Abstract
Cholangiocarcinomas (CCA) are heterogeneous biliary tract tumors with dismal prognosis. Perihilar cholangiocarcinoma (pCCA) involves the large bile ducts of the hepatic hilum, and is the most common type of CCA. Primary sclerosing cholangitis (PSC) is an established risk factor for pCCA. Although the diagnosis of pCCA is challenging, recent advances have been made including cytologic techniques such as fluorescence in situ hybridization. Endoscopic ultrasound with sampling of regional lymph nodes is emerging as a valuable diagnostic modality in the diagnosis and staging of pCCA. Curative treatment options are limited to early stage disease, and include surgical resection and liver transplantation after neoadjuvant therapy. This underscores the importance of early detection, and the need for development of innovative diagnostic tools such as biomarkers. A dense desmoplastic tumor stroma plays an integral role in pCCA progression. The tumor stroma represents an additional target for development of new therapies. Herein, we discuss these advances in the diagnosis and treatment of pCCA.
Keywords: Fluorescence in situ hybridization, liver transplantation, perihilar cholangiocarcinoma, primary sclerosing cholangitis, tumor microenvironment
Introduction
Cholangiocarcinomas (CCAs) are heterogeneous tumors arising from the biliary tree with features of biliary epithelial differentiation [1]. Perihilar cholangiocarcinoma (pCCA) involves the large bile ducts in the hepatic hilum, and is anatomically separated proximally from intrahepatic cholangiocarcinoma (iCCA) by the second-order biliary ducts and distally from distal cholangiocarcinoma (dCCA) by the cystic duct insertion [2]. pCCA is the most common type of cholangiocarcinoma, accounting for 50% of CCA cases with dCCA and iCCA representing approximately 40% and less than 10% of cases, respectively [3]. Epidemiologic registries have indicated a global rise in the incidence and mortality rates from iCCA and concomitant fall in pCCA + dCCA rates over the past 3 decades [4]. These trends may partly reflect a misclassification of perihilar tumors as iCCAs under the second edition of the International Classification of Diseases for Oncology [4,5].
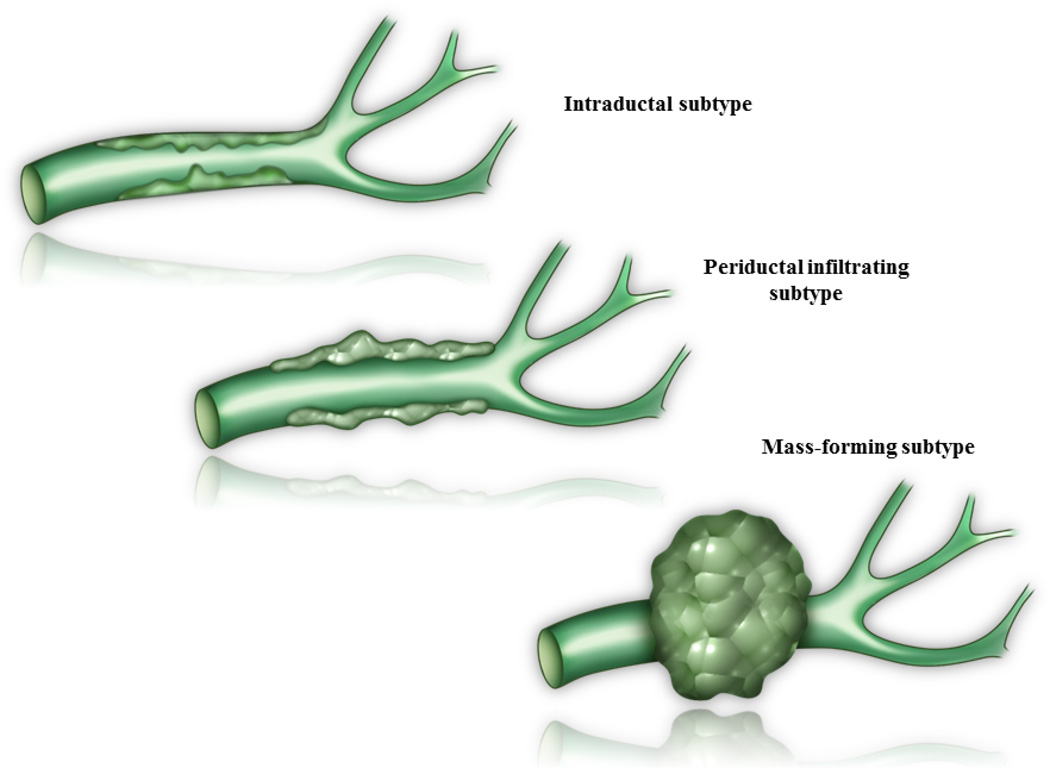
pCCAs are classified morphologically based on primary growth patterns. These include the intraductal, periductal infiltrating, and mass-forming subtypes (Figure 1) [2,6]. The periductal infiltrating subtype is the most common type of pCCA, and results in development of bile duct stricturing and obstruction. Initially, these tumors display a tropism for bile with a periductal longitudinal growth pattern. With tumor progression, radial growth away from the bile duct and resultant mass-forming lesions occur [2]. Histologically, pCCAs are typically highly desmoplastic, mucin-producing adenocarcinomas with an abundant fibrous stroma. Intraductal (polypoid) pCCAs are often well-differentiated neoplasms with a characteristic papillary growth pattern and mucin production. Intraductal tubular neoplasms, a recently described intraductal subtype, have predominantly a tubular architecture and scant mucin production [7]. These two intraductal subtypes have a more favorable prognosis than nodular or periductal infiltrating tumors.
Figure 1.
Perihilar cholangiocarcinoma (pCCA) growth patterns. The primary growth patterns in pCCA are intraductal, periductal infiltrating, and mass-forming subtypes.
In an effort to standardize pCCA reporting, a surgical staging system was recently introduced. This staging system expands on the traditional Bismuth-Corlette classification and takes into consideration bile duct involvement (common bile duct, hepatic duct confluence, right or left hepatic duct, both hepatic ducts), tumor size (<1 cm, 1–3 cm, ≥3 cm), tumor form (sclerosing, mass, mixed or both types, and polypoid), portal vein involvement, hepatic artery involvement, liver remnant volume, underlying liver disease (e.g. fibrosis, primary sclerosing cholangitis), lymph node involvement (none, hilar and/or along the hepatic artery, periaortic), and presence of metastases [8].
