Summary
Primary Leishmania major infection typically produces cutaneous lesions that heal but that harbor persistent parasites. While the opposing roles of CD4+ T cell-derived IFN-γ and IL-10 in promoting parasite killing and persistence have been well established, how these responses develop from naïve precursors has not been directly monitored throughout the course of infection. We used peptide:Major Histocompatibility Complex II (pMHCII) tetramers to investigate the endogenous, parasite-specific primary CD4+ T cell response to L. major in mice resistant to infection. Maximal frequencies of IFN-γ+ CD4+ T cells were observed in the spleen and infected ears within a month after infection and were maintained into the chronic phase. In contrast, peak frequencies of IL-10+ CD4+ T cells emerged within 2 weeks of infection, persisted into the chronic phase, and accumulated in the infected ears but not the spleen, via a process that depended on local antigen presentation. T helper type-1 (Th1) cells, not Foxp3+ regulatory T cells, were the chief producers of IL-10 and were not exhausted. Therefore, tracking antigen-specific CD4+ T cells revealed that IL-10 production by Th1 cells is not due to persistent T cell antigen receptor stimulation, but rather driven by early antigen encounter at the site of infection.
Keywords: Leishmania major, CD4+ T cells, tetramer, IL-10, IFN-γ
Introduction
The adaptive immune response to intracellular parasites or bacteria is in large part dependent upon the generation of primed CD4+ T cells in secondary lymphoid organs. The clonal proliferation that accompanies priming provides for a greater number of antigen-specific cells that can differentiate into effector cells capable of homing to sites of infection. In the case of L. major, the differentiation of CD4+ T cells into Th1 cells that can migrate to the site of parasite inoculation by infected sand fly bite in the skin is required to activate infected macrophages and promote healing of the cutaneous lesion [1]. The number and function of Th1 cells can be limited by the activation of regulatory T cells (Treg) and other populations of IL-10 secreting cells, including Th1 cells, which may restrain immunopathology while preventing the complete elimination of the parasite [2]. In chronically infected mice, the number of persistent organisms and the severity of the chronic cutaneous lesions are determined by the balance of immune activating and deactivating cells and cytokines in the site [3].
The expansion of parasite specific CD4+ T cells, their homing to and function within peripheral sites of infection, and their maintenance, contraction, or exhaustion during the chronic stage, remain poorly characterized in any Leishmania infection model. The adoptive transfer of naïve TCR transgenic CD4+ T cells responding to OVA expressing L. major (Lm-OVA), revealed their early proliferation in the draining lymph node and generation of both central and effector memory cells during the first 14d of infection [4]. This study did not follow the cells in the inoculation site, nor their fate during the chronic stage of infection. Furthermore, the transfer of large, non-physiologic numbers of T cells with the same specificity can distort their proliferation and differentiation programs [5, 6], a phenomenon borne out in the response of OT-II transgenic T cells to Lm OVA [4]. Peptide-MHC class II (pMHCII) tetramer based approaches that permit detection of rare endogenous precursors in normal mice have been applied in Lm infection models to study the activation and expansion of CD4+ T cells specific for the Lm antigen LACK [7]. The studies were again confined to lymph node cells during the acute stage of infection, and did not consider the polyfunctionality of these cells.
In the current studies, we used a sensitive pMHCII tetramer–based approach that allowed detection of polyclonal pMHCII-specific CD4+ T cells in normal mice after intra-dermal infection with L. major. We applied this approach to enumerate the expansion, contraction and tissue distribution of parasite-specific CD4+ T cells throughout the course of the infection. Most informatively, we have been able to define the dynamics of IFN-γ and IL-10 secreting effector and Treg cells that contribute to the chronicity of the infection, and to the balance of immunity and pathology in the inflammatory site.
Results
Detection of CD4+ T cells specific for an Lm-derived model antigen
We generated L. major parasites (Lm 2W) that express a secreted chimeric protein consisting of the 2W peptide and the L. donovanii 3’ nucleotidase/nuclease, an antigen expressed in the promastigote and amastigote stages [8] to directly visualize an endogenous polyclonal, antigen-specific CD4+ T cell response to Lm. The 2W peptide is a variant of peptide 52-68 from the I-E alpha chain (EAWGALANWAVDSA) [9]. The relatively large naive precursor size of the 2W:I-Ab-specific T cell repertoire provided a technical advantage over tracking CD4+ T cells specific for a chicken ovalbumin-derived peptide (323-339) bound to I-Ab [10, 11] with a previously generated recombinant L. major strain [8]. C57BL/6 mice were primed in the footpad with either Lm 2W or Lm SP-OVA and boosted in the ears 8 weeks later with the homologous recombinant L. major strain used in the primary infection to determine if endogenous CD4+ T cell responses to L. major-derived antigens could be detected with I-Ab tetramers. One week after boosting, we stained single-cell suspensions with fluorochromeconjugated 2W:I-Ab and pooled OVA323-339:I-Ab and OVA265-280:I-Ab tetramers [12], and used anti-fluorochrome magnetic beads to enrich specific T cells from the ears and ear-draining lymph nodes (dLN) of uninfected and Lm-infected mice. T cells in the enriched fraction were identified as CD3+ cells that did not bind a cocktail of lineage-specific antibodies (Figure 1A). 2W:I-Ab-specific T cells were detected in the CD4+ T cell compartment but not among the MHCI-restricted CD8+ T cells (Figure 1B, C), suggesting that the tetramer bound to T cells via the TCR. 2W:I-Ab-specific CD4+ T cells were found in the dLN of uninfected and SP-OVA-infected mice, but were absent in the ears of those mice (Figure 1C). Furthermore, the tetramer-binding cells in these mice were CD44low, as expected for naive cells. In contrast, large numbers of CD44high 2W:I-Ab-specific CD4+ T cells were detected in the dLN and ears of Lm-2W-infected mice (Figure 1C). Importantly, the expanded 2W:I-Ab+ T cell population did not bind the OVAp:I-Ab tetramers (Figure 1D), again indicating the tetramer staining was TCR-specific. These results indicate that endogenous pMHCII-specific T cell responses to L. major-derived model antigens can be monitored with I-Ab tetramers.
