Abstract
Purpose
To examine the effects of presurgical corticosteroid treatment for normal-risk penetrating keratoplasty (NRPK), high-risk penetrating keratoplasty (HRPK), and high-risk penetrating keratoplasty plus lensectomy.
Methods
We used 3 corneal transplantation models (NRPK, HRPK, and high-risk penetrating keratoplasty plus lensectomy). For each model, we tried to compare the effect of corticosteroid treatment according to different timetables as follows: The first trial began with a corticosteroid injection given 2 weeks before the PK and continued until 4 weeks after the PK (group 1). The second trial started with a corticosteroid injection given on the day of the PK and continued for 4 weeks after the PK (group 2). The third trial started with a corticosteroid injection administered on the day of the PK and continued for 8 weeks after the PK (group 3). After harvesting and immunostaining of corneas, graft survival, neovascularization (NV), and lymphangiogenesis (LY) were compared among the groups. A P value <0.05 was considered as being statistically significant.
Results
With respect to graft survival, group 1 had improved graft survival compared with that of group 3 in the HRPK model (P = 0.025). In all the 3 PK models, groups 2 and 3 demonstrated a similar graft survival (P > 0.05). With respect to NV and LY, in NRPK, group 1 showed less NV than did group 2 (P < 0.001) and group 3 (P = 0.016). In HRPK, group 1 also demonstrated less NV and LY than did group 3 (P = 0.045 and 0.044, respectively).
Conclusions
The initiation time point of the corticosteroid treatment is important for graft survival. Corticosteroid pretreatment is an effective means to increase graft survival for HRPK and to decrease NV and LY for both NRPK and HRPK.
Keywords: corticosteroid pretreatment, angiogenesis, penetrating keratoplasty
Corticosteroids have remained the mainstay of preventive and active immunologic rejection therapy for penetrating keratoplasty (PK) since their introduction in the 1960s. Approximately, 50% of rejection episodes respond to topical corticosteroid treatment alone.1–3
The antiinflammatory effects of corticosteroids are initiated through the inhibition of chemotaxis and phagocytosis by the blockade of the synthesis of prostaglandins in the phospholipase A2 pathway.1,2,4,5 Corticosteroids inhibit vascularization and have an immunosuppressive action by reducing the number of circulating T lymphocytes.1,2,4,5 Corticosteroids also exert an antilymphangiogenic effect.1 We previously revealed the positive effect of corticosteroid pretreatment in decreasing neovascularization (NV) in a PK model.6 In this study, we hypothesized that there would be a difference in NV and inflammation based on when the corticosteroids were administered. We divided the time period around PK into 3 periods: before the PK, soon after the PK, and late after the PK. Corticosteroid treatment soon after PK is essential and indispensable in almost every case.2,7 We tested the additive effect of corticosteroid pretreatment and extended the treatment after the PK. We first compared the induced NV and the inflammatory reaction among the 3 PK models. Second, in each model, we tested which of the 2 treatments, pretreatment or extended treatment, was more effective in decreasing the NV and lymphangiogenesis (LY) and increasing graft survival when added to the corticosteroid treatment of “soon after the PK.”
MATERIALS AND METHODS
The experiments were performed in accordance with the guidelines of the Association for Research in Vision and Ophthalmology and were approved by the Institutional Animal Care and Use Committee of the Catholic University of Korea, St Vincent’s Hospital.
We used 3 corneal transplantation models: The models were normal-risk penetrating keratoplasty (NRPK), high-risk penetrating keratoplasty (HRPK), and high-risk penetrating keratoplasty plus lensectomy (HRPKL).