Primary Sclerosing Cholangitis
Primary sclerosing cholangitis (PSC) with associated chronic inflammation is a well-established risk factor for CCA, particularly pCCA. The lifetime risk of CCA development among PSC patients is 5–10% [9,10]. Approximately 50% of patients with CCA in the setting of PSC are diagnosed within 1–2 years of initial PSC diagnosis [11,12]. Thereafter, the risk is lower with CCA development in 9% of patients 10 and 20 years after PSC diagnosis [9]. A number of risk factors have been reported to confer a higher risk of CCA in PSC patients. Among these are smoking and alcohol use, older age at PSC diagnosis, long-standing associated inflammatory bowel disease, concomitant colorectal cancer/dysplasia in the setting of ulcerative colitis, proctocolectomy, variceal bleeding, hyperbilirubinemia, cholelithiasis, and NKG2D gene polymorphisms [10]. However, definitive data are lacking and further studies are needed to confirm these associations.
Clinical Features and Diagnostic Criteria
Clinical Features
pCCA involvement of the large bile ducts and ensuing cholestasis results in an earlier presentation compared to iCCA. Painless jaundice is the most frequent presentation, occurring in 90% of patients. pCCA manifests as bacterial cholangitis in 10% of patients [13]. Nonspecific symptoms such as weight loss, malaise, cachexia, and abdominal pain also occur. Vascular encasement by pCCA can lead to atrophy of the affected lobe with compensatory hypertrophy of the contralateral, unaffected lobe. This “atrophy-hypertrophy” complex can present as a palpable lobe on physical examination [13].
Laboratory and Imaging Studies
Laboratory analyses are typically not specific in pCCA as elevations of alkaline phosphatase, bilirubin, and the tumor marker carbohydrate antigen 19-9 (CA 19-9) may be a reflection of the tumor itself or the resultant obstructive cholestasis [2]. In patients with PSC, when using a cutoff value of 129 U/mL for CA 19-9, the sensitivity and specificity for CCA diagnosis is 79% and 98%, respectively [14]. Also, only two-thirds of patients with a sustained elevated CA 19-9 > 129 u/L have pCCA – one-third do not appear to develop this cancer [10]. The sensitivity is 76% in patients without PSC when using a CA 19-9 cutoff value of 100 U/mL [2]. It is important to note that 7% of the general population will have undetectable CA 19-9 levels due to absence of the Lewis antigen [10].
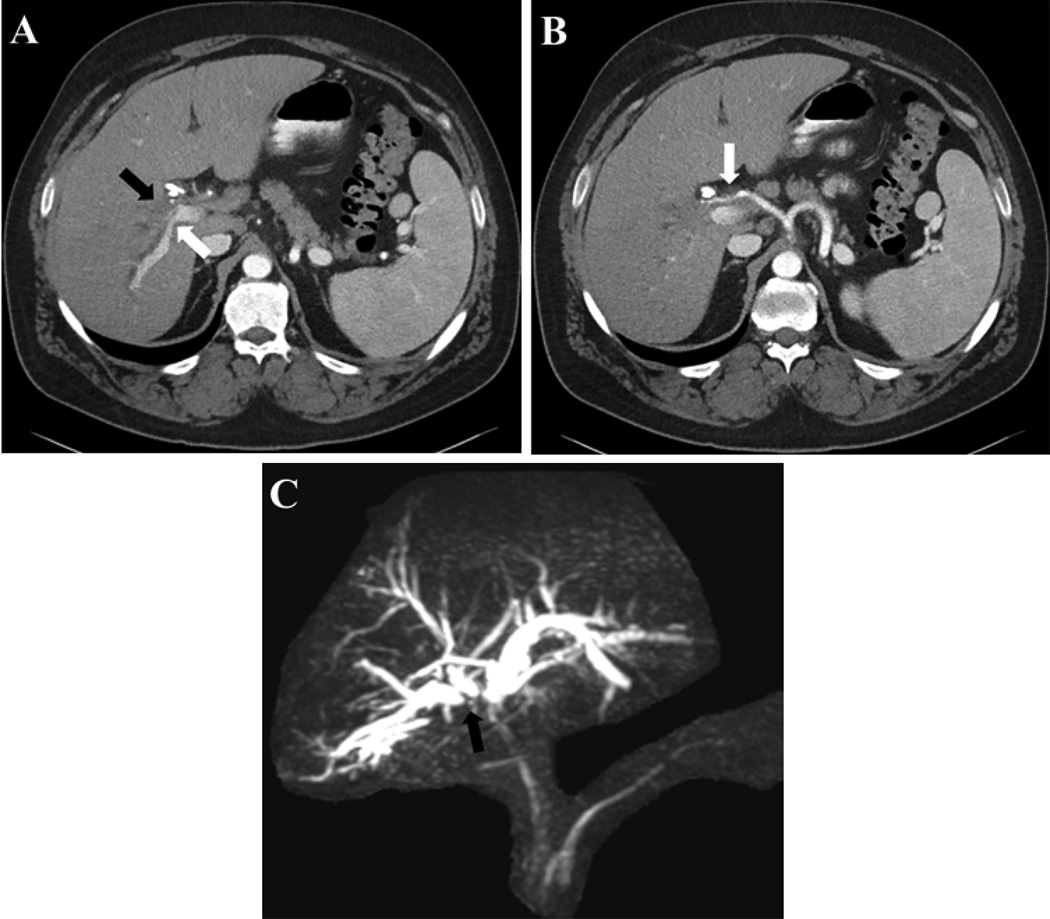
Imaging modalities utilized for pCCA diagnosis include computed tomography (CT) and magnetic resonance imaging (MRI) (Figure 2). The diagnostic accuracy of CT in evaluating biliary extent of tumor is 86% [15]. Additionally, CT has adequate accuracy for assessment of portal vein and hepatic artery involvement but is subpar in determining nodal involvement [15]. MRI, on the other hand, has a suboptimal accuracy (67–73%) in ascertaining vascular encasement [16,17]. In PSC patients, the combination of MRI and magnetic resonance cholangiopancreatography (MRCP) has a sensitivity of 89% and accuracy of 76% in detecting CCA, which is comparable to endoscopic retrograde cholangiography (ERC) with a sensitivity and accuracy 91% and 69%, respectively (Figure 2C) [18]. Positron emission tomography (PET) has insufficient diagnostic utility in pCCA; in patients with pCCA or dCCA PET scanning had a sensitivity of 55% and specificity of 33% [19]. Clinically, we evaluate all pCCA patients with an MRI/MRCP.
