean (± SD) age was 23.5 ± 7.3 years and 24.9 ± 5.5, respectively, in 2008–2009 and 2011–2012. In both cohorts, 80% of women reported using state public health insurance and approximately 90% reported being U.S.-born and not married. Racial/ethnic profiles differed by cohort but these differences were not statistically significant.
MDLs for individual PBDE and PCB congeners were stable between cohorts and generally within a factor of two while MDLs for OH-compounds were consistently lower for the second cohort (Table 2). PBDEs were widely detected in the study population. In 2008–2009, all five PBDE congeners were detected in all participants; whereas in 2011–2012, BDE-47 was the only PBDE congener detected in all participants. BDE-47 was also the most abundant PBDE congener in both cohorts. Median concentrations of ΣPBDEs were significantly lower in 2011–2012 compared to 2008–2009 (p = 0.001). Similar trends were observed for all five PBDE congeners except differences in concentrations across cohorts were less pronounced and only marginally significant for BDE-153 (p = 0.07). Detection frequency and concentrations of all three OH-PBDEs declined between 2008–2009 and 2011–2012 (Table 2).
Table 2.
Comparison of serum PBDE, PCB, and OH-PBDE concentrations between the first and second cohorts of second trimester pregnant women recruited from San Francisco General Hospital, San Francisco, California
| Chemical analyte | 2008–2009 (n=25) | 2011–2012 (n=36)a | pc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MDL | % > MDL | GM (GSD)b | 50th % | 95th % | MDL | % > MDL | GM (GSD)b | 50th % | 95th % | ||
| PBDEs (ng/g lipid) | |||||||||||
| BDE-28 | 0.66 | 100 | 2.32 (1.68) | 2.40 | 6.97 | 0.77 | 78 | 1.38 (1.96) | 1.49 | 4.11 | 0.002 |
| BDE-47 | 11.7 | 100 | 43.1 (1.70) | 42.1 | 123.2 | 5.85 | 100 | 25.75 (2.03) | 21.8 | 102.2 | 0.002 |
| BDE-99 | 3.63 | 100 | 11.5 (1.82) | 9.80 | 27.2 | 4.92 | 61 | 6.68 (2.08) | 5.89 | 36.00 | 0.001 |
| BDE-100 | 0.91 | 100 | 9.00 (1.86) | 8.96 | 23.3 | 1.08 | 97 | 4.66 (2.21) | 4.24 | 19.84 | 0.001 |
| BDE-153 | 0.93 | 100 | 15.5 (1.82) | 16.5 | 38.3 | 1.54 | 97 | 10.87 (2.17) | 11.7 | 37.91 | 0.08 |
| ΣPBDEs | 85.8 (1.59) | 82.9 | 192.8 | 51.9 (1.92) | 47.9 | 189.4 | 0.001 | ||||
| PCBs (ng/g lipid) | |||||||||||
| PCB-74 | 0.63 | 76 | 0.89 (0.1) | 0.94 | 1.75 | 0.77 | 53 | 0.7 (2.07) | 0.81 | 1.79 | 0.23 |
| PCB-118 | 0.69 | 88 | 1.66 (0.12) | 1.82 | 3.79 | 0.92 | 100 | 1.54 (1.81) | 1.52 | 3.32 | 0.28 |
| PCB-138 | 1.63 | 64 | 2.38 (0.13) | 2.52 | 6.40 | 0.77 | 100 | 2.17 (1.82) | 1.82 | 6.05 | 0.59 |
| PCB-153 | 1.52 | 80 | 3.51 (0.15) | 3.84 | 13.56 | 0.77 | 100 | 3.53 (2.03) | 3.12 | 11.84 | 0.66 |
| PCB-180 | 1.44 | 92 | 3.19 (0.14) | 3.05 | 10.00 | 0.92 | 86 | 2.02 (2.14) | 1.95 | 6.32 | 0.02 |
| ΣPCBs | 12.3 (1.77) | 13.3 | 34.1 | 10.4 (1.86) | 9.75 | 26.8 | 0.18 | ||||
| OH-PBDEs (ng/ml) | |||||||||||
| 5-OH-BDE47 | 0.012 | 83 | 0.028 (3.52) | 0.036 | 0.131 | 0.002 | 64 | 0.004 (4.25) | 0.007 | 0.028 | <0.0001 |
| 6-OH-BDE47 | 0.010 | 92 | 0.025 (2.40) | 0.023 | 0.147 | 0.002 | 61 | 0.004 (3.43) | 0.007 | 0.021 | <0.0001 |
| 4′-OH-BDE49 | 0.010 | 50 | 0.008 (2.01) | 0.010 | 0.020 | 0.002 | 6 | -- | <MDL | 0.008 | <0.0001 |
| ΣOH-PBDEs | 0.072 (2.20) | 0.069 | 0.268 | 0.010 (3.05) | 0.014 | 0.051 | <0.0001 | ||||
| OH-PCBs (ng/ml) | |||||||||||
| 4′-OH-CB107 | 0.010 | 8 | -- | <MDL | 0.025 | 0.005 | 25 | -- | <MDL | 0.026 | -- |
| 4′-OH-CB146 | 0.006 | 13 | -- | <MDL | 0.007 | 0.005 | 47 | -- | <MDL | 0.053 | -- |
| 4′-OH-CB187 | 0.014 | 34 | -- | <MDL | 0.026 | 0.005 | 44 | -- | <MDL | 0.024 | -- |
| ΣOH-PCBs | 0.019 (1.72) | 0.019 | 0.055 | 0.017 (2.44) | 0.017 | 0.080 | 0.43 | ||||
Abbreviations: MDL = method detection limit; GM = geometric mean; GSD = geometric standard deviation.