Figure 1. Antigen-dependent response of 2W:I-Ab-specific CD4+ T cells after Lm-2W infection.
Mice were infected in the right hind footpad with recombinant Lm expressing OVA (SP-OVA) or 2W. Eight weeks after primary infection, mice were challenged in the ears with the homologous recombinant L. major strain. One week later, 2W:I-Ab+ and OVAp:I-Ab+ (pooled OVA265-280 and OVA323-339 T cells from dLN and ears were magnetically enriched. (A) Flow cytometry plots show strategy to detect T cells in tetramer-enriched dLN of an uninfected mouse. (B) 2W:I-Ab versus CD44 staining within CD8+ T cells in tetramer-enriched dLN of an uninfected mouse. (C) 2W:I-Ab versus CD44 staining of CD4+ T cells in tetramer-enriched dLN or ears of uninfected, SP-OVA-infected, or Lm-2W-infected mice. (D) 2W:I-Ab versus OVAp:I-Ab on CD4+ T cells in the ears. Numbers in plots indicate the percentage of tetramer-binding cells within the CD4+ or CD8+ T cell compartments of the tetramer-enriched samples. Data shown are from a single experiment.
Kinetics of primary 2W:I-Ab-specific T cell response to Lm-2W infection
The course of lesion development as well as parasite growth and clearance obtained following intra-dermal ear injection of 105 metacyclic promastigotes of the Lm-2W parasites were comparable to the wild type Lm FV1 strain, with pathology peaking at 6-8 weeks, and mean parasite numbers peaking at 4-5 weeks post-infection in the ear (Lm FV1 2.33×106 +/− 3.87×106; Lm-2W 2.37×106 +/− 2.97×106), and in the local draining LN (Lm FV1 4.01×104 +/− 2.74×104; Lm-2W 2.96 ×104 +/− 1.12 ×104). At 5 weeks post-infection, none of the Lm FV1 infected mice and only one of four of the Lm 2W infected mice presented any detectable parasites in the spleen. The maintenance of a low number of Lm 2W parasites in the skin following healing of the lesion, as has been reported for the wild type strain [13], was confirmed by detection of between 1.28 × 102 and 2.48 × 103 parasites in a total of 6 ears from mice examined at 11 and 17 weeks post-infection.
We enumerated 2W:I-Ab-specific CD4+ T cells in the ear-draining lymph nodes, spleen, and ears in response to intra-dermal infection with Lm-2W to gain insight into the development, maintenance, and tissue distribution of the parasite-specific CD4+ T cell response. 2W:I-Ab-specific T cells were detected in the spleen and dLN and absent from the ears of uninfected mice (Figure 2A, B), and as expected for naive cells, these were virtually all CD44low (Figure 2A). CD44high 2W:I-Ab-specific T cells were detected in the dLN on day 3 post-infection (Figure 2B), indicating that priming was underway. This is consistent with a previous report showing expansion of LACKp:I-Ad-specific T cells in BALB/c mice [7]. On day 3, however, no 2W:I-Ab-specific CD4+ T cells had reached the ears (Figure 2B). By day 7, the number of 2W:I-Ab-specific T cells had increased about 10-fold in the ear-draining lymph nodes and spleen over those in naive mice, and about 70-fold 2W:I-Ab-specific T cells had accumulated in the ears (Figure 2A, B). 2W:I-Ab-specific T cells expanded about 100-fold in the draining lymph nodes within the first 2 weeks of the infection and then declined by day 32 to about 9% of peak numbers. By day 56, the number of 2W:I-Ab-specific T cells was about 3.5% of peak numbers, a value that was maintained for at least two months into the chronic phase of the infection. In contrast, peak numbers of 2W:I-Ab-specific T cells in the spleen and ears were achieved 3 weeks post-infection and were at least 3 times greater than in the lymph nodes. 2W:I-Ab-specific T cells in the spleen and ears gradually declined during the following 35 days to about 11% of peak numbers, values that were maintained for at least two months. Most of the 2W:I-Ab-specific T cells enumerated at any given time point were found in the spleen, indicating that this tissue is a large reservoir of parasite-specific T cells. These results demonstrate that parasite-specific CD4+ T cells undergo expansion, contraction, and numerically stable phases in response to a persistent infection, and that the tempos of these processes differ for the dLN, spleen, and ears.
Figure 2. Kinetics of the primary 2W:I-Ab-specific T cell response to Lm-2W.
Mice were infected in the ears with Lm-2W. (A) Flow cytometry plots of 2W:I-Ab versus CD44 staining on CD4+ T cells in the dLN, ears, or tetramer-enriched spleens from uninfected and infected mice. Numbers in plots indicate the percentage of tetramer-binding CD4+ T cells in the stained sample. (B) Absolute numbers of 2W:I-Ab+ T cells in each indicated tissue. Pooled data from more than 3 independent experiments collectively containing 2 - 12 mice per time point are shown.
Preferential expansion of T-bet+ Foxp3− CD4+ T cells in response to Lm-2W infection
We then sought to determine which T cell subsets were present within the tetramer-binding cells in the chronic phase of the infection. We focused our analysis on Th1 cells and Foxp3+ Tregs because Lm infection in B6 mice induces a potent Th1 response [1] and thymus-derived parasite-specific Tregs have been implicated in parasite persistence [14, 15]. The 2W:I-Ab-specific CD4+ T cell repertoire is amenable for these studies because it can generate Th1 cells [16] and about 8% of the tetramer-binding cells in naïve B6 mice are Helios+ Foxp3+ Tregs [17], suggesting a thymic origin [18]. Naive CD44low 2W:I-Ab-specific T cells in the secondary lymphoid tissues do not express T-bet [16]. Seventy-seven to 94% of the CD44high 2W:I-Ab-specific T cells in the ears, dLN, and spleen, respectively, expressed T-bet and not Foxp3 eight weeks post-infection (Figure 3B, C), indicating that these were Th1 cells [19] and not Treg cells [20, 21]. In contrast, 40 – 65% of the CD44high tetramer− CD4+ T cells in the ears, dLN, and spleen were Th1 cells, demonstrating that the tetramer-binding T cells are enriched for these cells. Less than 7% of the tetramer-binding cells in these tissues expressed Foxp3, and a small fraction co-expressed T-bet (Figure 3B, C), as has been described for Mycobacterium tuberculosis infection [22]. Contrary to 2W:I-Ab+ cells, 5 - 27% of the tetramer− compartment was comprised of Tregs. 2W:I-Ab+ Th1 cells were 20 - 100 times more abundant than 2W:I-Ab+ Tregs in the sampled tissues (Figure 3D). Similar results were observed by tracking OVAp:I-Ab-specific T cells in response to SP-OVA infection (Figure 3E), demonstrating that they were not unique to the 2W:I-Ab-specific CD4+ T cell repertoire.