Figure 2.
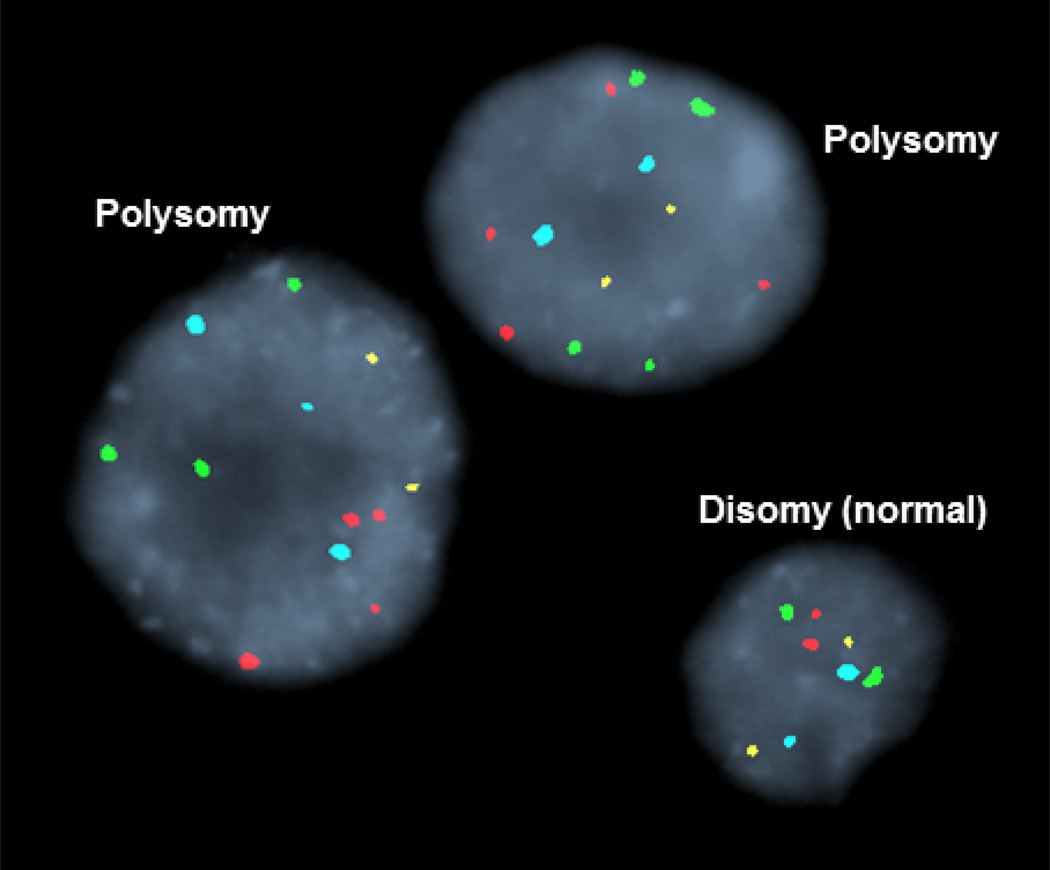
Fluorescence in site hybridization (FISH) analysis of biliary brushings. Each color signal denotes one chromosome. Normal or disomic cells have two signals of the same color. Cells with polysomy have three signals of the same color.
Endoscopic ultrasound
Endoscopic ultrasound (EUS) is emerging as a valuable diagnostic modality for the diagnosis and staging of cholangiocarcinoma, particularly in the evaluation of unresectable perihilar cholangiocarcinoma for liver transplantation [20]. EUS has a tumor detection rate of 94% in CCA patients, albeit it has a significantly higher detection rate for distal tumors (100%) compared to proximal tumors (83%) [21]. Fine-needle aspiration (FNA) of the primary tumor has a diagnostic sensitivity of 73% when the presence of malignancy is indicated by ‘positive’ as well as ‘suspicious’ cytologic results [21]. EUS-FNA sensitivity is greater in dCCA compared to pCCA (81% vs. 59%) [21]. EUS-FNA does carry a significant risk of tumor seeding. This risk was assessed in a study of 191 patients with locally unresectable CCA being evaluated for liver transplantation [22]. Of these, 16 patients underwent transperitoneal FNA, and malignancy was confirmed in six. Peritoneal metastasis was detected in five of these six patients (83%) compared to zero of nine patients without malignancy (0%) [22]. Furthermore, peritoneal metastases were noted in only 8% (14 of 175) of patients who did not undergo transperitoneal FNA. Although obtaining a tissue diagnosis remains challenging, the risk of tumor seeding is significant and should deter from performing EUS-FNA of primary tumor. At our center, this is an absolute contraindication to liver transplantation [20]. Lymph node involvement by CCA portends a poor outcome, as locoregional nodal metastasis precludes liver transplantation and distant nodal involvement is a contraindication for both liver transplantation and curative resection [20]. Accordingly, assessment of nodal metastasis is essential in CCA staging. The value of EUS in staging was highlighted in a study of 47 potential liver transplant candidates with unresectable perihilar CCA. Regional lymph nodes were identified in all patients and EUS guided fine-needle aspiration (FNA) detected the presence of malignant lymph nodes in eight patients. In contrast, CT and/or MRI identified malignant lymph nodes in only two of these eight patients. Furthermore, staging exploratory laparotomy confirmed that 91% of lymph nodes identified as benign by EUS-FNA were in fact benign. The study also found that EUS imaging features of lymph nodes had a poor predictive value for distinguishing benign from malignant nodes. This underscores the utility of sampling visualized lymph nodes regardless of their morphological features [23].