OH-PBDE and OH-PCB concentrations were missing for 1 participant in the second cohort
GM and GSD only calculated for chemicals with detection frequencies ≥ 50 percent.
Differences in median chemical concentrations by cohort evaluated by Wilcoxon Rank Sum test.
In contrast, detection frequency for PCB and OH-PCB congeners did not vary by cohort. PCB congeners were widely detected, but individual OH-PCBs were detected in less than 50% of the population. Median concentrations for individual PCB congeners and ΣPCBs were lower in 2011–2012 compared to 2008–2009, but differences in concentrations across cohorts were only statistically significant for PCB-180. ΣOH-PCB concentrations were similar between cohorts (Table 2).
ΣPBDEs concentrations were correlated with ΣOH-PBDEs concentrations (Pearson r = 0.62, p < 0.0001) but not with ΣPCBs concentrations (Pearson r = 0.08, p > 0.10). BDE-47 was highly correlated with other PentaBDE congeners (e.g. BDE-28, -99, and -100) (Pearson r = 0.9, p < 0.0001), but less correlated with BDE-153 (minor component of PentaBDE and OctaBDE) (Pearson r = 0.44, p = 0.0003). ΣPCBs showed a modest correlation with BDE-153 (Pearson r = 0.34, p = 0.0007) but little correlation with PentaBDE congeners (Pearson r range = −0.17 to 0.18, p > 0.10) (Figure S1).
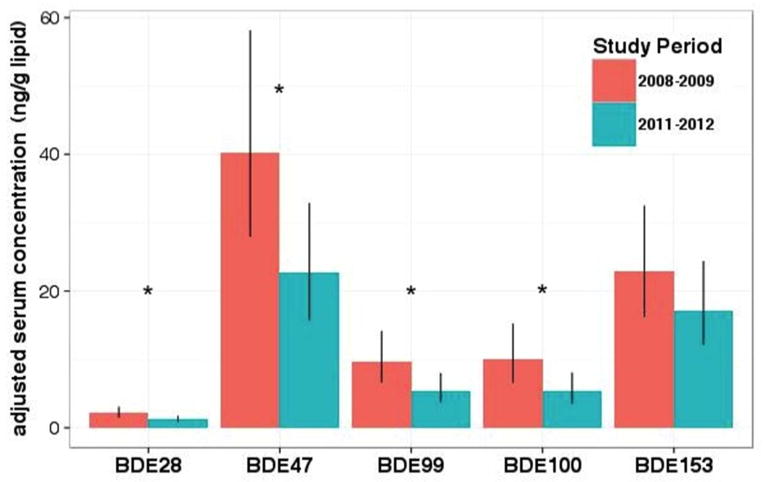
After adjusting for covariates, LSGM concentrations of BDE-47 were 77% lower (95% CI: 23, 156) in 2011–2012 compared to 2008–2009 (p = 0.004). Reductions in other PentaBDE congeners (BDE-28, -99, and -100) were similar in magnitude. LSGM concentrations of BDE-153 declined 36% (95% CI: 3, 92) from 2008–2009 to 2011–2012 but this difference was only marginally significant (p = 0.10) (Figure 1 and Table 3).
Figure 1.
Least square geometric mean serum concentration for individual PBDE congeners by cohort after adjustment by maternal age, gestational age, race/ethnicity, parity, and insurance status. Error bars indicate 95% confidence intervals and asterisks reflect statistically significant differences (p < 0.05).
Table 3.
