Abstract
As actual stem cell application quickly approaches tissue engineering and regenerative medicine, aspects such as cell attachment to scaffolds and biomaterials become important and are often overlooked. Here, we compare the effects of several attachment proteins on the adhesion, proliferation and stem cell identity of three promising human stem cell types: human adipose-derived stem cells (hASCs), human embryonic stem cells (hESCs), and human induced pluripotent stem cells (hiPSCs). Traditional tissue culture polystyrene plates (TCPS), Matrigel (Mat), laminin (Lam), fibronectin (FN), and poly-L-lysine (PLL) were investigated as attachment protein surfaces. For hASCs typically cultured on TCPS, laminin resulted in the greatest cell attachment and proliferation with largest cell areas, indicating favorability by cell spreading. However, mesenchymal stem cell markers indicative of hASCs were slightly more expressed on surfaces with lowest cell attachment, corresponding to increased cell roundness, a newly observed attribute in hASCs possibly indicating more stem cell-like character. hESCs preferred Matrigel as a feeder-free culture surface. Interestingly, hiPSCs favored laminin over Matrigel for colony expansion, shown by larger cell colony area and perimeter lengths, although cell numbers and stem cell marker expression level remained highest on Matrigel. These data provide a practical reference guide for selecting a suitable attachment method for using human induced pluripotent, embryonic or adipose stem cells in tissue engineering and regenerative medicine applications.
Keywords: cell adhesion, surface functionalization, stem cells, laminin, fibronectin, extracellular matrix
Stem cells have great potential as a cell source for many engineered tissues and in regenerative medicine treatments. In tissue engineering, using stem cells to create tissue constructs generally begins with adhesion to a patterning material surface or a scaffold. In regenerative medicine applications, it was once believed that stem cells could simply be injected into the affected site or niche to initiate healing, but it is now known that the stem cells either die or dissipate from the local area. Hence, researchers in this area are looking to either synthetic or natural biomaterials as stem cell delivery vehicles. An issue often overlooked is that of cell adhesion to tissue engineering surfaces; it is often assumed that cells will readily attach on their own. Generally cells, especially stem cells, do not inherently attach to any surface – there must be some surface functionalization to promote adhesion. Means of attachment not only affect cell adhesion, but also growth and differentiation, factors imperative to directing stem cell phenotype in tissue engineering applications. Thus, it is important that the proper attachment method is chosen for the desired outcome. Here, we investigate different means of stem cell attachment, information pertinent for promoting adhesion to several tissue engineering surfaces or biomaterials for regenerative medicine. We compare the efficacy of several attachment proteins to their current standard culturing methods for three human stem cell types: human adipose-derived stem cells (hASCs), human embryonic stem cells (hESCs), and human induced pluripotent stem cells (hiPSCs). hASCs were chosen for their abundance, ease of procurement and non-controversial nature. hESCs and hiPSCs were chosen for their pluripotent capabilities, and hence exceptional potential to be used in many different tissue applications. The following protein surface treatments were investigated for their effects on cell expansion and stem cell identity retainment or differentiation: traditional tissue culture polystyrene plates (TCPS), laminin (Lam), fibronectin (FN), Matrigel (Mat), and poly-L-lysine (PLL).
Historically, embryonic stem cells (ESCs) are cultured on a feeder layer of mouse embryonic fibroblasts. This creates several issues, namely the complexity of co-culturing and the difficulty of removing the contaminating animal-derived fibroblasts once the ESCs have expanded enough for use. The next advancement involved the cell culture surface extracellular matrix (ECM) extract, termed Matrigel, containing many proteins and growth factors which are able to support proliferation and maintain stem cell phenotype (Xu et al., 2001). However, Matrigel is mouse derived and thus is not safe nor practical for human studies and in addition is expensive. Similarly, induced pluripotent stem cells (iPSCs) are also typically cultured on a feeder layer or on Matrigel (Takahashi et al., 2007). An effective alternative is needed to eliminate the presence of possible xenogenic factors in the stem cell cultures, but should also be able to promote proliferation and allow stem cell identity maintenance. Adipose-derived stem cells are gaining popularity as a potential stem cell source due to their abundance and non-controversial nature. ASCs are typically cultured on standard tissue culture plastic plates (TCPS) as a default, though other approaches to optimize attachment have not been investigated.