ERC and Conventional Cytology
ERC is essential in the evaluation and management of pCCA as it delineates the biliary anatomy, allows for acquisition of biliary brushings for subsequent cytologic evaluation, and serves as a therapeutic tool for dilation and stenting of biliary strictures. ERC detection of a dominant stricture with or without associated proximal biliary ductal dilatation, or the presence of a polypoid lesion is indicative of the presence of pCCA. These findings should prompt further cytological evaluation via biliary brushings. Cytological assessment includes conventional cytology and fluorescence in situ hybridization (FISH). The potential findings on conventional cytology include negative, atypical, suspicious, or diagnostic of malignancy. Conventional cytology is fraught with sampling errors, paucicellular or acellular specimens, and pathologic misinterpretations secondary to subtle differences between benign and malignant cells. Moreover, the presence of inflammation secondary to PSC or associated infection can pose a dilemma on cytologic evaluation as reactive cells can mimic cancer cells [24]. Consequently, conventional cytology has a low sensitivity; 15% when only positive for malignancy results are used and 38% if positive and suspicious for malignancy results are used [24].
Fluorescence in situ hybridization
Chromosomal instability, a hallmark of cancer, results in structural chromosomal abnormalities and aneuploidy or abnormalities in the number of chromosomes within a cell. FISH employs fluorescently labeled DNA probes to identify chromosomal aberrations in cells. FISH utilizing directly labeled DNA probes to the pericentromeric regions of chromosome 3, 7, 17, and the chromosomal locus 9p21 has an increased sensitivity (34% to 58%) and preserved specificity for detection of FISH polysomy (chromosomal amplification) compared to routine cytology [25–27]. FISH analyses which include probes to detect deletion of 9p21 increased diagnostic yield to 93% [25]. FISH results can be categorized as follows: negative or disomy, trisomy 7 (≥10 cells with 3 copies of chromosome 7 and two or fewer copies of the other 3 probes), polysomy (≥5 cells with ≥3 signals in 2 or more of the 4 probes (Figure 3) [27]. High mitotic rates during the M phase of mitosis can yield tetrasomy and thus this finding should be interpreted with caution [2]. Trisomy 7 is frequently detected in the setting of inflammation of the biliary tree. Patient with FISH trisomy or tetrasomy have outcomes similar to those with negative FISH analysis [28]. FISH polysomy in the setting of a dominant stricture is strongly suggestive of the presence of pCCA [1]. The occurrence of FISH polysomy in the absence of other corroborative evidence of malignancy poses a diagnostic challenge. PSC patients with FISH polysomy in the absence of other diagnostic features had a significantly higher risk of CCA development if polysomy was confirmed on subsequent FISH analysis compared to patients with non-serial polysomy (69% vs. 18%) [29]. In the majority of individuals with serial FISH polysomy, a diagnosis of CCA was attained within 6 months of the initial detection of polysomy, although in 4 patients CCA was not diagnosed until 1–2.7 years after initial polysomy result. Hence, patients with polysomy should have close and prolonged surveillance.
Figure 3.
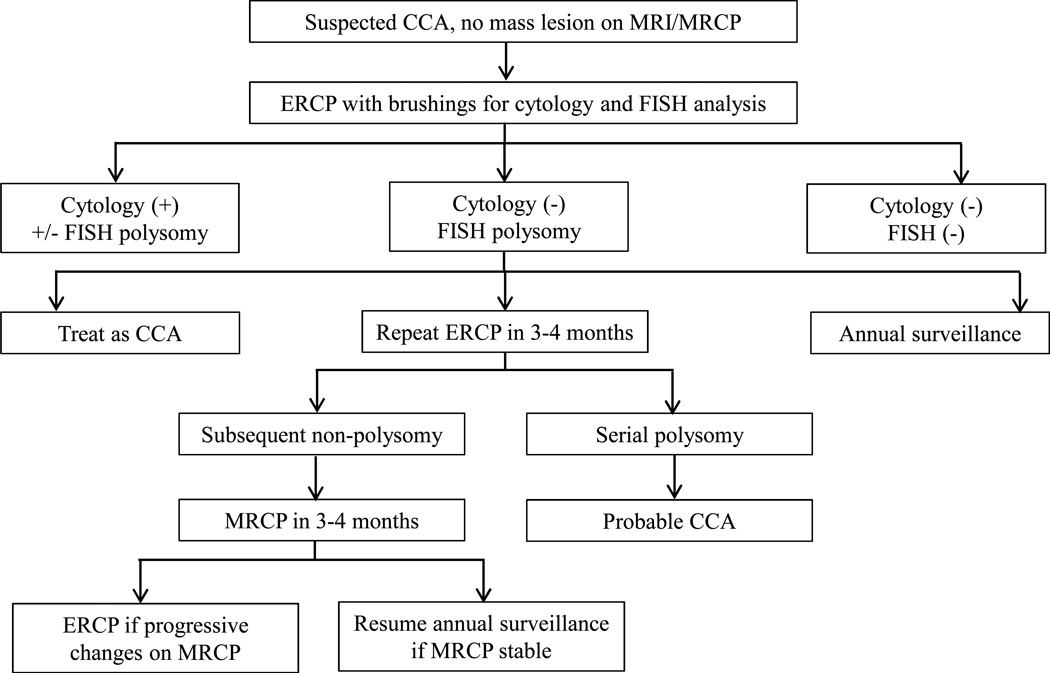
Approach to management of abnormal FISH and cytology results.
In the absence of imaging criteria for pCCA, equivocal (atypical or suspicious) cytology is another challenging clinical scenario. In a study of 102 PSC patients with equivocal cytology and no baseline imaging features of CCA, 42% of patients with suspicious cytology were ultimately diagnosed with CCA compared to 23% with atypical cytology [30]. Moreover, FISH polysomy in conjunction with equivocal cytology had a positive predictive value of 76%. Interestingly, all patients with FISH polysomy and CA 19-9 levels ≥ 129 U/mL had eventual CCA development (90% within the first 2 years).
In summary, conventional cytology positive for malignancy is diagnostic of pCCA. Likewise, the following scenarios are highly indicative of a pCCA diagnosis: presence of FISH polysomy with a CA 19-9 ≥ 129 U/mL, FISH polysomy in conjunction with suspicious cytology, or FISH polysomy with a dominant stricture on imaging. In contrast, pCCA is unlikely in the setting of FISH trisomy 7 or tetrasomy (Figure 4).
Figure 4.