REVISED. Comparison of Chemical Analyte Concentrations between Cohorts Calculated from Multivariate Regression Models Adjusted for Age, Gestational Age, Race/Ethnicity, Parity, And Insurance Statusa,c
| chemical analyte | n | % differenceb | 2008–2009 LSGM (95% CI) | 2011–2012 LSGM (95% CI) | p |
|---|---|---|---|---|---|
| PBDEs (ng/g lipid) | |||||
| BDE-28 | 59 | −43 (−60, −17) | 2.15 (1.49, 3.09) | 1.23 (0.85, 1.78) | 0.004 |
| BDE-47 | 59 | −44 (−61, −18) | 40.3 (27.9, 58.1) | 22.7 (15.7, 32.9) | 0.003 |
| BDE-99 | 59 | −44 (−61, −17) | 9.69 (6.64,14.2) | 5.48 (3.74, 8.02) | 0.004 |
| BDE-100 | 59 | −47 (−65, −19) | 10.0 (6.60,15.2) | 5.31 (3.48, 8.09) | 0.004 |
| BDE-153 | 59 | −25 (−47, 6) | 23.0 (16.2, 32.5) | 17.2 (12.1, 24.4) | 0.10 |
| ΣPBDEs | 59 | −39 (−56, −15) | 90.0 (64.7, 125.2) | 54.6 (39.2, 76.2) | 0.004 |
| PCBs (ng/g lipid) | |||||
| PCB-74 | 59 | −26 (−46, 1) | 1.26 (0.92, 1.72) | 0.93 (0.68, 1.27) | 0.06 |
| PCB-118 | 59 | −10 (−34, 24) | 2.04 (1.49, 2.81) | 1.85 (1.34, 2.54) | 0.53 |
| PCB-138 | 59 | −6 (−31, 29) | 2.96 (2.17, 4.05) | 2.79 (2.04, 3.82) | 0.70 |
| PCB-153 | 59 | −9 (−36, 30) | 4.34 (3.05, 6.17) | 3.96 (2.78, 5.65) | 0.61 |
| PCB-180 | 59 | −42 (−59, −18) | 3.83 (2.72, 5.38) | 2.23 (1.59, 3.15) | 0.003 |
| ΣPCBs | 59 | −20 (−40, 7) | 15.1 (11.3, 20.1) | 12.0 (8.98, 16.1) | 0.13 |
| OH-PBDEs (ng/mL) | |||||
| 5-OH-BDE47 | 58 | −83 (−92, −66) | 0.042 (0.020, 0.088) | 0.007 (0.003, 0.014) | <0.0001 |
| 6-OH-BDE47 | 58 | −84 (−91, −71) | 0.039 (0.022, 0.070) | 0.006 (0.004, 0.011) | <0.0001 |
| ΣOH-PBDEs | 58 | −86 (−92, −75) | 0.099 (0.055, 0.179) | 0.014 (0.008, 0.025) | <0.0001 |
| ΣOH-PCBs (ng/mL) | 58 | −8 (−41, 43) | 0.020 (0.013, 0.031) | 0.018 (0.012, 0.028) | 0.71 |
Chemical analyte concentrations were natural log-transformed in multivariate regression models.
Reference group is 2008−2009 cohort.
Abbreviations: CI = confidence intervals; LSGM = least-squares geometric mean.
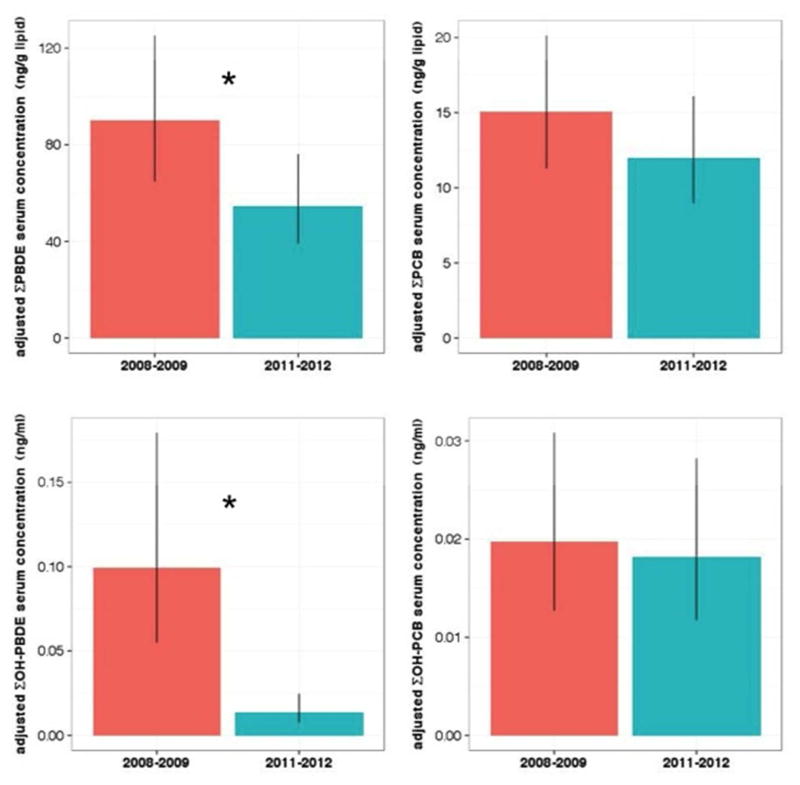
LSGM concentrations of ΣPBDEs decreased 65% (95% CI: 18, 130) from 90.0 ng/g lipid (95% CI: 64.7, 125.2) in 2008–2009 to 54.6 ng/g lipid (95% CI: 39.2, 76.2) in 2011–2012 (p = 0.004). LSGM concentrations of ΣOH-PBDEs declined 620% (95% CI: 302, 1192) from 0.099 ng/ml (95% CI: 0.055, 0.179) in 2008–2009 to 0.014 ng/ml (95% CI: 0.008, 0.025) (p < 0.0001); reductions in individual metabolites 5-OH-BDE47 and 6-OH-BDE47 were similar in magnitude to the summary measure.