Corneal Transplantation
The NRPK was performed as follows: Mice (8–12 weeks old) were anesthetized by giving an intramuscular injection with a 1:1 mixed solution of tiletamine and zolazepam (30 mg/kg, Zoletil 50; Virbac Korea Co, Ltd, Seoul, Korea) and xylazine (10 mg/kg). The donor (C57BL/6 strain) cornea was marked with a 2-mm trephine. The cornea was cut with a microsurgical knife and scissors and placed in balanced salt solution (Alcon Laboratories, Inc, Fort Worth). One percent tropicamide ophthalmic solution was used to dilate the pupil, and a 0.5% proparacaine ophthalmic solution was used to anesthetize the recipient (BALB/c strain) cornea. After dissection of the recipient cornea, the donor graft was sutured into the recipient bed using interrupted sutures (10-0 nylon; CS140-6, Ethicon, Inc). After the PK was performed, the eye was covered with 0.5% ofloxacin ophthalmic ointment, and the lid was sutured with 8-0 coated vicryl (BV130-5, Ethicon, Inc). The sutures remained in the recipient’s eye for 1 week after transplantation. In the HRPK, to induce the vascularization of the recipient corneal bed before the PK, 2 corneal sutures (10-0 nylon, CS140-6, Ethicon, Inc) were placed between the corneal center and the limbus at the 12- and 6-o’clock positions. After 2 weeks, the 2 corneal sutures were removed, and a PK was performed according to the methods used in the NRPK. In the HRPKL model, we removed the lens just after trephination of the recipient cornea by puncturing the anterior lens capsule with a sharp needle, and we extracted the lens inside of the lens capsule. The rest of the procedures were performed as previously described for the HRPK.
Clinical Evaluation of Rejection
The mice were examined and photographed weekly through postoperative week 8 under general anesthesia (inhaled isoflurane) using an operating microscope (OPMI 9-FC, Zeiss, Germany) and a camera (CVMV-K59, Ecwox, China). Microscopic examination was done to determine corneal clarity. We evaluated clinical graft rejection according to the grading system for orthotopic corneal graft opacity and NV as previously described.8 The opacity was graded (from 0 to 5), and grades 3 and above were considered as graft rejection. Mice that developed complications, such as severe inflammation and hemorrhage, were excluded from the study.
Comparison of NV and Inflammatory Infiltration in the 3 Corneal Transplantation Models
Three PK models were compared with respect to NV and inflammatory cell infiltration: NRPK (4 eyes), HRPK (4 eyes), and HRPKL (4 eyes). In all the 3 PK model groups, the sutures were removed 8 days after the PK, and the eyes were harvested the next day. After harvesting, double staining (CD 31 and CD11b) was done to compare NV and inflammatory cell infiltration among the groups as follows: Corneas were dissected, rinsed in phosphate-buffered saline (PBS) for 30 minutes, and fixed in 100% acetone for 20 minutes. After washing in phosphate-buffered saline with Tween 20 (0.1% Tween 20/PBS), nonspecific binding was blocked with 3% bovine serum albumin (BSA)/PBS for 3 nights at 4°C. Overnight incubation with 1:200 fluorescein isothiocyanate–conjugated monoclonal antimouse CD31 antibody (558738, BD Pharmingen) and 1:100 Alexa Fluor 647 rat antimouse CD11b antibody (557686, BD Pharmingen) in 3% BSA/PBS was done at 4°C. This was followed by washing in PBST at room temperature. Corneas were mounted with an antifading agent.
To evaluate NV, images of the corneal vasculature were captured using a camera attached to a fluorescent microscope (Olympus BX51, Tokyo, Japan). NV was quantified by setting a threshold level of fluorescence above which only vessels were captured and processed using Image J (National Institutes of Health). The total corneal area was outlined using the innermost vessel of the limbal (rim of the cornea) arcade. The total area of the NV was calculated as follows: Total neovascularization (%) = (neovascularized area of total cornea/total cornea area) × 100
To evaluate inflammatory infiltration with confocal microscope pictures, 3 to 4 sites from each cornea were chosen in the NRPK (12 sites), the HRPK (15 sites), and the HRPKL group (13 sites). A confocal microscope was used to quantify the area of inflammatory infiltration (LSM 510 META, Carl Zeiss, Germany). Horizontal sections (objective magnification ×10) of 17 to 19 images were obtained from the top surface to the bottom of the cornea at 5-μm intervals and were stacked to create the final stack images. In each stack image, inflammatory infiltration was quantified by setting a threshold level of fluorescence above which the cells were captured and processed using Image J (National Institutes of Health). The percentage area of CD 11b+ cell infiltration was analyzed in each stack image using the pixel area.