MRI images of pCCA mass with (A) Occlusion of the right anterior portal vein (black arrow) and narrowing of right posterior portal vein (white arrow), (B) right hepatic artery encasement, (C) separation of the right anterior and right posterior bile ducts (black arrow).
Treatment
Surgical Resection
Curative resection for pCCA remains an arduous task with five year survival rates of 11–41% [31]. The surgery is complex and entails lobar or extended lobar hepatic and bile duct resection, regional lymphadenectomy, and Roux-en-Y hepaticojejunostomy. Exclusion criteria for resection of pCCA includes bilateral involvement of the second order bile ducts, bilateral or contralateral hepatic artery or portal vein encasement, intra- or extra-hepatic metastasis, and distant lymph node metastasis. Regional lymphadenopathy (cystic, portal, hepatic arterial, pericholedochal, and posterior pancreaticoduodenal nodes) does not necessarily preclude resection, albeit patient outcomes are less favorable in this scenario [31]. pCCA occurring in the setting of PSC is also deemed unresectable owing to the propensity for skip lesions, the field defect, and underlying parenchymal liver disease [1]. Portal vein embolization is employed in instances when a tumor is potentially resectable but the remnant lobe has limited volume. In this technique, embolization of the portal vein in the affected lobe is carried out, and this stimulates hypertrophy of the contralateral unaffected lobe [31]. Approximately one-third of patients undergoing portal vein embolization may not have adequate hypertrophy of the remnant lobe and thus are unable to undergo a resection. Recently, the associating liver partition and portal vein ligation for staged hepatectomy or ALPPS procedure has been described as another manner of inducing increase in the remnant lobe volume [32]. Portal vein ligation is combined with parenchymal transection along the falciform ligament in this procedure. Isolated parenchymal transection following failed portal vein embolization has also been reported [33].
Neoadjuvant Chemoradiation and Liver Transplantation
Orthotopic liver transplantation alone as a potentially curative treatment for CCA, albeit a promising endeavor, has been a futile practice. Reported five-year survival rates for liver transplantation for this indication have been 20–30%, with recurrence rates of 53–84% [34,35]. With the high recurrence rates, pCCA became a contraindication to orthotopic liver transplantation.
Reports of palliative efficacy of radiotherapy provided grounds for the University of Nebraska transplant team to pioneer an approach utilizing high-dose neoadjuvant brachytherapy and 5-fluorouracil (5-FU) followed by liver transplantation [35]. Subsequently, the Mayo Clinic developed a protocol combining radiosensitizing chemotherapy with 5-FU, external beam radiation therapy, brachytherapy with endoscopically placed iridium-192 beads, maintenance chemotherapy with capecitabine, staging laparotomy to assess for presence of metastasis, followed by orthotopic liver transplantation [34]. This protocol employs rigorous selection of early stage pCCA patients, with the following inclusion criteria: confirmed diagnosis of pCCA, radial tumor diameter less than 3 cm, absence of intra- or extrahepatic metastasis, unresectability in non-PSC patient, and pCCA in a PSC patient [1]. Exclusion criteria include transperitoneal tumor biopsy, prior radiation or attempted resection with disruption of the bile ducts, and uncontrolled infection [36]. With reports of survival rates approaching 70%, the United Network of Organ Sharing approved prioritization of pCCA by allocation of a model of end-stage liver disease (MELD) exception score in 2009 [34]. This exception score increases every 3 months, reflecting the 10% expected waitlist mortality. To test the appropriateness of this allocation, a large study analyzing data from 12 high-volume transplant centers was conducted [34]. pCCA patients treated with neoadjuvant therapy followed by liver transplantation had intent-to-treat two-year and five-year survival rates of 68% and 53%, respectively. Similarly, two-year and five-year recurrence free survival rates were 78% and 65%, respectively. Dropout rate prior to liver transplantation was 11.5% after 3.5 months of therapy [34]. Outcomes were significantly worse in patients with prior transperitoneal tumor biopsy, metastatic disease, and tumor mass > 3 cm. This study confirmed not only the excellent clinical outcomes for neoadjuvant chemoradiation followed by liver transplantation and appropriateness of the MELD allocation system for pCCA but also validated the stringent selection criteria for this protocol.
Predictors of pretransplant dropout and posttranplant recurrence were assessed in a recent study of 199 patients enrolled in the neoadjuvant chemoradiation protocol in anticipation of liver transplantation [37]. The pretransplant dropout rate was 31%, and predictors included CA 19-9 levels ≥ 500 U/mL, radial diameter of tumor mass ≥ 3 cm, brushing or biopsy result positive for malignancy, and MELD score ≥ 20. Five-year posttranplant recurrence free survival was 68%, and predictors of recurrence included elevated CA 19-9, portal vein encasement, and residual tumor on explant, with the latter being the most strongly associated (hazard ratio 9.8). Pretreatment pathological confirmation of pCCA by conventional cytology or endoscopic biopsy is not a requisite as it does not result in lower rates of residual CCA in explants or lower recurrence after transplantation [38]. Despite its rigor, patients who underwent the neoadjuvant chemoradiation followed by liver transplantation protocol for pCCA reported excellent quality of life, at par or better than those who underwent liver transplantation for other indications [39].
Treatment of Advanced pCCA
Systemic chemotherapy is the mainstay of treatment for patients who are not candidates for curative resection or neoadjuvant therapy followed by liver transplantation. The combination of gemcitabine and cisplatin is the preferred regimen in this setting. Compared to gemcitabine alone, the addition of cisplatin confers a survival advantage with median progression free survival of eight months without a significant increase in toxicity [40]. Relief of biliary obstruction of jaundice is advocated to enhance patients’ tolerability to chemotherapy. An optimal bilirubin level following endoscopic intervention with stenting is ≤ 2 mg/dL. In patients who fail to reach this level following stenting, repeat biliary intervention should be considered in 6 weeks if their pre-stent bilirubin level was ≥ 10 mg/dL or 3 weeks if the present bilirubin level was < 10 mg/dL [41]. Metal stents are a more durable and cost-effective option as they have a superior patency to plastic stents (5.56 months versus 1.86 months) with patients requiring less frequent procedures [42]. Accordingly, if a patient is not a candidate for curative surgical treatment and has a life expectancy of at least 4–6 months, then metal stents should be utilized [42].