TCPS was chosen because it is the cell culture standard. Matrigel was included since it is the current convention for culturing hESCs and hiPSCs. Laminin and fibronectin are natural components of the basement membrane, with key functions of cell attachment, cell spreading, and cell growth; thus, these proteins are often used in cell culture to promote adhesion. Poly-L-lysine is a synthetic molecule with a highly positively charged amino acid chain which enhances cell adhesion by altering surface charges on the culture substrate (McKeehan, 1984). PLL is often used as a coating agent to encourage cell adhesion in different applications.
hESCs and hiPSCs were obtained as H7 and Thomson cell lines, respectively, and hASCs were isolated from liposuction waste material from female flank tissue as detailed in the Supporting Information. To identify any variability across cell lines, an additional cell source (male chest for hASCs) and cell lines (H9 hESCs and primary skin fibroblast hiPSCs) were also investigated (Supporting Figure 2–3). To ensure that the different proteins were deposited onto a neutral surface, culture plates were first coated with poly(dimethylsiloxane) (PDMS), a hydrophobic and inert polymer which cells will not readily adhere to. Lam, FN, Mat, and PLL were then coated onto the neutral PDMS surface. All stem cells were cultured on all 5 culture surfaces (TCPS, Lam, FN, Mat, and PLL). Unmodified TCPS was used as the control for culturing hASCs, while Matrigel was the control for both hESCs and hiPSCs. Cells were cultured until confluence was reached (hASCs; 125 h), or differentiation began to occur (hESCs, hiPSCs; 190 h). Stem cells were counted daily to obtain growth curves. Since hESCs and hiPSCs grow in colonies, cells were scored as a 1 for single cells and as a 1 for each colony. Morphology measures were taken for hASCs, hESCs and hiPSCs by calculating cell area, cell perimeter length, and circularity. Cell area and perimeter length indicate the amount of cell spreading, reflecting the cells’ preference for a particular surface. Circularity data describes the roundness of the cell, and reveals the degree of cellular extensions and hence the preference of the cells to the attachment protein. The closer the circularity number is to 1, the rounder the cell and the least preference for that attachment protein. The opposite is true for circularity numbers near 0. To determine the level of stem cell-like character maintenance on the various attachment proteins, quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed on all stem cell types on all attachment proteins at the end of the time course to detect expression of stem cell markers (5 days for hASCs; 8 days for hESCs and hiPSCs). Mesenchymal stem cell markers CD29, CD90 and Sca-1 gene expression levels were measured in the hASCs. Nanog, Oct4 and Klf4 were probed in the hESCs and hiPSCs. Additionally, hESCs and hiPSCs were evaluated midway through the cell culture period (day 4) to determine stem cell identity maintenance.
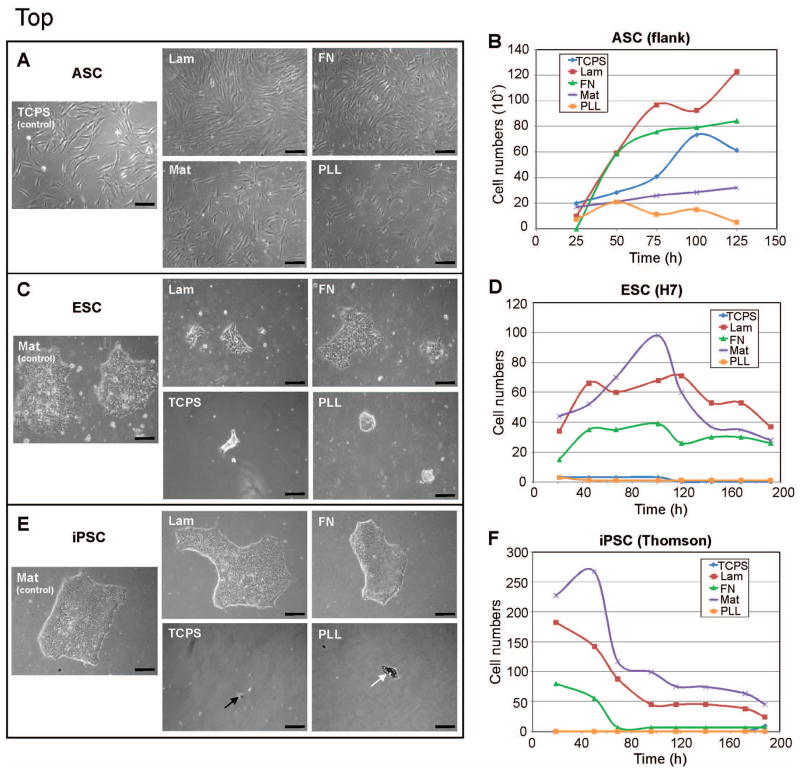
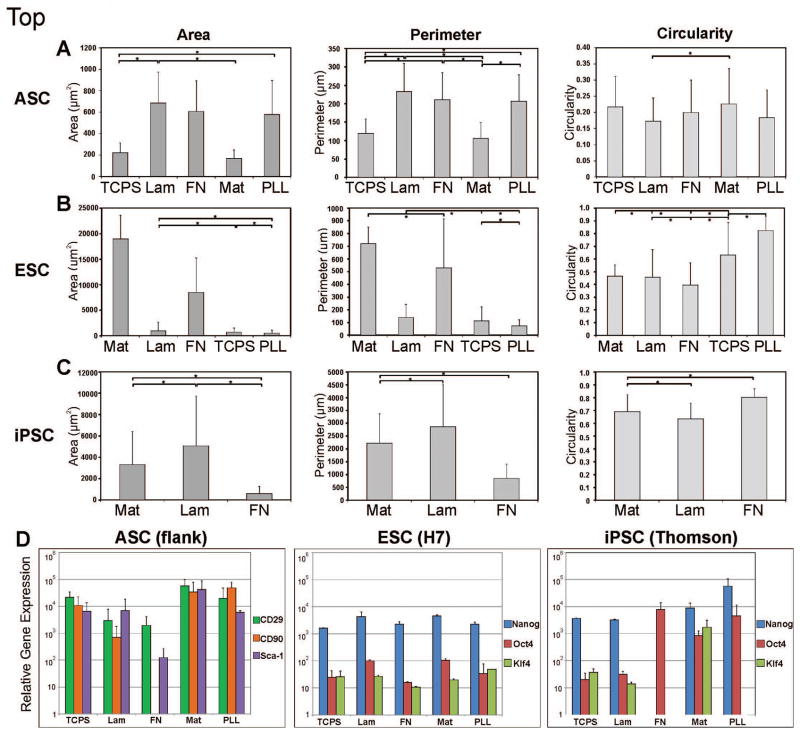
Human ASCs were able to attach and grow with normal cell morphology on all 5 surfaces (Figure1A). The hASCs grew steadily on the control tissue culture plastic plates, with an increase in cell number around 75 h in culture followed by a decrease at 100 h (Figure 1B). Proliferation was notably increased by culture on laminin and fibronectin compared to that on the traditional culture plates. Both laminin and fibronectin promoted initial proliferation at about the same rate. However, by 48 hours, cell growth on laminin had surpassed that on fibronection, continuing on at about the same proliferation rate whereas growth on fibronectin plateaued. By 125 hours, cultures on TCPS, laminin and fibronectin had reached confluence, with laminin resulting in the greatest cell numbers. hASCs attached and proliferated less well on Matrigel and poly-L-lysine. Greatest hASC area and perimeter were seen on laminin, followed by fibronectin (Figure 2A). hASCs on PLL exhibited the third highest area and perimeter numbers, though as seen with the circularity data, hASCs on PLL remained more rounded in comparison and additionally had much lower cell numbers (Figure 1B). hASCs interestingly had lowest area and perimeter lengths on the typically used TCPS. In comparison to laminin and fibronectin, hASCs on TCPS were more round. Cells on Matrigel followed similar morphometrical trends as those on TCPS (Figure 2A), though had lower cell numbers overall (Figure 1B). Interestingly, mesenchymal stem cell markers were most expressed by the hASCs on Matrigel and PLL, which also resulted in lowest cell numbers (Figure 1B and 2D). Stem cell genes were least expressed in hASCs on fibronectin. ASCs from an additional source (chest) also exhibited overall similar growth characteristics and stem cell marker gene expression on the protein surfaces, though stem cell gene expression differed on fibronectin between cell sources (Figure 2D, Supporting Figures 2–3).