LSGM concentrations of ΣPCBs decreased 25% (95% CI: −68, 7) from 15.1 ng/g lipid (95% CI: 11.3, 20.1) in 2008–2009 to 12.0 ng/g lipid (95% CI: 8.98, 16.1) in 2011–2012 but this difference was not statistically significant (p = 0.13) (Figure 2 and Table 3). Similar, non-significant or marginally significant reductions in LSGM concentrations were observed for PCB-74, -118, -138, and -153 but not PCB-180. LSGM concentrations of PCB-180 declined 71% (95% CI: 22, 141) (p = 0.003) between the first and second cohorts. LSGM estimates for ΣOH-PCBs concentrations showed the least amount of change among the analytes analyzed and were statistically similar across cohorts (Figure 2 and Table 3). None of the sensitivity analyses showed qualitatively different results (Table S2).
Figure 2.
Least square geometric mean serum concentration for ΣPBDEs, ΣPCBs, ΣOH-PBDEs, and ΣOH-PCBs by cohort after adjustment by maternal age, gestational age, race/ethnicity, parity, and insurance status. Error bars indicate 95% confidence intervals and asterisks reflect statistically significant differences (p < 0.05).
DISCUSSION
In this study, we find that serum concentrations of PBDEs and OH-PBDEs among second trimester pregnant women in San Francisco, California declined substantially between 2008–2009 and 2011–2012, consistent with the hypothesis the phase-out of PBDE use and production in California and the rest of the US (and consequent market shift to other flame retardants) would produce such a decline. In contrast, we observed only modest declines in serum concentrations of PCBs and virtually no change in OH-PCBs suggesting relatively stable exposures. To our knowledge, this is the first study to conduct a temporal comparison of PBDE body burden of pregnant women residing in California, a subpopulation with historically elevated exposures that may show enhanced vulnerability to the adverse effects of PBDE chemicals. While PBDE and OH-PBDE exposures in this study population declined significantly, the levels in our study population are still higher than those reported in other contemporary cohorts of US pregnant women.19, 36
Our results are consistent with two recent studies that find a decline in PBDE concentrations in the indoor environment coupled with a parallel rise in replacement flame retardants. The first study by Stapleton and colleagues found that only 1% of couches purchased after 2005 had detectable levels of PentaBDE compared to 39% of couches purchased before 2005.37 The second study by Dodson and colleagues employed a longitudinal study design to collect repeated household dust samples in 16 California homes and found that median dust concentrations of PentaBDE congeners declined between 2006 and 2011.24 In the present study, we did not measure replacement flame retardants so while PBDE exposures may be declining in this study population, it is likely that their exposures to replacement flame retardants may be on the rise.
Our results are in agreement with the reported elimination half-lives of PBDEs in the human body. Concentrations of PentaBDE congeners (BDE-47, -99, and -100) declined by 70–90% consistent with their estimated half-lives of 1–3 years; and BDE-153 declined by 34% consistent with its estimated half-life of 7–15 years. Our findings may also reflect shifts in the relative contribution of different exposure pathways to body burden such that PBDE exposures from dust may be decreasing more rapidly than dietary exposures in response to the PBDE phase-out. House dust is a major exposure pathway for BDE-47, -99, and -100 but not BDE-153.17 Diet is a contributing factor for many PBDEs18 but the relative contribution of diet may be more substantial for BDE-153.15, 19 For example, previous studies suggest that breast milk is more enriched with BDE-153 than BDE-47 when normalized by the composition of PentaBDE.15, 39 In our study population, ΣPCB concentrations showed a higher correlation with BDE-153 than with PentaBDE congeners suggesting that BDE-153 and PCBs may have overlapping exposure sources such as diet. The reasons underlying observed temporal patterns for the various PBDE analytes warrant further research since we cannot identify sources of exposure in our study participants nor distinguish between the relative contributions of elevated exposures in the past and current intake.
Our finding of greater reductions in concentrations of OH-PBDEs than for the parent compounds may in part be explained by differences in physicochemical properties. OH-PBDEs have faster oxidative transformation rates and shorter half-lives than PBDEs so they are less persistent40 and presumably more reflective of recent exposures. Additionally, the substantially lower concentrations of OH-PBDEs in the second cohort may indicate a non-linear relationship between concentrations of parent PBDE and OH-PBDEs consistent with the Michaelis-Menten model of enzyme kinetics and in-vitro metabolism studies of BDE-47 and -99.41, 42
PCB and OH-PCB concentrations in our study population were substantially lower than those reported in other contemporary populations of US reproductive-aged women.43, 44 The extent of decline that we observed for PCB congeners are generally in agreement with the Ritter et al. study45 which estimated elimination half-lives of 10–15 years for low-level, background exposures to PCBs. Consistent with prior studies, our findings suggests that global PCB emissions are declining at a slower rate in recent years compared to the more rapid declines observed in the 1970s following restrictions and phase-out of production in the US and Western Europe. 26, 27, 46, 47 One anomalous result in our study was that we observed differences between cohorts for concentrations of PCB-180 but not PCB-153 even though both congeners are highly chlorinated chemicals that persist in the environment. PCB-153 is typically the most abundant congener found in the food supply48, 49 so it is possible that ongoing dietary exposures to PCB-153 are greater than those of PCB-180 and substantial enough to counteract any potential decline in body burden that may arise from elimination decay during our relatively short study period. Another possible explanation is the unique chemical properties of PCB-180. Compared to the other PCB congeners in this study, PCB-180 is the most persistent in human adipose tissue46 and has the highest octanol-water partitioning coefficient (log Kow).50 Since PCBs were phased out several decades ago, it is plausible that the other PCB congeners have already undergone significant reductions such that contemporary serum concentrations for those congeners are in steady state with internal and external environments whereas the rate of decline for PCB-180 may be ongoing and span a greater time period. Future work is needed to confirm our observed finding of differences in temporal patterns between PCB-180 and other PCB congeners. All OH-PCB congeners were infrequently detected, and did not differ between cohorts, possibly because OH-PCBs (like the OH-PBDE analogues) are less persistent than the parent compounds and thus more reflective of recent exposures. Additionally, the low-level background PCB exposures characteristic of our study population may not be high enough to elicit metabolic production.