Future Directions
Despite recent advances, diagnosis of pCCA at a stage early enough to warrant eligibility for curative treatment options remains a significant challenge. Survival rates for this highly lethal malignancy remain low, as treatment options are limited, particularly for inoperable tumors. The only effective treatment options are surgical resection and combined neoadjuvant therapy with liver transplantation for the subset of patients with early stage pCCA. Advances in diagnosis enabling early detection of pCCA are needed. Utilization of a stool assay for methylated genes and mutant Kirsten rat sarcoma viral oncogene homolog (KRAS) to diagnose pancreatic cancer was recently reported [43]. pCCA is known to have aberrant KRAS expression [44]. Hence, one can envision detection of CCA genetic signatures, such as mutant KRAS, in biological specimens including bile, serum, or stool.
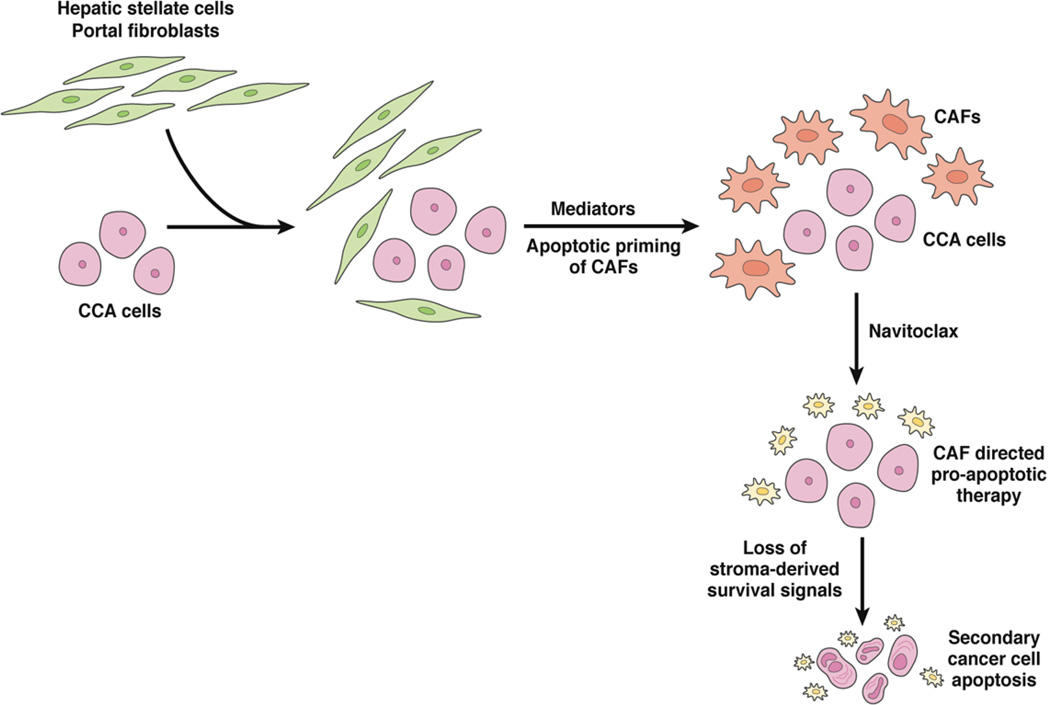
Emerging evidence has highlighted the critical role of the tumor microenvironment in tumor progression, metastases, and therapeutic resistance [45]. An intense tumoral desmoplastic stroma with a striking accumulation of α-smooth muscle actin expressing cancer-associated fibroblasts (CAF) is a characteristic feature of pCCA. To date, therapeutic efforts have been directed at the cancer cell with limited consideration of the tumor stroma. Stromal depletion via selective targeting of CAF utilizing a BH3 mimetic has been reported recently [46]. Combining cancer cell directed therapy with stromal CAF deletion represents a novel therapeutic approach in pCCA (Figure 5).
Figure 5.
Therapeutic Deletion of CAF in CCA. In the course of tumorigenesis, stromal fibroblasts acquire a modified phenotype and become activated or primed for apoptosis. Navitoclax, a BH-3 mimetic, causes apoptotic cell death in activated CAFs [46]. This targeted deletion of CAFs leads to secondary cancer cell apoptosis and reduction in tumor size.
Acknowledgements
The authors would like to thank Ms. Courtney Hoover for outstanding secretarial support.
This work was supported by National Institutes of Health grants DK59427 (G.J.G.) and T32DK007198 (S.R.), and the Mayo Foundation.