Comparison of Graft Survival, Angiogenesis, and LY According to the Timetable of Corticosteroid Treatment
In each of the 3 models, we tested the groups according to the timetable of corticosteroid treatment. Dexamethasone sodium phosphate (5 mg/1 mL, Yuhan, Corp, Seoul, Korea) was injected into the subconjunctival space of each eye. Figure 1 shows the 3 scheduled corticosteroid treatments. In all the 3 PK models, each group was composed of 7 eyes. The first trial (group 1) began with a subconjunctival corticosteroid injection given twice a week, 2 weeks before the PK was performed. The injections were maintained weekly until 4 weeks after the PK (pretreatment plus early postoperative treatment). The second trial (group 2) included weekly subconjunctival corticosteroid injections from the day of the PK until 4 weeks after the PK (early postoperative treatment). The third trial (group 3) included weekly subconjunctival corticosteroid injections from the day of the PK to 8 weeks after the PK (early postoperative treatment plus late postoperative treatment). The 3 groups were compared for graft survival, NV (% total NV area/total corneal area), and LY (% total LY area/total corneal area).
FIGURE 1.
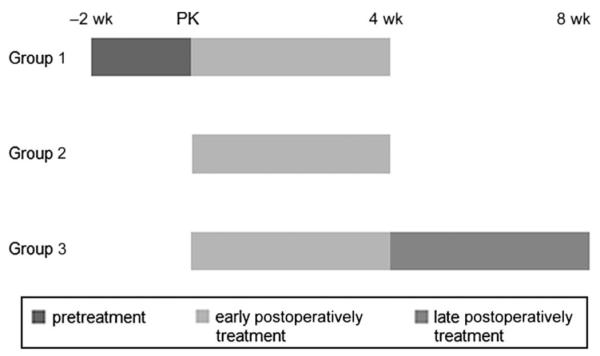
The 3 groups according to the scheduled corticosteroid treatments (group 1, pretreatment added on early treatment; group 2, early treatment alone; and group 3, extended treatment added on early treatment).
Analysis of Angiogenesis and LY
After the planned injections and observation periods, mouse eyes were harvested, and the corneas were trimmed of any remaining limbus and iris. Immunohistochemical staining for vascular and lymphatic endothelial cells was performed on corneal flat mounts. Fresh corneas were dissected, rinsed in PBS for 30 minutes, and were fixed in 100% acetone (Sigma) for 20 minutes. After washing in PBST (0.1% Tween 20/PBS), nonspecific binding was blocked with 3% BSA/PBS for 3 nights at 4°C. Incubation with 1:500 fluorescein isothiocyanate–conjugated monoclonal antimouse CD31 antibody (558738, BD Pharmingen) and 1:200 rabbit anti-LYVE-1 (ab 14,917, Abcam Inc, Cambridge, MA) in 3% BSA/PBS at 4°C overnight was followed by 1:1000 goat antirabbit antibody-Alexa Fluor 546 (A11071, Invitrogen, Corp, Carlsbad, CA) for 1 hour with subsequent washes in PBST at room temperature. The corneas were mounted with the antifading agent Gel Mount. After immunochemical staining for vascular endothelial cells and flat mounting of the cornea, images of the corneal vasculature were captured using a camera attached to a fluorescent microscope (Olympus BX51). NV and LY were quantified using Image J as described above. The total area of the NV and LY was calculated as above and Total lymphangiogenesis (%) = (lymphangiogenesis area of total cornea/total cornea area) × 100.
Statistical Analysis
Statistical analysis was performed using SPSS 11.5 (Chicago, IL). Postoperative survival of the allografts was analyzed using Kaplan–Meier survival curves and the log rank test. The NV and LY of each group were compared with those of the corresponding control group using an unpaired 2-tailed t test and the Mann–Whitney U test. A P value <0.05 was considered to be statistically significant.