Figure 1. Light micrographs and growth curves of the stem cells on each of the 5 culture surfaces.
A–B) hASCs proliferated most on Lam (red line) and FN (green line), even more than on the traditional tissue culture plate (blue line). C–D) hESCs attached and proliferated well on Mat (purple line) and Lam (red line), and less well on FN (green line); hESCs did not attach to TCPS nor PLL (blue and orange lines). E) hiPS attached and grew on Mat, Lam and FN, but not on TCPS nor PLL (black arrow indicates small, singular attached stem cell; white arrow indicates previously attached cell detaching and dying); F) hiPSCs had highest cell/colony numbers on Mat (purple line), closely followed by Lam (red line); FN (green line) showed moderate iPS cell attachment initially but declined to zero by day 3; TCPS (blue line) results are hidden by PLL (orange line) results- both showed no hiPSC attachment. TCPS = tissue culture polystyrene; Lam = laminin; FN = fibronectin; Mat = Matrigel; PLL = poly-L-lysine. Scale bar = 100 μm.
Figure 2. Stem cell morphometry and relative gene expression on the different culture surfaces.
Morphometric results for area, perimeter length and circularity. A) hASCs exhibited largest cell areas on laminin, closely followed by fibronectin; on surfaces where cell attachment was low, such as Matrigel, hASCs had high circularity indicating little cell spreading and low preference to that surface. B) hESCs preferred Matrigel as an attachment surface, evidenced by high cell/colony area and perimeter measurements, along with low circularity or high cell spreading. C) hiPSCs preferred laminin over the standard feeder-free culture surface Matrigel, shown by largest cell/colony areas and perimeter lengths, and lowest circularity. * indicates statistical significance, p<0.05. D) Quantitative real-time polymerase chain reaction results for stem cell gene expression for hASCs at day 5; and for hESCs and hiPSCs at day 8. hASCs were probed for gene expression of mesenchymal stem cell markers CD29 (green bars), CD90 (orange bars) and Sca-1 (purple bars). ASC gene expression of these genes on the different proteins was consistently high, except on fibronectin. hESCs and hiPSCs were probed for Nanog (blue bars), Oct4 (red bars) and Klf4 (green bars) expression. ESC gene expression was highest on laminin and Matrigel, while iPSC gene expression was highest on Matrigel and PLL. TCPS = tissue culture polystyrene; Lam = laminin; FN = fibronectin; Mat = Matrigel; PLL = poly-L-lysine. Error bars = standard deviation.
Human ESCs grew well with many colonies on Matrigel as expected, fewer colonies on laminin and fibronectin, and very little cell attachment on TCPS and PLL (Figure 1C). Interestingly, over the first 48 hours in culture, hESCs grew fastest on laminin (Figure 1D). However, cell growth on Matrigel quickly overtook growth on laminin, with a rapid increase from 48 to 100 hours in culture, followed by a decline for the remainder of the culture period. This is due to the fact that hESCs grow in a colony, and as each colony expands it combines with neighboring colonies to create one large colony. Since each colony was scored as a 1, this would decrease the number count as colonies integrated and grew larger. The morphology data in Figure 2B shows that cell colonies on Matrigel had a much greater area than those on the other attachment proteins. As a stem cell colony grows larger the cells begin to differentiate, losing their pluripotency; hence allowing colonies to continue to grow is undesirable. Cells are typically passaged prior to colonies growing too large, although the more often this is done, the higher the passage number of the cells, and the greater the possibility for phenotypic and/or genotypic drift. Thus in cases where retaining stemness is of concern, using laminin or fibronectin may be the better culture substrate. In contrast to cell types that grow individually in culture, for cell colonies, cell area gives an indication of colony expansion (i.e. cell proliferation), while perimeter reveals how much the colony has spread. Area and perimeter length were greatest for colonies on Matrigel, followed by fibronectin, laminin, TCPS, and lastly PLL. Circularity indicates how much the cells have extended out of the colony, indicating cell-to-surface preference. Cells on PLL were the most round, followed by those on TCPS. Given that few cells grew on these surfaces, this further confirms that these substrates not suitable for hES cell culture. As