Our most significant limitation is that we could not collect repeated biological samples in the same participants. However, the demographic and biological similarities between groups suggest that our two separate cohorts reflect the same underlying population. Another limitation is the fact that the analyses of samples from the two cohorts were conducted at two different times (albeit by the same analysts), reflecting the two distinct recruitment periods. Ideally, samples should have been analyzed together and blindly. Nevertheless, the analytical methods were similar across cohorts and the laboratory is proficient in these analyses. The only difference in analytical methods between cohorts is the improved methodology for OH-PBDEs and OH-PCBs that the laboratory was using by the time the second cohort samples were processed. Our quality control documentation (control charts on method blanks, recoveries and precision) demonstrates the validity of our data. By using instrumentation with increased sensitivity (GC-NCI/MS), we were able to quantify concentrations of OH-PBDEs in the second cohort, in spite of the drastically reduced levels. Additionally, lipids were measured at different laboratories; however, both laboratories use standard methods and the mean lipid content did not vary by cohort. We also have a modest sample size with data at only two time points and some of our estimates have wide confidence intervals. Differences in ΣPCBs and BDE-153 concentrations between cohorts might have reached statistical significance with a larger sample size, but the percent difference in concentrations of ΣPCBs and BDE-153 would likely still be smaller than that of PentaBDEs and OH-PBDEs. Another limitation is that our first cohort did not include smokers while our second cohort did. However, our sensitivity analysis suggests that this difference in study design does not likely explain our findings. Moreover, smoking has actually been associated with higher PBDE and PCB exposures in prior studies of pregnant or postpartum women.51, 52 Therefore, the absence of smokers from the first cohort may lead to an underestimation of the true decline between 2008–2009 and 2011–2012. Women obtaining second trimester pregnancy terminations are unique and likely differ from pregnant women who continue their pregnancy. Therefore, our results may not be generalizable to other populations, but from a health policy perspective, it is encouraging to see exposure reductions in this vulnerable population. Future studies should examine temporal trends of human exposure to flame retardants in populations that are more representative of California and the US population.
This study has several important strengths that reiterate the importance of our findings. To our knowledge, we are the first study to conduct a temporal comparison of PBDE body burdens in the U.S. after the phase-out. Two prior studies that examined temporal trends in human PBDE exposure only included data before the phase-out.53, 54 This is also the first human study to conduct a temporal comparison of exposures to OH-PBDEs which may be more potent neurotoxicants11 than the parent congeners. Lastly, the estimated differences in PBDEs and OH-PBDEs between cohorts were large, highly statistically significant, and consistent in various sensitivity analyses; all of which suggest a robust effect.
In conclusion, we found substantial declines in PBDE and OH-PBDE serum concentrations among ethnically diverse, low-income pregnant women from California - a population with historically elevated PBDE exposures. The substantial decline in PBDEs and OH-PBDE concentrations are encouraging from a public policy perspective and demonstrates the power of using biomonitoring to gauge the efficacy of regulatory or public health interventions. Our findings for both PBDEs and PCBs suggest regulations targeting chemical use and production may result in a faster decline of those chemicals with shorter half-lives. Despite the phase-out of both PBDEs and PCBs, these chemicals continue to pose a public health challenge given their ubiquitous detection and increasing evidence that co-exposures to PBDEs and PCBs can increase health risks more than exposure to either chemical alone.55 Furthermore, the relative stability of PCB exposures in this population coupled with their structural similarities to PBDEs23 suggest that the rate of decline in PBDE body burden may eventually plateau and that these chemicals will persist in the serum for decades. Our findings can help evaluate public policies and inform future strategies for public health interventions.
Supplementary Material
Acknowledgments
We thank the staff and faculty at San Francisco General Hospital’s Women’s Options Center, who assisted in specimen collection. We thank Dr. Darcy Tarrant and Greg Yeh at the DTSC for sharing their analytical expertise in OH-PCB confirmation and serum preparation. This work was supported by the Passport Foundation Science Innovation Fund, the National Institute of Environmental Health Sciences (NIEHS) K99ES019881 and ViCTER supplement to 5R01ES010026.
Footnotes
Disclaimer
Views expressed here are those of the authors and they do not necessarily represent those of the DTSC.