Figure 3. Preferential expansion of Foxp3− T-bet+ parasite-specific T cells after Lm infection.
Mice were uninfected (F and G) or infected in the ears with Lm-2W (A-D, F and G) or SP-OVA (E). Ear-draining lymph nodes (dLN), spleens, and ears were harvested at 3 (F and G) or 8 weeks after infection (A-D). (A) 2W:I-Ab versus CD44 staining on CD4+ T cells from dLN, ears, or tetramer-enriched spleens. (B) Foxp3 versus Tbet staining on CD44high 2W:I-Ab+ or CD44high 2W:I-Ab− CD4+ T cells gated in (A). Numbers in plots depict the percentages of cells within each quadrant. (C and F) Scatter plots show the percentage of Foxp3+ (left) or Foxp3− Tbet+ (right, 8 weeks only) 2W:I-Ab− or 2W:IAb+ CD4+ T cells. Scatter plots of the absolute numbers of Foxp3+ or Foxp3- Tbet+ 2W:I-Ab+ T cells (D and G) or OVAp:I-Ab+ T cells (pooled OVA-2:I-Ab, OVA-3:I-Ab, and OVA265:I-Ab tetramers) (E). Statistical significance was determined with an unpaired, two-tailed Student's t test, * p < 0.05, ** p < 0.01, *** p < 0.001. Data are pooled from two independent experiments with 2 - 5 mice per group (A-D) or one experiment with 3-5 mice per group (F and G).
To explore the possibility that expansion of parasite-specific Treg may have occurred earlier in infection, Foxp3 and Tbet expression were analyzed on 2W:I-Ab+ and 2W:I-Ab− cells recovered at 3 weeks post-infection, and in comparison to the frequency of these cells in naïve mice. Again, less than 5% of the tetramer binding cells in the dLN, spleen or ear expressed Foxp3 (Figure 3F), significantly less than the frequency of the Foxp3+ cells in the tetramer− population (9-26%), and less than the frequency of these cells in the tetramer binding population of cells present in the dLN and spleen of uninfected mice (note that no tetramer binding cells were detected in the naïve ear). In addition, no increase in the total number of tetramer binding, Foxp3+ cells was observed in the dLN or spleen compared to naïve mice (Figure 3G). The low but detectable numbers of 2W:I-Ab-specific Foxp3+ cells in the infected skin likely reflects inflammation-induced recruitment of these cells. By contrast, there was a massive expansion of the 2W:I-Ab-specific Foxp3−Tbet+ cells in dLN and spleen over the numbers present in naïve mice, and they were 29 times more abundant than the 2W:I-Ab+ Tregs in the ear.
These findings indicate that Th1 cells comprise by far the largest T cell subset both early and during the chronic stage of the infection, and suggest that parasite-specific thymic Tregs are not preferentially expanded in response to this infection.
Kinetics of IFN-γ and IL-10 production during primary Lm-2W infection
Helper T cell-derived IFN-γ and IL-10 exert opposite effects on parasite burden [13]. We evaluated IFN-γ and IL-10 production by 2W:I-Ab-specific T cells throughout the infection to determine when these responses develop and assessed the tissue distribution of the cells capable of making these cytokines upon restimulation in vivo. Both Lm-specific Th1 cells and Lm-specific Tregs can produce IL-10 [14, 15, 23]. Since most of the tetramer+ cells in the chronic phase expressed T-bet and not Foxp3, Th1 cells were the most likely source of IL-10. Within the first two months of infection, about 15% of the 2W:I-Ab-specific T cells in the ears made IFN-γ in the absence of in vivo peptide re-stimulation above the levels of those cells found in the spleen (Figure 4A – C). This is in agreement with a report of IFN-γ production by CD4+ T cells in the skin of mice immunized intra-dermally with protein emulsified in Incomplete Freund's Adjuvant [24]. In contrast, no IL-10 could be detected without the intravenous injection of 2W peptide (Figure 4A, B, and D). Two – 4 hours after intravenous injection of 2W peptide, IFN-γ and IL-10 could be detected in tetramer-binding cells. The frequency of IFN-γ+ 2W:I-Ab+ T cells in the spleen rose from 25% on day 14 to 65% on day 21, and then remained at around 50% for at least 70 more days (Figure 4A, C). The frequency of IFN-γ+ 2W:I-Ab+ T cells in the ears also increased sharply between days 14 and 28, from 45% to 65%, and then gradually increased to about 80% by day 91 after infection. About 5% of the tetramer-binding cells in the spleen 14 or 21 days post infection made IL-10 after peptide stimulation (Figure 4A, D). By day 28 the frequency dropped to background levels. In contrast, ~20% of the tetramer-binding cells in the ears at all of the time points analyzed made IL-10 after peptide stimulation (Figure 5B, D). On day 14, about half of the IL-10+ cells in the spleen and ears were IFN-γ+ (Figure 4A - C). However, by day 21, IFN-γ+ cells were the major source of IL-10 within tetramer-binding cells. This trend became more pronounced by day 28 in the ears.
Figure 4. Kinetics of IFN-γ and IL-10 production by 2W:I-Ab+ T cells during course of Lm-2W infection.