RESULTS
Comparison of the 3 Corneal Transplantation Models
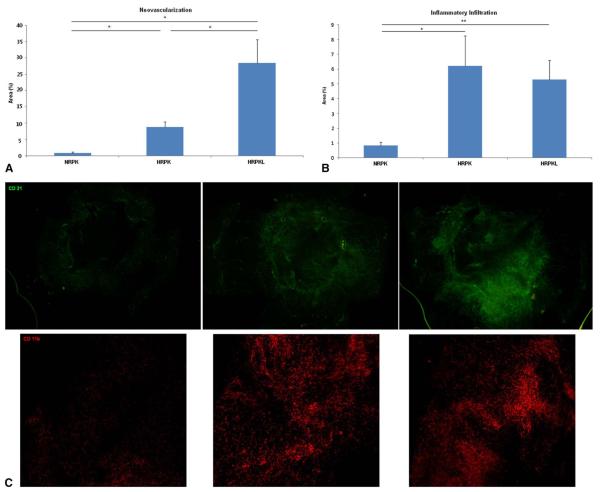
Figure 2 shows the comparison of the NV and inflammatory cell infiltration in the 3 corneal transplantation models.
FIGURE 2.
Comparison of NV (A), inflammatory cell infiltration (B) in the 3 corneal transplantation models. C, Representative pictures. *P < 0.05, **P < 0.01.
Neovascularization
The NRPK model showed less NV than did the HRPK model (P = 0.016, Mann–Whitney U test) and the HRPKL model (P = 0.016). The HRPK model showed less NV than did the HRPKL model (P = 0.032).
Inflammatory Cell Infiltration (CD 11b+ Cells)
The NRPK model showed less inflammatory infiltration than did the HRPK model (P = 0.027, unpaired 2-tailed t test) and the HRPKL model (P = 0.003). There was no difference in inflammatory infiltration between the HRPK and HRPKL (P = 0.712, unpaired 2-tailed t test).
Comparison of the 3 Corticosteroid Treatments According to the Treatment Schedule in Each PK Model
Normal Risk Penetrating Keratoplasty
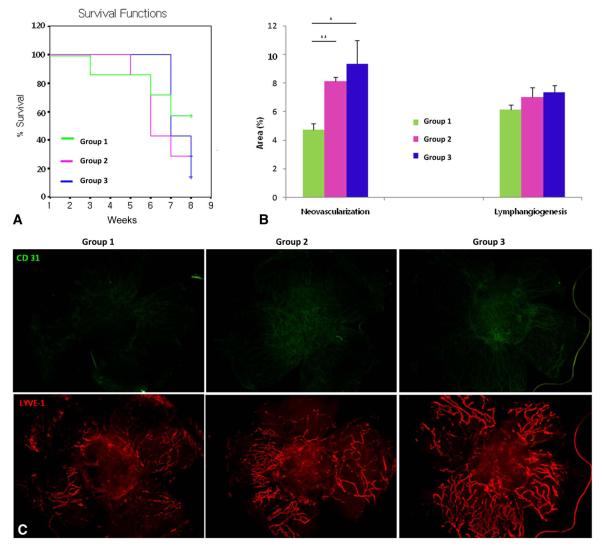
Figure 3 shows the comparison of graft survival, NV, and LY according to the corticosteroid treatment schedule in the NRPK model.
FIGURE 3.
Comparison of graft survival (A), NV and LY (B), according to corticosteroid treatment schedule in the NRPK model. C, Representative pictures (group 1, pretreatment added on early treatment; group 2, early treatment alone; and group 3, extended treatment added on early treatment). +Censored data, *P < 0.05, and **P < 0.01.
Graft Survival
There was no difference among the groups in graft survival in the NRPK model (P > 0.05, log rank test).
Neovascularization
In the NRPK model, the pretreatment group (group 1) demonstrated less NV than did the early treatment group (group 2; P = 0.000, unpaired 2-tailed t test) and the extended treatment group (group 3; P = 0.016).
Lymphangiogenesis
Pretreatment showed some trend toward decreasing LY but was not statistically significant (P > 0.05, unpaired 2-tailed t test).