evidenced by the lowest circularity numbers, hESCs preferred growing on fibronectin, closely followed by laminin and Matrigel (Figure 2B). Laminin resulted in higher numbers of hESCs than fibronectin, although average cell area was much lower on laminin than on fibronectin. More cells attached initially on laminin compared to fibronectin, although hESCs were able to subsequently grow larger colonies on fibronectin. hESCs maintained their stem cell character relatively well on all substrates as seen by Nanog, Oct4 and Klf4 expression, with highest levels seen on laminin and Matrigel (Figure 2D). Interestingly, halfway through the culture period cells on TCPS expressed the highest levels of these stem cell markers (Supporting Figure 4). One possibility for the difference in gene expression between days 4 and 8 may be because after day 4, individual cells had divided enough to become full cell colonies, and individual cells are more similar to non-stem cell-like cells and cells in colonies are more stem cell-like. These data indicate that hESCs proliferate the most on Matrigel and results in the largest colonies. This may raise some concern about differentiation and the loss of stem cell-like character in the larger colonies. Laminin and fibronectin colonies, in contrast, remained smaller while still promoting proliferation, indicating that depending on the specific application, if rapid stem cell number expansion is desired, Matrigel may be the preferred culture surface. However, if long-term, low passage number hESC culture is the aim, then laminin or fibronectin may be the better culture substrate.
Human iPSCs attached well to Matrigel, laminin and fibronectin, with almost no cell attachment onto TCPS or PLL (Figure 1E). Cell numbers were greatest on Matrigel, followed by laminin (Figure 1F). Fibronectin had few cells attach, although the cells that did were able to grow into noticeable colonies. hiPS cell colonies were larger on Matrigel and laminin, than on fibronectin (Figure 1E). Other than an early spike on Matrigel, growth rates gradually decreased on all surfaces and then plateaued around 96 hours (Figure 1F). Cells began to differentiate after 190 hours as evidenced by the iPS cells no longer growing in colonies but rather as individual cells (Supporting Figure 1). Interestingly, colonies on laminin were significantly larger in area than those on Matrigel (Figure 2C), even though cell numbers were greater on Matrigel. This phenomenon suggests that human iPS cells favor laminin for colony expansion, but Matrigel for initial attachment. Cell perimeter length was significantly larger for iPS cells on laminin compared to Matrigel. Fibronectin resulted in the lowest area and perimeter lengths. Cells were most round and hence had the least number of cellular extensions on fibronectin, followed by Matrigel and laminin, as indicated by circularity. Essentially no cells attached on TCPS and PLL (Figure 1E), and thus morphometry data could not be taken. By day 8, Nanog, Oct4 and Klf4 were expressed overall most by iPS cells cultured on Matrigel and PLL (Figure 2D). Stem cell marker expression in the hiPSCs varied between day 4 and 8 in which genes increased or decreased in expression, though expression on PLL remained relatively high (Figure 2D, Supporting Figure 4). On day 4, relative expression on Matrigel was low and increased by day 8. Similar to hESCs, this may be evidence of the implicit difference between stemness of individual cells (near day 4) and those in a colony (at day 8). Other than the early spike in cell numbers on Matrigel, the growth curves on laminin and Matrigel had approximately the same slope, or declined at about the same rate, further supporting that initial cell attachment was greatest on Matrigel, though colony expansion was best on laminin.
Matrigel is a relatively new standard for culturing embryonic and induced pluripotent stem cells without feeder cells (Xu et al., 2001; Takahashi et al., 2007), although it is quite costly. Matrigel, a basement membrane derivative, contains ECM proteins (laminin, 56%; collagen IV, 31%; entactin, 8%) and several growth factors (bFGF, EGF, IGF-1, TGF-β, etc.) (Kleinman, et al., 1982). It is now well known that Matrigel can support attachment and stem cell maintenance of hESCs and hiPSCs, however it is also known to allow stem cell differentiation (Chambers, et al., 2009). Colony expansion is inhibited by differentiation, which may be why hiPSC colony area was lower on Matrigel compared to laminin.