The supporting information includes: chemical-specific MDLs by cohort; percent of samples above the IDT and MDL, respectively, by cohort; regression results of sensitivity analyses; and correlation matrix of chemical analytes. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Zota AR, Park JS, Wang Y, Petreas M, Zoeller RT, Woodruff TJ. Polybrominated diphenyl ethers, hydroxylated polybrominated diphenyl ethers, and measures of thyroid function in second trimester pregnant women in California. Environ Sci Technol. 2011;45(18):7896–905. doi: 10.1021/es200422b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chevrier J, Harley KG, Bradman A, Gharbi M, Sjodin A, Eskenazi B. Polybrominated diphenyl ether (PBDE) flame retardants and thyroid hormone during pregnancy. Environ Health Perspect. 2010;118(10):1444–9. doi: 10.1289/ehp.1001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stapleton HM, Eagle S, Anthopolos R, Wolkin A, Miranda ML. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environ Health Perspect. 2011;119(10):1454. doi: 10.1289/ehp.1003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbstman JB, Sjodin A, Apelberg BJ, Witter FR, Halden RU, Patterson DG, Panny SR, Needham LL, Goldman LR. Birth delivery mode modifies the associations between prenatal polychlorinated biphenyl (PCB) and polybrominated diphenyl ether (PBDE) and neonatal thyroid hormone levels. Environ Health Perspect. 2008;116(10):1376–82. doi: 10.1289/ehp.11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbstman JB, Sjödin A, Kurzon M, Lederman SA, Jones RS, Rauh V, Needham LL, Tang D, Niedzwiecki M, Wang RY. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect. 2010;118(5):712. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, Trujillo C, Sjödin A, Bradman A. In utero and childhood polybrominated diphenyl ether (pbde) exposures and neurodevelopment in the CHAMACOS Study. Environ Health Perspect. 2013;121(2):257. doi: 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szabo DT, Richardson VM, Ross DG, Diliberto JJ, Kodavanti PRS, Birnbaum LS. Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicol Sci. 2009;107(1):27–39. doi: 10.1093/toxsci/kfn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoker TE, Laws SC, Crofton KM, Hedge JM, Ferrell JM, Cooper RL. Assessment of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture, in the EDSP male and female pubertal protocols. Toxicol Sci. 2004;78(1):144–155. doi: 10.1093/toxsci/kfh029. [DOI] [PubMed] [Google Scholar]
- 9.Hallgren S, Sinjari T, Hakansson H, Darnerud PO. Effects of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) on thyroid hormone and vitamin A levels in rats and mice. Arch Toxicol. 2001;75(4):200–208. doi: 10.1007/s002040000208. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert ME, Rovet J, Chen Z, Koibuchi N. Developmental thyroid hormone disruption: Prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology. 2012;33(4):842–852. doi: 10.1016/j.neuro.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Dingemans MML, van den Berg M, Westerink RHS. Neurotoxicity of Brominated Flame Retardants: (In)direct Effects of Parent and Hydroxylated Polybrominated Diphenyl Ethers on the (Developing) Nervous System. Environ Health Perspect. 2011;119(7):900–907. doi: 10.1289/ehp.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu N, Herrmann T, Paepke O, Tickner J, Hale R, Harvey E, La Guardia M, McClean MD, Webster TF. Human exposure to PBDEs: associations of PBDE body burdens with food consumption and house dust concentrations. Environ Sci Technol. 2007;41(5):1584–1589. doi: 10.1021/es0620282. [DOI] [PubMed] [Google Scholar]
- 13.Lorber M. Exposure of Americans to polybrominated diphenyl ethers. J Expo Sci Environ Epidemiol. 2008;18(1):2–19. doi: 10.1038/sj.jes.7500572. [DOI] [PubMed] [Google Scholar]
- 14.Trudel D, Scheringer M, von Goetz N, Hungerbühler K. Total consumer exposure to polybrominated diphenyl ethers in North America and Europe. Environ Sci Technol. 2011;45(6):2391–2397. doi: 10.1021/es1035046. [DOI] [PubMed] [Google Scholar]
- 15.Stapleton HM, Eagle S, Sjödin A, Webster TF. Serum PBDEs in a North Carolina Toddler Cohort: Associations with Handwipes, House Dust, and Socioeconomic Variables. Environ Health Perspect. 2012;120(7):1049. doi: 10.1289/ehp.1104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.La Guardia MJ, Hale RC, Harvey E. Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame-retardant mixtures. Environ Sci Technol. 2006;40(20):6247–6254. doi: 10.1021/es060630m. [DOI] [PubMed] [Google Scholar]