Abbreviations
- 5-FU
5-fluorouracil
- CA 19-9
carbohydrate antigen 19-9
- CAF
cancer-associated fibroblast
- CCA
cholangiocarcinoma
- CT
computed tomography
- dCCA
distal cholangiocarcinoma
- ERC
endoscopic retrograde cholangiography
- EUS
endoscopic ultrasound
- FISH
fluorescence in situ hybridization
- FNA
fine-needle aspiration
- KRAS
Kirsten rat sarcoma viral oncogene homolog
- MELD
model of end-stage liver disease
- MRCP
magnetic resonance cholangiopancreatography
- MRI
magnetic resonance imaging
- pCCA
perihilar cholangiocarcinoma
- PSC
primary sclerosing cholangitis
Footnotes
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215–1229. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nature reviews Gastroenterology & hepatology. 2011;8:512–522. doi: 10.1038/nrgastro.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ, Schulick RD. Cholangiocarcinoma: Thirty-one-year experience with 564 patients at a single institution. Annals of surgery. 2007;245:755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan SA, Emadossadaty S, Ladep NG, Thomas HC, Elliott P, Taylor-Robinson SD, Toledano MB. Rising trends in cholangiocarcinoma: Is the icd classification system misleading us? Journal of hepatology. 2012;56:848–854. doi: 10.1016/j.jhep.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Welzel TM, McGlynn KA, Hsing AW, O'Brien TR, Pfeiffer RM. Impact of classification of hilar cholangiocarcinomas (klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the united states. Journal of the National Cancer Institute. 2006;98:873–875. doi: 10.1093/jnci/djj234. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita Y, Takahashi M, Kanazawa S, Charnsangavej C, Wallace S. Hilar cholangiocarcinoma. An evaluation of subtypes with ct and angiography. Acta radiologica. 1992;33:351–355. [PubMed] [Google Scholar]
- 7.Katabi N, Torres J, Klimstra DS. Intraductal tubular neoplasms of the bile ducts. The American journal of surgical pathology. 2012;36:1647–1655. doi: 10.1097/PAS.0b013e3182684d4f. [DOI] [PubMed] [Google Scholar]
- 8.Deoliveira ML, Schulick RD, Nimura Y, Rosen C, Gores G, Neuhaus P, Clavien PA. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology. 2011;53:1363–1371. doi: 10.1002/hep.24227. [DOI] [PubMed] [Google Scholar]
- 9.Claessen MM, Vleggaar FP, Tytgat KM, Siersema PD, van Buuren HR. High lifetime risk of cancer in primary sclerosing cholangitis. Journal of hepatology. 2009;50:158–164. doi: 10.1016/j.jhep.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Razumilava N, Gores GJ, Lindor KD. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology. 2011;54:1842–1852. doi: 10.1002/hep.24570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boberg KM, Bergquist A, Mitchell S, Pares A, Rosina F, Broome U, Chapman R, Fausa O, Egeland T, Rocca G, Schrumpf E. Cholangiocarcinoma in primary sclerosing cholangitis: Risk factors and clinical presentation. Scandinavian journal of gastroenterology. 2002;37:1205–1211. doi: 10.1080/003655202760373434. [DOI] [PubMed] [Google Scholar]
- 12.Chapman MH, Webster GJ, Bannoo S, Johnson GJ, Wittmann J, Pereira SP. Cholangiocarcinoma and dominant strictures in patients with primary sclerosing cholangitis: A 25-year single-centre experience. European journal of gastroenterology & hepatology. 2012;24:1051–1058. doi: 10.1097/MEG.0b013e3283554bbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blechacz B, Gores GJ. Cholangiocarcinoma: Advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48:308–321. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD. The value of serum ca 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Digestive diseases and sciences. 2005;50:1734–1740. doi: 10.1007/s10620-005-2927-8. [DOI] [PubMed] [Google Scholar]
- 15.Ruys AT, van Beem BE, Engelbrecht MR, Bipat S, Stoker J, Van Gulik TM. Radiological staging in patients with hilar cholangiocarcinoma: A systematic review and meta-analysis. The British journal of radiology. 2012;85:1255–1262. doi: 10.1259/bjr/88405305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manfredi R, Barbaro B, Masselli G, Vecchioli A, Marano P. Magnetic resonance imaging of cholangiocarcinoma. Seminars in liver disease. 2004;24:155–164. doi: 10.1055/s-2004-828892. [DOI] [PubMed] [Google Scholar]
- 17.Masselli G, Manfredi R, Vecchioli A, Gualdi G. Mr imaging and mr cholangiopancreatography in the preoperative evaluation of hilar cholangiocarcinoma: Correlation with surgical and pathologic findings. European radiology. 2008;18:2213–2221. doi: 10.1007/s00330-008-1004-z. [DOI] [PubMed] [Google Scholar]
- 18.Charatcharoenwitthaya P, Enders FB, Halling KC, Lindor KD. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008;48:1106–1117. doi: 10.1002/hep.22441. [DOI] [PubMed] [Google Scholar]
- 19.Petrowsky H, Wildbrett P, Husarik DB, Hany TF, Tam S, Jochum W, Clavien PA. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. Journal of hepatology. 2006;45:43–50. doi: 10.1016/j.jhep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Levy MJ, Heimbach JK, Gores GJ. Endoscopic ultrasound staging of cholangiocarcinoma. Current opinion in gastroenterology. 2012;28:244–252. doi: 10.1097/MOG.0b013e32835005bc. [DOI] [PubMed] [Google Scholar]
- 21.Mohamadnejad M, DeWitt JM, Sherman S, LeBlanc JK, Pitt HA, House MG, Jones KJ, Fogel EL, McHenry L, Watkins JL, Cote GA, Lehman GA, Al-Haddad MA. Role of eus for preoperative evaluation of cholangiocarcinoma: A large single-center experience. Gastrointestinal endoscopy. 2011;73:71–78. doi: 10.1016/j.gie.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 22.Heimbach JK, Sanchez W, Rosen CB, Gores GJ. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2011;13:356–360. doi: 10.1111/j.1477-2574.2011.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gleeson FC, Rajan E, Levy MJ, Clain JE, Topazian MD, Harewood GC, Papachristou GI, Takahashi N, Rosen CB, Gores GJ. Eus-guided fna of regional lymph nodes in patients with unresectable hilar cholangiocarcinoma. Gastrointestinal endoscopy. 2008;67:438–443. doi: 10.1016/j.gie.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Barr Fritcher EG, Kipp BR, Slezak JM, Moreno-Luna LE, Gores GJ, Levy MJ, Roberts LR, Halling KC, Sebo TJ. Correlating routine cytology, quantitative nuclear morphometry by digital image analysis, and genetic alterations by fluorescence in situ hybridization to assess the sensitivity of cytology for detecting pancreatobiliary tract malignancy. American journal of clinical pathology. 2007;128:272–279. doi: 10.1309/BC6DY755Q3T5W9EE. [DOI] [PubMed] [Google Scholar]