Mice were infected in the ears with Lm-2W. Some mice were injected intravenously with 2W peptide 2 - 4 hours prior to harvesting spleens and ears. Flow cytometry plots of IFN-γ versus IL-10 on 2W:I-Ab+ CD44high CD4+ T cells from tetramer-enriched spleens (A) or unenriched ears (B) of 2W peptide-stimulated or unstimulated mice. Numbers in plots indicate the percentage of cells within each quadrant. (Left) Line graphs show kinetics of IFN-γ (C) and IL-10 (D) production by 2W:I-Ab+ T cells. (Right) Scatter plots depict the percentages of IFN-γ+ (C) and IL-10+ (D) 2W:I-Ab+ T cells on days 28 - 91. (E) Line graph shows the difference in IFN-γ mean fluorescence intensity (MFI) of peptide-stimulated IL-10+ IFN-γ+ 2W:I-Ab+ T cells or IL-10− IFN-γ+ 2W:IAb+ T cells minus IFN-γ MFI of unstimulated 2W:I-Ab+ T cells in the ears. Statistical significance was determined with an unpaired, two-tailed Student's t test, ** p < 0.01, *** p < 0.001. Pooled data from at least 3 experiments with 2 - 5 mice per time point are shown.
Figure 5. Parasite-specific CD4+ T cells with enhanced capacity to produce IL-10 are enriched in site of antigen presentation.
Mice were simultaneously infected with Lm SP-OVA in one ear and Lm-2W in the other. Four or 12 weeks after infection, some mice were injected intravenously with 2W peptide 2 - 4 hours prior to harvesting spleens and ears. (A) Flow cytometry plots of IFN-γ versus IL-10 on 2W:I-Ab+ CD44high CD4+ T cells from tetramer-enriched spleens or ears of 2W peptide-stimulated or unstimulated mice. Numbers in plots depict the percentage of cells within each quadrant. Scatter plots show the percentages of IFN-γ+ (B) or IL-10+ (C) 2W:I-Ab+ T cells. Statistical significance was determined by one-way ANOVA with the Bonferroni post-test. n.s. p > 0.05, * p < 0.05, ** p < 0.01, *** p < 0.001. Data from two experiments containing 3 - 4 mice per group were combined.
IL-10 production by CD8+ T cells responding to a chronic LCMV infection has been associated with greater levels of T cell exhaustion, including a reduced capacity to make IFN-γ on a per cell level [25]. To determine if this property applied to CD4+ T cells responding to Lm, we measured IFN-γ mean fluorescence intensities in IL-10+ IFN-γ+ and IL-10− IFN-γ+ 2W:I-Ab-specific T cells in the ears at different times after Lm 2W infection (Figure 4E). IL-10+ cells were unimpaired in their capacity to produce IFN-γ after peptide stimulation in vivo. Collectively, these results show that the elaboration of an optimal Th1 response to Lm requires at least 3 – 4 weeks, while that of the IL-10 response occurs 1 – 2 weeks earlier. Furthermore, parasite-specific CD4+ T cells with enhanced cytokine production potential, in particular for IL-10, were enriched in the ears compared to the spleen.
Parasite-specific CD4+ T cells with enhanced IL-10 production potential are enriched in site of antigen-presentation
The observation that cytokine-producing 2W:I-Ab-specific T cells were enriched in the infected ears compared to the spleen prompted us to test whether cognate antigen presentation regulated the tissue distribution of these cells. Mice were simultaneously infected with Lm SP-OVA in one ear and Lm 2W in the other, and 2W:I-Ab-specific T cells in each ear and the spleen were evaluated for IFN-γ and IL-10 production 4 or 12 weeks post infection. In the absence of exogenous peptide, about 10% of the tetramer-binding T cells in the ear infected with Lm 2W produced IFN-γ above the levels of those achieved in the ear infected with Lm SP-OVA (Figure 5), suggesting that this basal level of IFN-γ is due to in situ pMHCII presentation [24]. This experiment also demonstrated that only a fraction of the ear-infiltrating cells are making detectable amounts of IFN-γ at any given time. Virtually no IL-10+ cells could be detected in the absence of exogenous 2W peptide (Figure 5). Two – 4 hours after intravenous injection of 2W peptide, ~40% of the 2W:I-Ab-specific T cells in the spleen made IFN-γ, whereas ~60% of those in the Lm SP-OVA and Lm 2W ears made IFN-γ. However, the differences between the spleen and each ear were not statistically significant. Importantly, the observation that similar frequencies of 2W:I-Ab-specific T cells in the Lm SP-OVA and Lm 2W ears produced IFN-γ after peptide re-stimulation suggests that IFN-γ production potential is not enhanced by local antigen presentation. In contrast, IL-10+ Th1 cells, were largely restricted to the Lm 2W ear, suggesting that IL-10 production potential is indeed regulated by local antigen presentation.
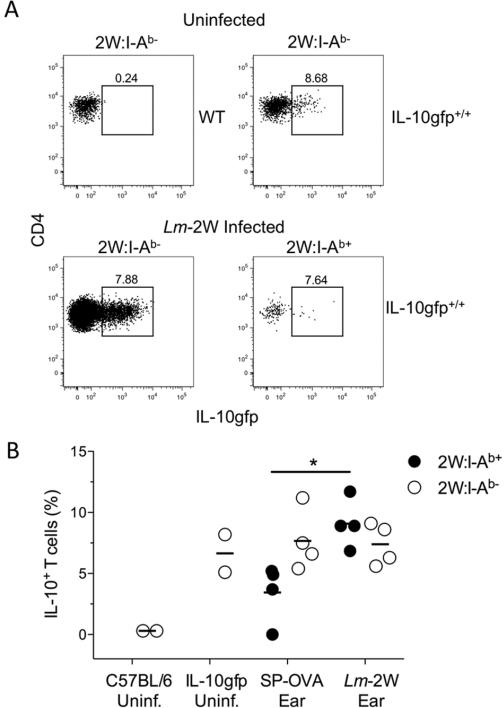
To address the possibility that the inability to detect 2W:I-Ab-specific T cell IL-10 production in the skin in the absence of exogenous peptide administration was due to the relative insensitivity of the intracellular IL-10 staining, we infected bicistronic IL-10 GFP reporter mice, designated Vert-X [26]. Reporter expression was observed in 5-8.6% of the CD4+CD44high 2W:I-Ab− cells recovered from the uninfected ears of Vert-X mice (Figure 6, A and B). No tetramer binding cells were observed in the ears of the uninfected reporter mice, similar to the wild type mice (data not shown). Following 5 weeks infection with Lm 2W in one ear and Lm SP-OVA in the contra-lateral ear, 7-12% of the 2W:I-Ab-specific T cells were IL-10 GFP+ in the 2W ear compared to 0-5.2% in the OVA ear (Figure 6, A and B). A greater absolute number of the CD4+CD44high T cells in the 2W:I-Ab− compartment in both ears acquired a IL-10 GFP+ phenotype, although their frequencies did not increase over those observed in the uninfected Vert-X mice. These data reinforce the conclusion that IL-10 production is regulated by local antigen presentation.