Tissue culture polystyrene is plasma-treated to create a net negative charge on the plastic’s surface, encouraging cell attachment (LaRocca and Barker, 1996). Matrigel promotes cell adhesion primarily through its ECM components laminin and collagen (Xu et al., 2001). Collagen utilizes its fibrils to bind to the integrin α2β1 domain (Jokinen, 2004). Laminins are a family of glycoproteins, which are secreted and incorporated into the ECM. Cell attachment to laminin is mediated by the α1β1, α2β1, α3β1, α6β1, and α7β1 integrins (Hynes, 1992; Vuoristo, et al., 2009). Human embryonic and induced pluripotent stem cell lines express α5, α6, αV, β1, and β5 integrin subunits; β1 integrins are involved in adhesion and proliferation of both hESCs and iPSCs on Matrigel (Rowland et al., 2010). Recombinant human laminin-511 has been recently shown to promote self-renewal of mouse embryonic stem cells and human induced pluripotent stem cells in culture, via interactions with the cells’ α6β1 and αVβ1 integrins (Domogatskaya et al., 2008; Rodin, et al., 2010). However, human laminin is approximately 20x more costly than Matrigel and thus is currently not a practical alternative solution for a stem cell self-renewal substrate in the laboratory nor as a cell adhesion substrate for tissue engineering, unless the cost is reduced in the future. The same issue applies to vitronectin, an ECM glycoprotein, which has also been found to support hESC self-renewal (Braam et al., 2008), though vitronectin is approximately 80x the cost of Matrigel. Fibronectin is found in the interstitial matrix and plasma, and mainly functions in cell migration during development and wound healing (Krammer et al., 2002). Cell adhesion is mediated by RGD (Arg-Gly-Asp) sequences on located on the fibronectin strand, which is recognized by α5β1 and αVβ3 integrins for binding. It is used in cell culture for cell attachment, spreading and proliferation for multiple cell types, and is also a regulator of cell growth and differentiation. Poly-L-lysine is used to promote cell attachment to plastic and glass surfaces (McKeehan, 1984). PLL alters the charge of a culture surface to a net positive charge to improve adhesive properties. PLL also enhances adsorption of serum or ECM proteins to the culture substrate. An advantage to using this synthetic polymer is that it is chemically inert and does not introduce impurities carried by natural polymers. However, its adhesion capabilities lack behind that of natural attachment proteins, such as laminin and fibronectin. Another synthesized polymer (poly[2-(methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide]) was found to promote long-term growth of hESCs (Villa-Diaz et al., 2010), though this polymer is not commercially available.
Through investigating cell attachment behavior, inherent differences between hESCs and hiPSCs become apparent, for two stem cell types which for many years were thought to be similar. For one, cell colonies for hESCs were larger on Matrigel than laminin, whereas hiPSC colonies were larger on laminin than Matrigel, showing the stem cell types’ distinct difference in mechanisms of cell attachment. By 190 hours in culture, hESCs began to differentiate readily on laminin and fibronectin, but less on Matrigel (Supporting Figure 1). In contrast, hiPSCs initiated differentiation on Matrigel, laminin and fibronectin by 190 hours.
Surprisingly, hASCs expressed stem cell markers the most on the two substrates resulting in the lowest cell numbers: Matrigel and PLL. Since hASC numbers were low on these proteins and thus cell density was low, this may be why cells on Matrigel and PLL were able to maintain a more stem cell-like phenotype. Our data suggests that hASCs maintain their stem cell identity the rounder they remain. Highest stem cell marker expression for hESCs was seen on Matrigel and laminin, and on Matrigel and PLL for hiPSCs. Both hESCs and hiPSCs exhibited an increase in stem cell gene expression on Matrigel over time, possibly signifying the level of stemness of these cells as they proliferate from individual cells to form colonies. The variations in the properties of the different stem cells may be an indication of the heterogeneity of the differentiation potential of these distinct stem cell types. hASCs are multipotent, as opposed to the pluripotent hESCs and hiPSCs. These findings suggest that there exists intrinsic differences between stem cells of varying potencies other than their differentiation capabilities.
There also appeared to be differences in stem cell response to the different protein substrates between cell lines. While growth curves remained similar for each stem cell type across lines (Supporting Figure 2), gene expression varied noticeably (Supporting Figure 3). For both ASC sources, mesenchymal gene expression was consistently high on all proteins, except for a difference on fibronection- cells from the flank region expressed less stem cell genes on fibronectin than cells from the chest (Figure 2D, Supporting Figure 3).