- 17.Johnson PI, Stapleton HM, Sjodin A, Meeker JD. Relationships between polybrominated diphenyl ether concentrations in house dust and serum. Environ Sci Technol. 2010;44(14):5627–5632. doi: 10.1021/es100697q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraser AJ, Webster TF, McClean MD. Diet contributes significantly to the body burden of PBDEs in the general US population. Environ Health Perspect. 2009;117(10):1520–1525. doi: 10.1289/ehp.0900817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horton MK, Bousleiman S, Jones R, Sjodin A, Liu X, Whyatt R, Wapner R, Factor-Litvak P, Wallin M, Sallsten G. Predictors of serum concentrations of polybrominated flame retardants among healthy pregnant women in an urban environment: a cross-sectional study. Environmental Health. 2013;12(1):23. doi: 10.1186/1476-069X-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol. 2004;38(4):945–56. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- 21.Zota AR, Rudel RA, Morello-Frosch RA, Brody JG. Elevated house dust and serum concentrations of PBDEs in California: unintended consequences of furniture flammability standards? Environ Sci Technol. 2008;42(21):8158–64. doi: 10.1021/es801792z. [DOI] [PubMed] [Google Scholar]
- 22.Zota AR, Adamkiewicz G, Morello-Frosch RA. Are PBDEs an environmental equity concern? Exposure disparities by socioeconomic status. Environ Sci Technol. 2010;44(15):5691–92. doi: 10.1021/es101723d. [DOI] [PubMed] [Google Scholar]
- 23.Shaw SD, Blum A, Weber R, Kannan K, Rich D, Lucas D, Koshland CP, Dobraca D, Hanson S, Birnbaum LS. Halogenated Flame Retardants: Do the Fire Safety Benefits Justify the Risks? Rev Environ Health. 2010;25(4):261. doi: 10.1515/reveh.2010.25.4.261. [DOI] [PubMed] [Google Scholar]
- 24.Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA. After the PBDE Phase-Out: A Broad Suite of Flame Retardants in Repeat House Dust Samples from California. Environ Sci Technol. 2012 doi: 10.1021/es303879n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrad S, Diamond M. New directions: exposure to polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs): current and future scenarios. Atmos Environ. 2006;40(6):1187–1188. [Google Scholar]
- 26.Breivik K, Sweetman A, Pacyna JM, Jones KC. Towards a global historical emission inventory for selected PCB congeners — a mass balance approach: 1.. Global production and consumption. Sci Total Environ. 2002;290(1–3):181–198. doi: 10.1016/s0048-9697(01)01075-0. [DOI] [PubMed] [Google Scholar]
- 27.Breivik K, Sweetman A, Pacyna JM, Jones KC. Towards a global historical emission inventory for selected PCB congeners — a mass balance approach: 2. Emissions Sci Total Environ. 2002;290(1–3):199–224. doi: 10.1016/s0048-9697(01)01076-2. [DOI] [PubMed] [Google Scholar]
- 28.Geyer HJ, Schramm KW, Darnerud PO, Aune M, Feicht EA, Fried KW, Henkelmann B, Lenoir D, Schmid P, McDonald TA. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Compounds. 2004;66:3867–3872. [Google Scholar]
- 29.Butte W, Heinzow B. Pollutants in house dust as indicators of indoor contamination. Rev Environ Contam T. 2002;175:1–46. [PubMed] [Google Scholar]
- 30.Shaw SD, Berger ML, Brenner D, Kannan K, Lohmann N, Päpke O. Bioaccumulation of polybrominated diphenyl ethers and hexabromocyclododecane in the northwest Atlantic marine food web. Sci Total Environ. 2009;407(10):3323–3329. doi: 10.1016/j.scitotenv.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 31.Rogers E, Petreas M, Park JS, Zhao GM, Charles MJ. Evaluation of four capillary columns for the analysis of organochlorine pesticides, polychlorinated biphenyls, and polybrominated diphenyl ethers in human serum for epidemiologic studies. J Chromatogr B: Anal Technol Biomed Life Sci. 2004;813(1–2):269–285. doi: 10.1016/j.jchromb.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 32.Park JS, Linderholm L, Charles MJ, Athanasiadou M, Petrik J, Kocan A, Drobna B, Trnovec T, Bergman A, Hertz-Picciotto I. Polychlorinated biphenyls and their hydroxylated metabolites (OH-PCBs) in pregnant women from eastern Slovakia. Environ Health Perspect. 2007;115(1):20–27. doi: 10.1289/ehp.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips DL, Pirkle JL, Burse VW, Bernert JT, Henderson LO, Needham LL. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18(4):495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- 34.Baccarelli A, Pfeiffer R, Consonni D, Pesatori AC, Bonzini M, Patterson DG, Bertazzi PA, Landi MT. Handling of dioxin measurement data in the presence of non-detectable values: overview of available methods and their application in the Seveso chloracne study. Chemosphere. 2005;60(7):898–906. doi: 10.1016/j.chemosphere.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 35.Helsel DR. Less than obvious - statistical treatment of data below the detection limit. Environ Sci Technol. 1990;24(12):1766–1774. [Google Scholar]
- 36.Chen A, Park J-S, Linderholm L, Rhee A, Petreas M, DeFranco EA, Dietrich K, Ho S-M. Hydroxylated Polybrominated Diphenyl Ethers in Paired Maternal and Cord Sera. Environ Sci Technol. 2013 doi: 10.1021/es3046839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A. Novel and High Volume Use Flame Retardants in US Couches Reflective of the 2005 PentaBDE Phase Out. Environ Sci Technol. 2012;46(24):13432–13439. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lupton SJ, McGarrigle BP, Olson JR, Wood TD, Aga DS. Human liver microsome-mediated metabolism of brominated diphenyl ethers 47, 99, and 153 and identification of their major metabolites. Chem Res Toxicol. 2009;22(11):1802–1809. doi: 10.1021/tx900215u. [DOI] [PubMed] [Google Scholar]