Figure 6. Detection of greater frequencies of IL-10+ parasite-specific CD4+ T in the site of antigen presentation does not require exogenous peptide administration.
Wild type C57BL/6 or Vert-X IL-10 reporter mice were uninfected or simultaneously infected with Lm SP-OVA in one ear and Lm-2W in the other. Five weeks after infection ears were processed in the presence of Brefeldin A. (A) Representative flow cytometry plots of CD4 versus IL-10 GFP on 2W:I-Ab+ CD44high CD4+ T cells. Numbers in plots depict the percentage of cells within the box gate. (B) Scatter plots show the percentages of IL-10 GFP+ 2W:IAb+ CD44high or 2W:I-Ab− CD44high CD4+ T cells. Statistical significance was determined with an unpaired, two-tailed Student's t test, * p=0.011. Data are from 1 experiment containing 2 - 4 mice per group.
Discussion
The present study describes how Lm-specific CD4+ T cell immunity develops. Parasite-specific CD4+ T cells expand in the infection-draining LN over the course of a few weeks, where they gradually polarize into IFN-γ-producing Th1 cells. After priming, these cells migrate to the infected tissue and spleen. A fraction of the parasite-specific CD4+ T cells rapidly acquire the capacity to make IL-10, which is largely restricted to the site of infection. IL-10+ IFN-γ− T cells appear early in the infection site but do not persist into the chronic phase. In contrast, IL-10+ Th1 cells preserve the ability to produce IFN-γ and IL-10 well into the chronic phase of the infection. Thus, the parasite-specific IL-10 and IFN-γ CD4+ T cell responses differ in their development and maintenance kinetics.
Enumerating tetramer-binding cells throughout the course of Lm infection revealed that the antigen-specific CD4+ T cell response to this pathogen was protracted compared to acute infections that induce Th1 responses. Tetramer-binding cells reached the peak of expansion about 1 – 2 weeks later than endogenous pMHCII-specific responses against LCMV Armstrong and L. monocytogenes and declined more slowly than T cells primed during these other infections [16, 27, 28]. The delayed kinetics of clonal expansion might be a consequence of slow pathogen growth compared to LCMV and L. monocytogenes, thereby limiting the amount of antigen available early in the infection, as has been demonstrated for Mycobacterium tuberculosis infection [29]. It is likely that using a lower, more physiological inoculum of Lm would produce an even more delayed CD4+ T cell response [13]. A stable population of pathogen-specific CD4+ T cells has also been documented in response to L. donovani [30] and to M. tuberculosis infection [31], suggesting that this might be a common feature of locally persistent infections. Sustained antigen presentation in the chronic phase might contribute to the numeric stability of the parasite-specific T cell pool by inducing iterative rounds of proliferation and/or recruiting new thymic emigrants [4, 32], although naïve Leishmania-specific T cells have been shown to respond poorly in the presence of previously primed cells [33]. Since sustained antigen presentation supports continued CD4+ T cell expansion [34-36], it is reasonable to surmise that this mechanism could contribute to the stability of pathogen-specific CD4+ T cell numbers in a chronic infection.
Very few Tregs were found within the parasite-specific T cell populations tested, either at 3 or 8 weeks post-infection. The few Foxp3+ cells within the 2W:I-Ab+ and OVAp:I-Ab+ compartments of uninfected mice expanded much less than the Foxp3− cells in response to infection, and the numbers of 2W:I-Ab+ Tregs appeared not to increase relative to the numbers found in the dLN and spleen of mice. These findings are surprising in light of the thymic-derived Lm-specific Tregs that have been previously described in B6 mice infected with the wild type Lm strain of the transgenic Lm used in this study [15]. The present findings suggest that the majority of the Tregs found in the infected skin are not parasite-specific. We cannot exclude the possibility that Treg cells might be more abundant within other Lm-specific populations besides those tested. Formally addressing this point will depend on the generation of pMHCII tetramers to directly track a variety of CD4+ T cell responses to endogenous Lm antigens. However, considering that 2W:I-Ab-specific Foxp3+ Tregs have been observed after intradermal immunization with peptide emulsified in Incomplete Freund's Adjuvant [24], the absence of 2W:I-Ab-specific Foxp3+ Tregs in the infected dermis points to likely differences in the cytokine milieu of Lm infection and IFA immunization that result in activation of functionally distinct CD4+ T cell subsets within the 2W:I-Ab-specific repertoire.
Most of the parasite-specific T cells detected with tetramer differentiated into Th1 cells, as illustrated by their expression of T-bet and ability to produce IFN-γ after peptide re-stimulation in vivo, and a fraction of these also made IL-10. Our results validate the well-established observation that Lm infection induces a robust Th1 response in mice genetically resistant to infection [37]. IL-10+ Th1 cells that can be recovered from the inflammatory site have also been described in the context of both healing and non-healing Lm infections, [13, 23], and have been described in other chronic infections including visceral leishmaniasis [30], toxoplasmosis [38] and malaria [2, 39]. The frequency of IFN-γ+ tetramer+ cells increased with time, supporting a model of progressive Th1 cell differentiation [40]. In contrast, the frequency of IL-10+ cells peaked early and was maintained thereafter. This result suggests that IL-10 synthesis is not necessarily a result of long-term stimulation. It is interesting that within the first 2 weeks of the infection, there were IL-10+ cells in the skin that did not make IFN-γ. It was also surprising to see that IL-10+ Th1 cells were not exhausted. This might be explained by the fact that in contrast to the CD8+ and CD4+ T cell exhaustion that occurs following chronic, systemic viral infections [41], Lm infection in B6 mice is restricted to the site of infection and to local LNs, such that circulating T cells might be only transiently stimulated. Similarly, parasite-specific CD8+ T cells in mice infected with Trypanosoma cruzi, which chiefly localize to muscle during the chronic phase, did not show impaired effector function [42]. Thus, Lm infection induces a robust Th1 response that develops slowly and is maintained in the chronic phase of the infection.