- 25.Gonda TA, Glick MP, Sethi A, Poneros JM, Palmas W, Iqbal S, Gonzalez S, Nandula SV, Emond JC, Brown RS, Murty VV, Stevens PD. Polysomy and p16 deletion by fluorescence in situ hybridization in the diagnosis of indeterminate biliary strictures. Gastrointestinal endoscopy. 2012;75:74–79. doi: 10.1016/j.gie.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 26.Kipp BR, Stadheim LM, Halling SA, Pochron NL, Harmsen S, Nagorney DM, Sebo TJ, Therneau TM, Gores GJ, de Groen PC, Baron TH, Levy MJ, Halling KC, Roberts LR. A comparison of routine cytology and fluorescence in situ hybridization for the detection of malignant bile duct strictures. The American journal of gastroenterology. 2004;99:1675–1681. doi: 10.1111/j.1572-0241.2004.30281.x. [DOI] [PubMed] [Google Scholar]
- 27.Moreno Luna LE, Kipp B, Halling KC, Sebo TJ, Kremers WK, Roberts LR, Barr Fritcher EG, Levy MJ, Gores GJ. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology. 2006;131:1064–1072. doi: 10.1053/j.gastro.2006.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bangarulingam SY, Bjornsson E, Enders F, Barr Fritcher EG, Gores G, Halling KC, Lindor KD. Long-term outcomes of positive fluorescence in situ hybridization tests in primary sclerosing cholangitis. Hepatology. 2010;51:174–180. doi: 10.1002/hep.23277. [DOI] [PubMed] [Google Scholar]
- 29.Barr Fritcher EG, Kipp BR, Voss JS, Clayton AC, Lindor KD, Halling KC, Gores GJ. Primary sclerosing cholangitis patients with serial polysomy fluorescence in situ hybridization results are at increased risk of cholangiocarcinoma. The American journal of gastroenterology. 2011;106:2023–2028. doi: 10.1038/ajg.2011.272. [DOI] [PubMed] [Google Scholar]
- 30.Barr Fritcher EG, Voss JS, Jenkins SM, Lingineni RK, Clayton AC, Roberts LR, Halling KC, Talwalkar JA, Gores GJ, Kipp BR. Primary sclerosing cholangitis with equivocal cytology: Fluorescence in situ hybridization and serum ca 19-9 predict risk of malignancy. Cancer cytopathology. 2013;121:708–717. doi: 10.1002/cncy.21331. [DOI] [PubMed] [Google Scholar]
- 31.Nagorney DM, Kendrick ML. Hepatic resection in the treatment of hilar cholangiocarcinoma. Advances in surgery. 2006;40:159–171. doi: 10.1016/j.yasu.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Horbelt R, Kroemer A, Loss M, Rummele P, Scherer MN, Padberg W, Konigsrainer A, Lang H, Obed A, Schlitt HJ. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Annals of surgery. 2012;255:405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 33.Tschuor C, Croome KP, Sergeant G, Cano V, Schadde E, Ardiles V, Slankamenac K, Claria RS, de Santibanes E, Hernandez-Alejandro R, Clavien PA. Salvage parenchymal liver transection for patients with insufficient volume increase after portal vein occlusion -- an extension of the alpps approach. Eur J Surg Oncol. 2013;39:1230–1235. doi: 10.1016/j.ejso.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Darwish Murad S, Kim WR, Harnois DM, Douglas DD, Burton J, Kulik LM, Botha JF, Mezrich JD, Chapman WC, Schwartz JJ, Hong JC, Emond JC, Jeon H, Rosen CB, Gores GJ, Heimbach JK. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 us centers. Gastroenterology. 2012;143:88–98. doi: 10.1053/j.gastro.2012.04.008. e83; quiz e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosen CB, Heimbach JK, Gores GJ. Surgery for cholangiocarcinoma: The role of liver transplantation. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2008;10:186–189. doi: 10.1080/13651820801992542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gores GJ, Darwish Murad S, Heimbach JK, Rosen CB. Liver transplantation for perihilar cholangiocarcinoma. Digestive diseases. 2013;31:126–129. doi: 10.1159/000347207. [DOI] [PubMed] [Google Scholar]
- 37.Darwish Murad S, Kim WR, Therneau T, Gores GJ, Rosen CB, Martenson JA, Alberts SR, Heimbach JK. Predictors of pretransplant dropout and posttransplant recurrence in patients with perihilar cholangiocarcinoma. Hepatology. 2012;56:972–981. doi: 10.1002/hep.25629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosen CB, Darwish Murad S, Heimbach JK, Nyberg SL, Nagorney DM, Gores GJ. Neoadjuvant therapy and liver transplantation for hilar cholangiocarcinoma: Is pretreatment pathological confirmation of diagnosis necessary? Journal of the American College of Surgeons. 2012;215:31–38. doi: 10.1016/j.jamcollsurg.2012.03.014. discussion 38–40. [DOI] [PubMed] [Google Scholar]
- 39.Darwish Murad S, Heimbach JK, Gores GJ, Rosen CB, Benson JT, Kim WR. Excellent quality of life after liver transplantation for patients with perihilar cholangiocarcinoma who have undergone neoadjuvant chemoradiation. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2013;19:521–528. doi: 10.1002/lt.23630. [DOI] [PubMed] [Google Scholar]
- 40.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J Investigators ABCT. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. The New England journal of medicine. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 41.Weston BR, Ross WA, Wolff RA, Evans D, Lee JE, Wang X, Xiao LC, Lee JH. Rate of bilirubin regression after stenting in malignant biliary obstruction for the initiation of chemotherapy: How soon should we repeat endoscopic retrograde cholangiopancreatography? Cancer. 2008;112:2417–2423. doi: 10.1002/cncr.23454. [DOI] [PubMed] [Google Scholar]
- 42.Raju RP, Jaganmohan SR, Ross WA, Davila ML, Javle M, Raju GS, Lee JH. Optimum palliation of inoperable hilar cholangiocarcinoma: Comparative assessment of the efficacy of plastic and self-expanding metal stents. Digestive diseases and sciences. 2011;56:1557–1564. doi: 10.1007/s10620-010-1550-5. [DOI] [PubMed] [Google Scholar]
- 43.Kisiel JB, Yab TC, Taylor WR, Chari ST, Petersen GM, Mahoney DW, Ahlquist DA. Stool DNA testing for the detection of pancreatic cancer: Assessment of methylation marker candidates. Cancer. 2012;118:2623–2631. doi: 10.1002/cncr.26558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersen JB, Spee B, Blechacz BR, Avital I, Komuta M, Barbour A, Conner EA, Gillen MC, Roskams T, Roberts LR, Factor VM, Thorgeirsson SS. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142:1021–1031. e1015. doi: 10.1053/j.gastro.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sirica AE, Gores GJ. Desmoplastic stroma and cholangiocarcinoma: Clinical implications and therapeutic targeting. Hepatology. 2013 doi: 10.1002/hep.26762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mertens JC, Fingas CD, Christensen JD, Smoot RL, Bronk SF, Werneburg NW, Gustafson MP, Dietz AB, Roberts LR, Sirica AE, Gores GJ. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer research. 2013;73:897–907. doi: 10.1158/0008-5472.CAN-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]