High-Risk Penetrating Keratoplasty
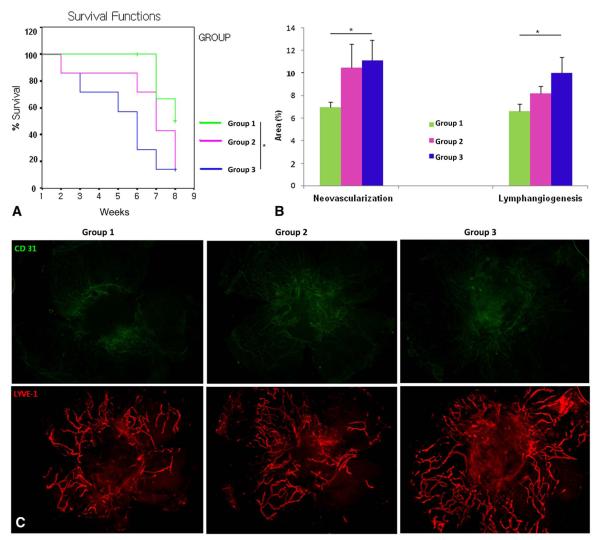
Figure 4 presents the comparison of graft survival, NV, and LY according to the corticosteroid treatment schedule in the HRPK model.
FIGURE 4.
Comparison of graft survival (A), NV and LY (B) according to corticosteroid treatment schedule in the HRPK model and representative pictures (C) (group 1, pretreatment added on early treatment: group 2, early treatment alone: group 3, extended treatment added on early treatment). +Censored data, *P < 0.05, and **P < 0.01.
Graft Survival
Group 1 had better graft survival than did group 3 (P = 0.025, log rank test). This suggests that pretreatment before the PK had a significant additive effect when compared with that of the extended treatment in the HRPK model. There was no significant difference between group 2 and group 3 for the HKPK model (P > 0.05).
Neovascularization
In the HRPK model, the pretreatment group (group 1) demonstrated less NV than did the extended treatment (group 3; P = 0.045, unpaired 2-tailed t test).
Lymphangiogenesis
The pretreatment group (group 1) showed less LY than did the extended treatment group (group 3; P = 0.044, unpaired 2-tailed t test).
High-Risk Penetrating Keratoplasty Plus Lensectomy
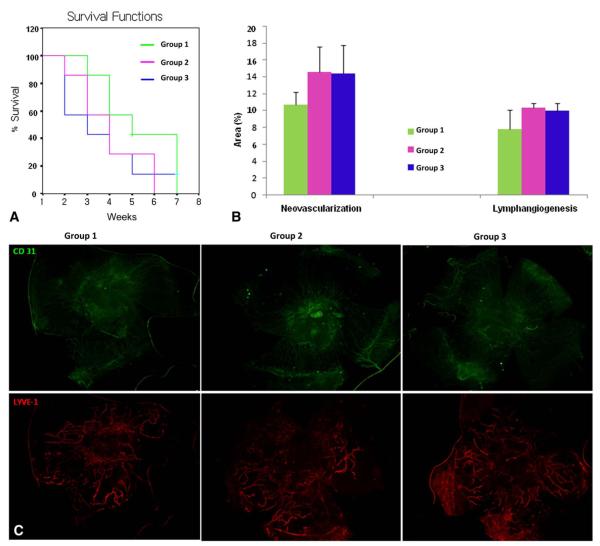
Figure 5 shows the comparison of graft survival, NV, and LY according to the schedule of steroid injections in the HRPKL model.
FIGURE 5.
Comparison of graft survival (A), NV and LY (B) according to the corticosteroid treatment schedule in the HRPKL model. C, Representative pictures (group 1, pretreatment added on early treatment; group 2, early treatment alone; group 3, extended treatment added on early treatment). +Censored data, *P < 0.05, and **P < 0.01.
Graft Survival
There was no difference among groups in graft survival in the HRPKL model (P > 0.05, log rank test).
Neovascularization
Pretreatment did not show any effect on the NV (P > 0.05, unpaired 2-tailed t test).
Lymphangiogenesis
Pretreatment showed a small trend toward decreasing LY, but it was not statistically significant (P > 0.05, unpaired 2-tailed t test).