Our study extends our understanding of how IL-10 production by Th1 cells is regulated in vivo. Following peptide re-stimulation in vivo, IL-10+ Th1 cells were largely restricted to the Lm 2W infected ear. The 2W:I-Ab-specific T cells present in the Lm OVA infected ear presumably home there as a consequence of the inflammation, similar to that described for human leishmaniasis skin lesions found to contain non-antigen specific T cells [43]. But upon their arrival, they do not appear to be locally activated by cognate antigen recognition to produce IL-10 in this site. Similar observations were made using the IL-10 GFP reporter mice in which even in the absence of exogenous peptide 2W:I-Ab-specific T cells that acquired an IL-10 GFP phenotype could be detected that were significantly more frequent in the Lm 2W infected ear compared to the Lm SP-OVA infected ear. Thus IL-10 production potential is chiefly restricted to the site of infection and implicates local antigen presentation in this process. Previous reports indicate that high and/or repeated TCR ligation can induce Th1 cells to make IL-10 [38, 44]. Of particular relevance may be the finding that rested T. gondii-specific IL-10+ Th1 clones produced IL-10 with delayed kinetics compared to recently stimulated ones [38], suggesting that IL-10 production might be transiently induced at local sites of antigen presentation and lost after cessation of cognate pMHCII-TCR interaction. IL-27, the IL-21R, ICOS, the aryl hydrocarbon receptor, and the transcription factor c-maf have also been shown to drive IL-10 expression [44-48]. Of note, the IL-27R was required for IL-10 production by Th1 cells in a non-healing model of Lm infection [49]. Collectively, our data and the published literature on IL-10 regulation suggest that signals emanating from the TCR, co-stimulatory receptors, and cytokine receptors during CD4+ T cell priming induce IL-10, while continued TCR signaling at the site of infection sustain IL-10 production potential.
The present finding that the protective IFN-γ+ T cell response develops slowly while the immunosuppresive IL-10+ response emerges relatively early and is sustained locally has important implications for achieving sterile cure. It suggests that early mobilization of large numbers of Th1 cells, preferably those lacking IL-10 potential, might be necessary to rapidly curtail Lm expansion.
Materials and Methods
Mice
Female C57BL/6 mice were purchased from Taconic Laboratories. Vert-X (C57BL/6 IL-10/eGFP) mice were generated as described [26] and were bred in the NIAID animal breeding facility. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Animal Care and Use Committee of the NIAID, NIH (protocol number LPD 68E). All mice were maintained at the NIAID animal care facility under specific pathogen-free conditions.
Parasites and infections
Lm SP-OVA transgenic parasites were generated by transfection of Lm FV1 (MHOM/IL/80/Friedlin) promastigotes as previously described [8]. For the generation of transgenic Lm expressing the 2W peptide, the pKS NEO plasmid was used to express a chimeric protein between the L. donovani 3’-nucleotidase/nuclease (Ld3’NT/NU) [50] and the C terminus of the 2W peptide (EAWGALANWAVDSA) [51]. To that end, the Ld3’NT/NU gene was amplified by PCR using the following primers. The forward primer, 5’-TGGACTAGTATG GCTCGAGCTCGTTTCCT TCAG-3’ contains a SpeI site followed by the first 24 nucleotides of the Ld3NT/NU gene. The reverse primer, 5’-CCAACTAGTC TACGCCGAGTCCACCGCCCAGTTCGCCAGCGCGCCCCACGCCTCA GCGCTGATGCCTTTCTGATCGTAG-3’ contains nucleotide 981 to 1005 of the Ld3’NT/NU gene followed by 42 nucleotides encoding the 2W peptide, a stop codon, and a SpeI cloning site. The codons used for the 2W peptide sequence in the reverse primer were chosen to reflect the GC-rich Leishmania codon bias. The PCR amplified product was cloned into the SpeI site of the pKS NEO plasmid and the sequence of the NT::2W open reading frame in the pKS NEO NT::2W plasmid was verified by nucleotide sequencing. Lm FV1 (MHOM/IL/80/Friedlin) promastigotes were transfected with pKS NEO NT::2W plasmid by electroporation and selected for growth at 26° C in medium 199 (Gibco BRL) supplemented with 20% heat-inactivated FBS (Gemini), 100 U/ml penicillin, 100 μg/ml streptomycin, 2mM L-glutamine, 40 mM HEPES 0.1 mM adenine (in 50 mM HEPES), 5 mg/ml hemin (in 50 %) triethanolamine, and 1 mg/ml 6-biotin (Sigma) in presence of geneticin (G418) (Sigma, St. Louis, MO) as previously described [52]. Expression of the chimeric protein was confirmed by SDS-PAGE and Western blotting of total parasite cell lysates and cultures supernatants using the anti-Ld3’NT/NU antibody as previously described [52].
For mouse infections, infective-stage metacyclic promastigotes were isolated from 4-5 day-old stationary cultures by density centrifugation on a Ficoll gradient [53]. Metacyclic promastigotes (1 × 105) were inoculated in the left hind footpad in a volume of 40 μl, or intradermally into the ear using a 27.5-gauge needle in a volume of ~5 μl.
pMHCII tetramer production
Soluble 2W:I-Ab, OVA323 339:I-Ab, OVA265-280:I-Ab , and peptide-register trapped OVA-2:I-Ab and OVA-3:I-Ab monomers corresponding to registers 325-335 and 327-338 of chicken ovalbumin, respectively, were produced and biotinylated in Drosophila melanogaster S2 cells and then combined with streptavidin-allophycocyanin or streptavidin-phycoerythrin (Prozyme) to make tetramers, as previously described [11, 17].