- 39.Antignac JP, Cariou R, Zalko D, Berrebi A, Cravedi JP, Maume D, Marchand P, Monteau F, Riu A, Andre F, Le Bizec B. Exposure assessment of French women and their newborn to brominated flame retardants: determination of tri- to deca- polybromodiphenylethers (PBDE) in maternal adipose tissue, serum, breast milk and cord serum. Environ Pollut. 2009;157(1):164–173. doi: 10.1016/j.envpol.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 40.Bastos PM, Eriksson J, Vidarson J, Bergman A. Oxidative transformation of polybrominated diphenyl ether congeners (PBDEs) and of hydroxylated PBDEs (OH-PBDEs) Environ Sci Pollut Res. 2008;15(7):606–613. doi: 10.1007/s11356-008-0045-9. [DOI] [PubMed] [Google Scholar]
- 41.Erratico CA, Szeitz A, Bandiera S. Oxidative metabolism of BDE-99 by human liver microsomes: predominant role of CYP2B6. Toxicol Sci. 2012 doi: 10.1093/toxsci/kfs215. [DOI] [PubMed] [Google Scholar]
- 42.Erratico CA, Szeitz A, Bandiera SM. Biotransformation of 2,2′,4,4′-Tetrabromodiphenyl Ether (BDE-47) by Human Liver Microsomes: Identification of Cytochrome P450 2B6 as the Major Enzyme Involved. Chem Res Toxicol. 2013;26(5):721–731. doi: 10.1021/tx300522u. [DOI] [PubMed] [Google Scholar]
- 43.Marek RF, Thorne PS, Wang K, DeWall J, Hornbuckle KC. PCBs and OH-PCBs in Serum from Children and Mothers in Urban and Rural US Communities. Environ Sci Technol. 2013 doi: 10.1021/es304455k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the US: NHANES 2003–2004. Environ Health Perspect. 2011;119(6):878–85. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ritter R, Scheringer M, MacLeod M, Moeckel C, Jones KC, Hungerbühler K. Intrinsic human elimination half-lives of polychlorinated biphenyls derived from the temporal evolution of cross-sectional biomonitoring data from the United Kingdom. Environ Health Perspect. 2011;119(2):225. doi: 10.1289/ehp.1002211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrad SJ, Sewart AP, Alcock R, Boumphrey R, Burnett V, Duarte-Davidson R, Halsall C, Sanders G, Waterhouse K, Wild SR. Polychlorinated biphenyls (PCBs) in the British environment: sinks, sources and temporal trends. Environ Pollut. 1994;85(2):131–146. doi: 10.1016/0269-7491(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 47.Bignert A, Olsson M, Persson W, Jensen S, Zakrisson S, Litzen K, Eriksson U, Haggberg L, Alsberg T. Temporal trends of organochlorines in Northern Europe, 1967–1995. Relation to global fractionation, leakage from sediments and international measures. Environ Pollut. 1998;99(2):177–198. doi: 10.1016/s0269-7491(97)00191-7. [DOI] [PubMed] [Google Scholar]
- 48.Llobet JM, Martí-Cid R, Castell V, Domingo JL. Significant decreasing trend in human dietary exposure to PCDD/PCDFs and PCBs in Catalonia, Spain. Toxicology Letters. 2008;178(2):117–126. doi: 10.1016/j.toxlet.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 49.Schecter A, Colacino J, Haffner D, Patel K, Opel M, Päpke O, Birnbaum L. Perfluorinated compounds, polychlorinated biphenyls, and organochlorine pesticide contamination in composite food samples from Dallas, Texas, USA. Environ Health Perspect. 2010;118(6):796. doi: 10.1289/ehp.0901347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hawker DW, Connell DW. Octanol-water partition coefficients of polychlorinated biphenyl congeners. Environ Sci Technol. 1988;22(4):382–387. [Google Scholar]
- 51.Herbstman JB, Sjodin A, Apelberg BJ, Witter FR, Patterson DG, Halden RU, Jones RS, Park A, Zhang Y, Heidler J, Needham LL, Goldman LR. Determinants of prenatal exposure to polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in an urban population. Environ Health Perspect. 2007;115(12):1794–800. doi: 10.1289/ehp.10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lind Y, Darnerud PO, Atuma S, Aune M, Becker W, Bjerselius R, Cnattingius S, Glynn A. Polybrominated diphenyl ethers in breast milk from Uppsala County, Sweden. Environ Res. 2003;93(2):186–194. doi: 10.1016/s0013-9351(03)00049-5. [DOI] [PubMed] [Google Scholar]
- 53.Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol. 2004;38(4):945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- 54.Sjodin A, Jones RS, Focant JF, Lapeza C, Wang RY, McGahee EE, 3rd, Zhang Y, Turner WE, Slazyk B, Needham LL, Patterson DG., Jr Retrospective time-trend study of polybrominated diphenyl ether and polybrominated and polychlorinated biphenyl levels in human serum from the United States. Environ Health Perspect. 2004;112(6):654–8. doi: 10.1289/ehp.112-1241957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller VM, Sanchez-Morrissey S, Brosch KO, Seegal RF. Developmental Coexposure to Polychlorinated Biphenyls and Polybrominated Diphenyl Ethers Has Additive Effects on Circulating Thyroxine Levels in Rats. Toxicol Sci. 2012;127(1):76–83. doi: 10.1093/toxsci/kfs089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.