DISCUSSION
Corneal transplantation has a 2-year success rate of >90% for initial grafts into an avascular cornea.9,10 However, in patients with high-risk characteristics, such as a vascularized corneal recipient bed, graft failure rates are between 60% and 90%.9,10 Vascularized and inflamed corneal tissue provides a conduit for both the blood and lymphatic systems to bring immune cells to and from the cornea, contributing to subsequent accelerated immunologic graft rejection.9–12
Corticosteroid therapy has been the standard antiinflammatory and antiangiogenic treatment for diseases associated with corneal NV and LY, including corneal transplantation.1,2,4,5,13 Some reports insist that the efficacy of a combined treatment with immunosuppressive agents and corticosteroids is no better at preventing rejection than is treatment with corticosteroids alone.2,14,15
In this study, we placed as much value on the treatment schedule of corticosteroids as on the total amount of administered corticosteroids. Starting antiinflammatory treatment after the insult occurs may not be sufficient to prevent or slow down angiogenesis.16,17 Therefore, starting antiinflammatory therapy before the insult occurs may decrease angiogenesis and improve outcomes. There are some clinical situations in which surgeons should consider the value of pretreatment before surgery, such as in patients with herpes keratitis and uveitis.18,19
Currently, there is no consensus on the best time to start immunosuppressive treatment or to end immunosuppressive tapering in patients who underwent a PK.20,21 We previously found that corticosteroid delivery before an angiogenic stimulus such as NRPK or a corneal suture is effective in reducing angiogenesis, LY, and inflammatory infiltration.6 Herein, we used 3 different corneal transplantation models to compare the effects of corticosteroid treatment, and in each model, the effect of corticosteroids was tested according to the time period in which it was administered.
The timing of antiangiogenic agent application after a PK has been discussed in several articles.6,16,17,21,22 There are reports that insist on the importance of early treatment especially in HRPK.6,16,17,22 In NRPK, allografts placed in normal-risk eyes do not generate sensitization until 2 to 4 weeks, likely reflecting the time needed for LY to develop. Grafts placed in high-risk eyes induce rapid donor specific sensitization (within 7 days), because they already have preestablished lymphatics.4,7,16,23,24 As a result, in HRPK, late treatment might not have an appreciable effect on the capacity of the eye to promote anterior chamber associated immune deviation. We suggest that fostering a good milieu in the recipient bed by suppressing inflammation and vascularization through corticosteroid pretreatment could improve corneal engraftment through decreased NV, LY, and inflammation, potentially resulting in improved graft survival. On the contrary, there are also reports that argue for the efficacy of prolonged corticosteroid treatment after a PK.20,21,25
There are several published differences between NRPK and HRPK.12,26–28 The graft survival rates between an NRPK and HRPK are different. Compared with NRPK, eyes that underwent an HRPK exhibited significantly higher levels of chemokines for NV induction in the early prerejection postoperative period.29 Accordingly, there are several published reports that insist on the early treatment of antiinflamamatory agents in HRPK.6,16,17,22 Regimens that result in significant degrees of angiostasis, but are delayed in onset, do not have an appreciable effect on the capacity of eye to promote deviant immunity.16 We observed similar results in this study. Early treatment alone was as effective as extended treatment in all 3 models. Moreover, pretreatment was more effective in decreasing NV than was early treatment alone in the NRPK model.
In the 3-model comparison, inflammation and NV were much greater in the HRPK model than in the NRPK model, as expected. When comparing the HRPK and HRPKL models, NV was much greater in the HRPKL model than in the HRPK model. However, unexpectedly, there was no significant difference when comparing inflammatory cell infiltration. It was different from our expectation that the lensectomy model would induce much greater corneal inflammation in the early postoperative period than that without lensectomy. We suggest the reason to be that in 3-model comparison, the ninth day after the PK may be too early to predict the final amount of inflammation lagging behind CD 11b+ cell infiltration.
After 8 weeks of follow-up, in the HRPKL model, the pretreatment did not affect NV, LY, or graft survival. The NV was significantly higher on the ninth day after the PK in the HRPKL model than in the other 2 models. The abundant new corneal vessels in the HRPKL models that developed after the preoperative period from both presuturing and intraoperative lensectomy may have obscured the effect of pretreatment on the NV, LY, and final graft survival.