Sample preparation, pMHCII tetramer staining, and magnetic enrichment
Individual spleens and retromaxillary (ear-draining) lymph nodes were removed and mechanically dissociated using a syringe plunger. Individual ear tissue was prepared by digestion with DMEM containing 200[.proportional]g Liberase CI enzyme blend (Roche Diagnostics Corp.) as previously described [54]. Single cell suspensions of tissue homogenates were filtered using a 70 mm-pore size Falcon cell strainer (BD Biosciences). 2W:I-Ab and OVAp:I-Ab tetramer staining and magnetic enrichment were performed as previously described [55]. Briefly, single cell suspensions of spleen, lymph nodes, or ears were stained with 10nM allophycocyanin- or phycoerythrin-labeled 2W:I-Ab-streptavidin or OVAp:I-Abstreptavidin tetramers for 1 hour at room temperature. Samples were then incubated with magnetic anti-fluorochrome microbeads and run through a magnetized LS column (Miltenyi Biotec).
Peptide re-stimulation for measurement of cytokine production in vivo
Mice were injected intravenously with 100 μg of 2W peptide (EAWGALANWAVDSA) (GenScript). Spleen and ears were harvested 2 – 4 hours later and homogenized on ice in media supplemented with Brefeldin A (10ug/ml) (SIGMA) [56].
Antibodies and flow cytometry
All antibodies were from eBioscience unless indicated. Samples were stained for 30 minutes at 4°C with Pacific Blue-, eFluor 450-, or PerCP-Cy5.5-conjugated anti-B220 (RA3-6B2), anti-CD11b (MI-70), anti-CD11c (N418), and anti-F4/80 (BM8, Invitrogen), Pacific Orange-conjugated anti-CD8α (5H10, Invitrogen), fluorescein isothiocyanate-conjugated anti-CD3ε (145-2C11), peridinin chlorophyll protein-cyanine 5.5-conjugated anti-CD3ε (145-2C11), or anti-CD4 (RM4-5), Alexa Fluor-conjugated anti-CD44 (IM7), allophycocyanin-Alexa Fluor 750 or allophycocyanin-eFluor 780-conjugated anti-CD4 (RM4-5). In some experiments, samples were treated with the Foxp3 Fixation/Permeabilization Buffer (eBioscience) per manufacturer's instructions, and then stained with eF450-conjugated anti-Foxp3 (FJK-16s) and PE- or AlexaFluor-647-conjugated anti-Tbet (eBio4B10). In other experiments, samples were fixed and permeabilized with BD Cytofix/Cytoperm (Becton-Dickinson) according to the manufacturer's instructions, and subsequently stained for 1 hour at 4°C with PE-conjugated anti-IL-10 (JES5-16E3) and PE-Cy7-conjugated anti-IFNγ (XMG1.2). Samples were run on LSRII or Fortessa flow cytometers (Becton-Dickinson) and analyzed with FlowJo (Tree Star).
Statistical analyses
Statistical tests were performed in Microsoft Excel or GraphPad Prism. Comparisons of absolute cell numbers were done on the log10 of each value to minimize differences in statistical variance of the raw values due to exponential growth. P values <0.05 were considered statistically significant. The two-tailed, unpaired Student's t test was used when comparing two groups, and a one-way analysis of variance (ANOVA) with Bonferroni's post-test was performed when comparing three groups.
Acknowledgements
We thank Kim Beacht, J. Walter and R. Speier for expert technical assistance. This work was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and by grants from the US National Institutes of Health, R37 AI027998, R01 AI039614, and R01 AI066016 (M.K.J.), T32 AI07313 (A.J.P.), and T32 CA9138 (M.P.), and a Frieda M. Kunze Fellowship from the Minnesota Medical Foundation (A.J.P.).
Abbreviations
- Lm
Leishmania major
- IFN-γ
Interferon-γ
- IL-10
Interleukin-10
- Th1
T helper type-1
- Treg
regulatory T cell
- dLN
Ear-draining lymph node
- OVA
chicken ovalbumin
- TCR
T cell antigen receptor
- B6
C57BL/6
Footnotes
Conflict of Interest: The authors have no conflicting financial interests.
References
- 1.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 2.Couper KN, Blount DG, Wilson MS, Hafalla JC, Belkaid Y, Kamanaka M, Flavell RA, de Souza JB, Riley EM. IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog. 2008;4:e1000004. doi: 10.1371/journal.ppat.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belkaid Y, Blank RB, Suffia I. Natural regulatory T cells and parasites: a common quest for host homeostasis. Immunol Rev. 2006;212:287–300. doi: 10.1111/j.0105-2896.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- 4.Colpitts SL, Scott P. The early generation of a heterogeneous CD4+ T cell response to Leishmania major. J Immunol. 2010;185:2416–2423. doi: 10.4049/jimmunol.1000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 6.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stetson DB, Mohrs M, Mallet-Designe V, Teyton L, Locksley RM. Rapid expansion and IL-4 expression by Leishmania-specific naive helper T cells in vivo. Immunity. 2002;17:191–200. doi: 10.1016/s1074-7613(02)00363-1. [DOI] [PubMed] [Google Scholar]
- 8.Bertholet S, Debrabant A, Afrin F, Caler E, Mendez S, Tabbara KS, Belkaid Y, Sacks DL. Antigen requirements for efficient priming of CD8+ T cells by Leishmania major-infected dendritic cells. Infect Immun. 2005;73:6620–6628. doi: 10.1128/IAI.73.10.6620-6628.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudensky A, Rath S, Preston-Hurlburt P, Murphy DB, Janeway CA., Jr. On the complexity of self. Nature. 1991;353:660–662. doi: 10.1038/353660a0. [DOI] [PubMed] [Google Scholar]
- 10.Shimonkevitz R, Colon S, Kappler JW, Marrack P, Grey HM. Antigen recognition by H-2-restricted T cells. II. A tryptic ovalbumin peptide that substitutes for processed antigen. J Immunol. 1984;133:2067–2074. [PubMed] [Google Scholar]
- 11.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeKruyff RH, Fang Y, Umetsu DT. Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J Immunol. 1998;160:2231–2237. [PubMed] [Google Scholar]