Overall, methods of cell attachment for culturing stem cells may be improved over the current standards. Adipose-derived stem cells did not proliferate as much on typically used traditional tissue culture plastic as on laminin. Fibronectin also promoted proliferation of hASCs more than TCPS. hASC stem cell marker expression was relatively high on all substrates except on fibronectin. Human iPS cell expansion was better on laminin rather than Matrigel, as evidenced by cell area. Human embryonic stem cells appeared to prefer the standard Matrigel as a culture surface in regards to cell proliferation numbers. hESCs were able to attach and develop colonies on laminin and fibronectin though in smaller numbers, showing that in cases where Matrigel use is undesired, laminin or fibronectin could be used as effective alternatives. These data serve as a quick reference guide for determining a proper protein culture surface for these three human stem cell types, information useful in many tissue engineering and regenerative medicine applications.
Supplementary Material
Acknowledgments
We thank the J. C. Wu laboratory for providing the hESC and hiPSC cell line cells. This work was supported by the Arthritis National Research Foundation, the Oak Foundation, the Hagey Laboratory for Pediatric Regenerative Medicine, and the National Institutes of Health grants RC1HL100490-02, RC2DE020771-01 and UO1HL099776-01.
Footnotes
Disclosure of Potential Conflicts of Interest
No competing financial interests exist.
References
- Braam SR, Zeinstra L, Litjens S, Ward-van Oostwaard D, van den Brink S, van Laake L, Lebrin F, Kats P, Hochstenbach R, Passier R, Sonnenberg A, Mummery CL. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells. 2008;26(9):2257–2265. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27(3):275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domogatskaya A, Rodin S, Boutaud A, Tryggvason K. Laminin-511 but not -332, -111, or -411 enables mouse embryonic stem cell self-renewal in vitro. Stem Cells. 2008;26(11):2800–2809. doi: 10.1634/stemcells.2007-0389. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jokinen J, Dadu E, Nykvist P, Käpylä J, White DJ, Ivaska J, Vehviläinen P, Reunanen H, Larjava H, Häkkinen L, Heino J. Integrin-mediated cell adhesion to type I collagen fibrils. J Biol Chem. 2004;279(30):31956–31963. doi: 10.1074/jbc.M401409200. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21(24):6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Krammer A, Craig D, Thomas WE, Schulten K, Vogel V. A structural model for force regulated integrin binding to fibronectin’s RGD-synergy site. Matrix Biol. 2002;21:139–147. doi: 10.1016/s0945-053x(01)00197-4. [DOI] [PubMed] [Google Scholar]
- LaRocca P, Barker S. Tissue culture surface treatments. The Cell/Line. 1996;6:1–2. [Google Scholar]
- McKeehan WL. Methods for Preparation of Media, Supplements, and Substrata for Serum-free Animal Cell Culture. A.R. Liss; New York, NY, USA: 1984. [Google Scholar]
- Rodin S, Domogatskaya A, Ström S, Hansson EM, Chien KR, Inzunza J, Hovatta O, Tryggvason K. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol. 2010;28(6):611–615. doi: 10.1038/nbt.1620. [DOI] [PubMed] [Google Scholar]
- Rowland TJ, Miller LM, Blaschke AJ, Doss EL, Bonham AJ, Hikita ST, Johnson LV, Clegg DO. Roles of integrins in human induced pluripotent stem cell growth on Matrigel and vitronectin. Stem Cells Dev. 2010;19(8):1231–1240. doi: 10.1089/scd.2009.0328. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Villa-Diaz LG, Nandivada H, Ding J, Nogueira-de-Souza NC, Krebsbach PH, O’Shea KS, Lahann J, Smith GD. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat Biotechnol. 2010;28(6):581–583. doi: 10.1038/nbt.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuoristo S, Virtanen I, Takkunen M, Palgi J, Kikkawa Y, Rousselle P, Sekiguchi K, Tuuri T, Otonkoski T. Laminin isoforms in human embryonic stem cells: synthesis, receptor usage and growth support. J Cell Mol Med. 2009;13(8B):2622–2633. doi: 10.1111/j.1582-4934.2008.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19(10):971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.