In clinical situations involving high-risk patients, topical corticosteroids are used frequently in the postoperative period followed by long-term indefinite use when there are no precluding contraindications.20,25 For this reason, high-risk patients are exposed to the potential complications of corticosteroids, such as cataracts, glaucoma, delayed wound healing, and infectious keratitis.7,14 Considering our findings, which demonstrated the improved effects of pretreatment compared with the extended treatment after the PK, pretreatment could be particularly valuable for high-risk patients. Effective pretreatment for a limited period could decrease the need for the prolonged use of corticosteroids and the resulting side effects. Additional work must be done in the future to determine how far ahead we need to start the corticosteroid pretreatment to get the best benefit with the least risk before this can be used in clinical practice.
In conclusion, we found that corticosteroid pretreatment is more effective in increasing graft survival than is extended treatment in HRPK. We also revealed that the pretreatment with corticosteroids decreased the NV in comparison with that in the extended treatment in both NRPK and HRPK.
ACKNOWLEDGMENTS
In this study, the relevance to human corneal transplantation could be limited because these results and methods do not support generalization to humans. This HRPKL model is different from that of the triple surgery (PK, lens extraction, and intraocular lens insertion) employed in humans in the clinical setting. Although we made the HRPKL model with an intact posterior capsule during lens extraction, we did not insert an intraocular lens, and we did not thoroughly clean up the retained cortex and viscoelastics.
Supported by St Vincent’s Hospital, Research Institute of Medical Science (SVHR-2012-10) (Y.K.C.).
Footnotes
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.Boneham GC, Collin HB. Steroid inhibition of limbal blood and lymphatic vascular cell growth. Curr Eye Res. 1995;14:1–10. doi: 10.3109/02713689508999908. [DOI] [PubMed] [Google Scholar]
- 2.Krachmer JH, Mannis MJ, Holland EJ. Cornea. 3rd ed. Vol 1. Mosby Elsevier; Philadelphia, PA: 2011. chap 121. [Google Scholar]
- 3.Tabbara KF. Pharmacologic strategies in the prevention and treatment of corneal transplant rejection. Int Ophthalmol. 2008;28:223–232. doi: 10.1007/s10792-007-9100-7. [DOI] [PubMed] [Google Scholar]
- 4.Krachmer JH, Mannis MJ, Holland EJ. Cornea. 3rd ed. Vol 1. Mosby Elsevier; Philadelphia, PA: 2011. chap 156. [Google Scholar]
- 5.Nauck M, Karakiulakis G, Perruchoud AP, et al. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol. 1998;341:309–315. doi: 10.1016/s0014-2999(97)01464-7. [DOI] [PubMed] [Google Scholar]
- 6.Cho YK, Uehara H, Young JR, et al. Effect of glucocorticoid (triamcinolone acetonide) pretreatment in a murine penetrating keratoplasty and suture model. Cornea. 2012;31:1468–1475. doi: 10.1097/ICO.0b013e3182473356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krachmer JH, Mannis MJ, Holland EJ. Cornea. Vol 2. Mosby Elsevier; Philadelphia, PA: 2011. chap 128. [Google Scholar]
- 8.Sonoda Y, Streilein JW. Orthotopic corneal transplantation in mice—evidence that the immunogenetic rules of rejection do not apply. Transplantation. 1992;54:694–704. doi: 10.1097/00007890-199210000-00026. [DOI] [PubMed] [Google Scholar]
- 9.Lam H, Dana MR. Corneal graft rejection. Int Ophthalmol Clin. 2009;49:31–41. doi: 10.1097/IIO.0b013e3181924e23. [DOI] [PubMed] [Google Scholar]
- 10.The collaborative corneal transplantation studies (CCTS) Effectiveness of histocompatibility matching in high-risk corneal transplantation. The Collaborative Corneal Transplantation Studies Research Group. Arch Ophthalmol. 1992;110:1392–1403. [PubMed] [Google Scholar]
- 11.Sano Y, Ksander BR, Streilein JW. Fate of orthotopic corneal allografts in eyes that cannot support anterior chamber-associated immune deviation induction. Invest Ophthalmol Vis Sci. 1995;36:2176–2185. [PubMed] [Google Scholar]