- 13.Belkaid Y, Hoffmann KF, Mendez S, Kamhawi S, Udey MC, Wynn TA, Sacks DL. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J Exp Med. 2001;194:1497–1506. doi: 10.1084/jem.194.10.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 15.Suffia IJ, Reckling SK, Piccirillo CA, Goldszmid RS, Belkaid Y. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J Exp Med. 2006;203:777–788. doi: 10.1084/jem.20052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, Jenkins MK. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol. 2010;11:83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moon JJ, Dash P, Oguin TH, 3rd, McClaren JL, Chu HH, Thomas PG, Jenkins MK. Quantitative impact of thymic selection on Foxp3+ and Foxp3-subsets of self-peptide/MHC class II-specific CD4+ T cells. Proc Natl Acad Sci U S A. 2011;108:14602–14607. doi: 10.1073/pnas.1109806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 20.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 21.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 22.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4(+)CD25(-)Foxp3(-) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med. 2007;204:285–297. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLachlan JB, Catron DM, Moon JJ, Jenkins MK. Dendritic cell antigen presentation drives simultaneous cytokine production by effector and regulatory T cells in inflamed skin. Immunity. 2009;30:277–288. doi: 10.1016/j.immuni.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madan R, Demircik F, Surianarayanan S, Allen JL, Divanovic S, Trompette A, Yogev N, Gu Y, Khodoun M, Hildeman D, Boespflug N, Fogolin MB, Grobe L, Greweling M, Finkelman FD, Cardin R, Mohrs M, Muller W, Waisman A, Roers A, Karp CL. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol. 2009;183:2312–2320. doi: 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 28.Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, Jenkins MK. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 2011;35:583–595. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, Ernst JD. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med. 2008;205:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stager S, Maroof A, Zubairi S, Sanos SL, Kopf M, Kaye PM. Distinct roles for IL-6 and IL-12p40 in mediating protection against Leishmania donovani and the expansion of IL-10+ CD4+ T cells. Eur J Immunol. 2006;36:1764–1771. doi: 10.1002/eji.200635937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiley WW, Shafiani S, Wittmer ST, Tucker-Heard G, Moon JJ, Jenkins MK, Urdahl KB, Winslow GM, Woodland DL. Distinct functions of antigen-specific CD4 T cells during murine Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A. 2010;107:19408–19413. doi: 10.1073/pnas.1006298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vezys V, Masopust D, Kemball CC, Barber DL, O'Mara LA, Larsen CP, Pearson TC, Ahmed R, Lukacher AE. Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J Exp Med. 2006;203:2263–2269. doi: 10.1084/jem.20060995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gray PM, Reiner SL, Smith DF, Kaye PM, Scott P. Antigen-experienced T cells limit the priming of naive T cells during infection with Leishmania major. J Immunol. 2006;177:925–933. doi: 10.4049/jimmunol.177.2.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med. 2005;201:1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yarke CA, Dalheimer SL, Zhang N, Catron DM, Jenkins MK, Mueller DL. Proliferating CD4+ T cells undergo immediate growth arrest upon cessation of TCR signaling in vivo. J Immunol. 2008;180:156–162. doi: 10.4049/jimmunol.180.1.156. [DOI] [PubMed] [Google Scholar]
- 36.Lee SJ, McLachlan JB, Kurtz JR, Fan D, Winter SE, Baumler AJ, Jenkins MK, McSorley SJ. Temporal expression of bacterial proteins instructs host CD4 T cell expansion and Th17 development. PLoS Pathog. 2012;8:e1002499. doi: 10.1371/journal.ppat.1002499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, Sher A. Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204:273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freitas do Rosario AP, Lamb T, Spence P, Stephens R, Lang A, Roers A, Muller W, O'Garra A, Langhorne J. IL-27 promotes IL10 production by effector Th1 CD4+ T cells: a critical mechanism for protection from severe immunopathology during malaria infection. J Immunol. 2012;188:1178–1190. doi: 10.4049/jimmunol.1102755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 41.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 42.Bustamante JM, Bixby LM, Tarleton RL. Drug-induced cure drives conversion to a stable and protective CD8+ T central memory response in chronic Chagas disease. Nat Med. 2008;14:542–550. doi: 10.1038/nm1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Da-Cruz AM, Oliveira-Neto MP, Bertho AL, Mendes-Aguiar CO, Coutinho SG. T cells specific to leishmania and other nonrelated microbial antigens can migrate to human leishmaniasis skin lesions. J Invest Dermatol. 130:1329–1336. doi: 10.1038/jid.2009.428. [DOI] [PubMed] [Google Scholar]
- 44.Saraiva M, Christensen JR, Veldhoen M, Murphy TL, Murphy KM, O'Garra A. Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity. 2009;31:209–219. doi: 10.1016/j.immuni.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 46.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 47.Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, Sharpe AH, Karp CL, Miaw SC, Ho IC, Kuchroo VK. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL, Kuchroo VK. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson CF, Stumhofer JS, Hunter CA, Sacks D. IL-27 regulates IL-10 and IL-17 from CD4+ cells in nonhealing Leishmania major infection. J Immunol. 2009;183:4619–4627. doi: 10.4049/jimmunol.0804024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Debrabant A, Gottlieb M, Dwyer DM. Isolation and characterization of the gene encoding the surface membrane 3′-nucleotidase/nuclease of Leishmania donovani. Mol Biochem Parasitol. 1995;71:51–63. doi: 10.1016/0166-6851(95)00035-y. [DOI] [PubMed] [Google Scholar]
- 51.Rees W, Bender J, Teague TK, Kedl RM, Crawford F, Marrack P, Kappler J. An inverse relationship between T cell receptor affinity and antigen dose during CD4(+) T cell responses in vivo and in vitro. Proc Natl Acad Sci U S A. 1999;96:9781–9786. doi: 10.1073/pnas.96.17.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Debrabant A, Ghedin E, Dwyer DM. Dissection of the functional domains of the Leishmania surface membrane 3′-nucleotidase/nuclease, a unique member of the class I nuclease family. J Biol Chem. 2000;275:16366–16372. doi: 10.1074/jbc.M908725199. [DOI] [PubMed] [Google Scholar]
- 53.Spath GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol. 2001;99:97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- 54.Peters NC, Kimblin N, Secundino N, Kamhawi S, Lawyer P, Sacks DL. Vector transmission of leishmania abrogates vaccine-induced protective immunity. PLoSPathog. 2009;5:e1000484. doi: 10.1371/journal.ppat.1000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moon JJ, Chu HH, Hataye J, Pagan AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK. Tracking epitope-specific T cells. Nat Protoc. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]