- 12.Niederkorn JY. High-risk corneal allografts and why they lose their immune privilege. Curr Opin Allergy Clin Immunol. 2010;10:493–497. doi: 10.1097/ACI.0b013e32833dfa11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hos D, Saban DR, Bock F, et al. Suppression of inflammatory corneal lymphangiogenesis by application of topical corticosteroids. Arch Ophthalmol. 2011;129:445–452. doi: 10.1001/archophthalmol.2011.42. [DOI] [PubMed] [Google Scholar]
- 14.Dana R. Comparison of topical interleukin-1 vs tumor necrosis factor-alpha blockade with corticosteroid therapy on murine corneal inflammation, neovascularization, and transplant survival (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2007;105:330–343. [PMC free article] [PubMed] [Google Scholar]
- 15.Unal M, Yücel I. Evaluation of topical ciclosporin 0.05% for prevention of rejection in high-risk corneal grafts. Br J Ophthalmol. 2008;92:1411–1414. doi: 10.1136/bjo.2008.143024. [DOI] [PubMed] [Google Scholar]
- 16.Dana MR, Streilein JW. Loss and restoration of immune privilege in eyes with corneal neovascularization. Invest Ophthalmol Vis Sci. 1996;37:2485–2494. [PubMed] [Google Scholar]
- 17.Cursiefen C, Cao J, Chen L, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci. 2004;45:2666–2673. doi: 10.1167/iovs.03-1380. [DOI] [PubMed] [Google Scholar]
- 18.Krachmer JH, Mannis MJ, Holland EJ. Cornea. Vol 2. Mosby Elsevier; Philadelphia, PA: 2011. chap 127. [Google Scholar]
- 19.Steinert RF. Cataract Surgery in Uveitis Patients. Saunders Elsevier; Philadelphia, PA: 2010. Cataract Surgery, Chapter 25. [Google Scholar]
- 20.Shimazaki J, Iseda A, Satake Y, et al. Efficacy and safety of long-term corticosteroid eye drops after penetrating keratoplasty: a prospective, randomized, clinical trial. Ophthalmology. 2012;119:668–673. doi: 10.1016/j.ophtha.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Claerhout I, Beele H, Verstraete A, et al. The effect of duration and timing of systemic cyclosporine therapy on corneal allograft survival in a rat model. Graefes Arch Clin Exp Ophthalmol. 2001;239:152–157. doi: 10.1007/s004170000242. [DOI] [PubMed] [Google Scholar]
- 22.Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamagami S, Hamrah P, Zhang Q, et al. Early ocular chemokine gene expression and leukocyte infiltration after high-risk corneal transplantation. Mol Vis. 2005;11:632–640. [PubMed] [Google Scholar]
- 24.Niederkorn JY, Larkin DF. Immune privilege of corneal allografts. Ocul Immunol Inflamm. 2010;18:162–171. doi: 10.3109/09273948.2010.486100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen NX, Seitz B, Martus P, et al. Long-term topical steroid treatment improves graft survival following normal-risk penetrating keratoplasty. Am J Ophthalmol. 2007;144:318–319. doi: 10.1016/j.ajo.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Dastjerdi MH, Saban DR, Okanobo A, et al. Effects of topical and subconjunctival bevacizumab in high-risk corneal transplant survival. Invest Ophthalmol Vis Sci. 2010;51:2411–2417. doi: 10.1167/iovs.09-3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bachmann BO, Bock F, Wiegand SJ, et al. Promotion of graft survival by vascular endothelial growth factor a neutralization after high-risk corneal transplantation. Arch Ophthalmol. 2008;126:71–77. doi: 10.1001/archopht.126.1.71. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, Grimaldo S, Yuen D, et al. Combined blockade of VEGFR-3 and VLA-1 markedly promotes high-risk corneal transplant survival. Invest Ophthalmol Vis Sci. 2011;52:6529–6535. doi: 10.1167/iovs.11-7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flynn TH, Ohbayashi M, Dawson M, et al. The effect of perioperative allergic conjunctivitis on corneal lymphangiogenesis after corneal transplantation. Br J Ophthalmol. 2011;95:1451–1456. doi: 10.1136/bjo.2010.201939. [DOI] [PubMed] [Google Scholar]