Abstract
Objective:
Treatment-resistant depression (TRD) represents a considerable global health concern. The goal of the InSight study was to investigate the prevalence of TRD and to evaluate its clinical characterization and management, compared with nonresistant depression, in primary care centres.
Methods:
Physicians completed a case report on a consecutive series of patients with major depressive disorder (n = 1212), which captured patient demographics and comorbidity, as well as current and past medication.
Results:
Using failure to respond to at least 2 antidepressants (ADs) from different classes as the definition of TRD, the overall prevalence was 21.7%. There were no differences in prevalence between men and women or among ethnicities. Patients with TRD had longer episode duration, were more likely to receive polypharmacy (for example, psychotropic, lipid-lowering, and antiinflammatory agents), and reported more AD related side effects. Higher rates of disability and comorbidity (axes I to III) were associated with treatment resistance. Obesity and being overweight were also associated with treatment resistance. While the selection and sequencing of pharmacotherapy by family physicians in this sample was in line with recommendations from evidence-based treatment guidelines, the wait time to make a change in treatment was 6 to 8 weeks in both groups, which exceeds guideline recommendations.
Conclusions:
These real-world data demonstrate the high prevalence of TRD in primary care settings, and underscore the substantial burden of illness associated with TRD.
Keywords: treatment-resistant depression, prevalence, risk factors, primary care
Abstract
Objectif :
La dépression réfractaire au traitement (DRT) représente un problème de santé considérable sur le plan mondial. Le but de l’étude InSight était de rechercher la prévalence de la DRT et d’en évaluer la caractérisation et la gestion cliniques, comparativement à la dépression non réfractaire, dans les centres de soins primaires.
Méthodes :
Les médecins ont procédé à une étude de cas sur une série consécutive de patients souffrant de trouble dépressif majeur (n = 1212), qui comprenait les données démographiques et les comorbidités des patients, ainsi que leurs médicaments présents et passés.
Résultats :
En utilisant la non réponse à au moins 2 antidépresseurs (AD) de différentes classes comme définition de la DRT, la prévalence globale était de 21,7 %. Il n’y avait pas de différence de prévalence entre hommes et femmes ou entre groupes ethniques. Les patients souffrant de DRT avaient des épisodes de plus longue durée, étaient plus susceptibles de recevoir une polypharmacie (par exemple, des agents psychotropes, des hypolipidémiants, et antiinflammatoires), et déclaraient plus d’effets secondaires liés aux AD. Des taux élevés d’invalidité et de comorbidité (axes I à III) étaient associés à la résistance au traitement. L’obésité et l’embonpoint étaient aussi associés à la résistance au traitement. Même si la sélection et la séquence de la pharmacothérapie par les médecins de famille dans cet échantillon étaient conformes aux recommandations des lignes directrices basées sur les données probantes du traitement, le temps d’attente pour effectuer un changement de traitement était de 6 à 8 semaines dans les 2 groupes, ce qui excède les recommandations des lignes directrices.
Conclusions :
Ces données en milieu réel démontrent la prévalence élevée de la DRT dans les soins de première ligne, et soulignent le fardeau substantiel de la maladie associé à la DRT.
Major depressive disorder is a disabling condition that results in significant economic and social burden.1–3 Much of this burden can be attributed to TRD,4 which is associated with a 40% to 50% increase in direct and indirect medical care costs, compared with nonresistant depression.5,6
Although there is no consensus on the definition of TRD, failure to respond to 2 or more adequate trials from different classes of ADs is the minimum requirement.7,8 The difficulty in defining TRD partly reflects the difficulty in obtaining an accurate medication history (adequacy of dose and duration for each trial), as well as incorporating new treatment strategies in the definition, such as augmentation and combination treatments. In the large STAR*D trial, the estimate of TRD, based on a failed response to at least 2 ADs, was about 30%.9 As these patients were recruited for treatment from primary care and psychiatric clinics, it is unclear whether this accurately reflects prevalence rates in the community. To our knowledge, no study has been reported that primarily evaluates the prevalence, determinants, and associated features of TRD within general practice.
By its very nature, TRD represents a chronic and complex illness that requires long-term management from health care professionals, usually in the form of multiple medications.10 This requires knowledge of adequate medication dose and duration, as well as potential drug interactions. Atypical APs are increasingly used as augmentation agents, owing to demonstrated efficacy in TRD samples.11–14 Other strategies for TRD include AD combination trials,10 as well as approved and experimental neurostimulation therapies, including electroconvulsive therapy, vagus nerve stimulation, repetitive transcranial magnetic stimulation, and deep brain stimulation.15
To date, few studies have examined the clinical and neurobiological differences between nonresistant and resistant MDD,16 although risk factors for resistance, including high recurrence rates (up to 80%),17 failure of initial AD, psychiatric comorbidity,18 undetected hypomanic symptoms,9 increased cardiac morbidity,19 and mortality,17 have been identified. There are also preliminary data from small studies to suggest TRD is associated with biological determinants, including hyperactivity in the anterior cingulate cortex, striatum and amygdala,20 altered neurotransmitter levels,21,22 and genetic polymorphisms.23–25
Clinical Implications
The high level of TRD in primary care highlights the necessity to provide ongoing psychiatric education for physicians.
Treatment of TRD should also address medical comorbidities, including chronic pain and obesity, which could exacerbate depressive symptoms.
Standardized assessments should be incorporated into the clinical interview to more objectively assess AD outcome.
Limitations
The sample was not selected at random.
AD compliance was based on patient self-report.
The retrospective design precluded longitudinal follow-up.
The large population of patients with MDD seen in primary care provides an opportunity to estimate TRD prevalence and its associated characteristics. While it may be assumed that the presentation of MDD in primary care clinics is not as severe or chronic, several investigations have reported a lack of demographic or symptom differences between primary care and tertiary samples of MDD.26,27 Further, studies28–31 have shown that at least 10% of primary care visits are related to depression: 1 group demonstrated that during the year of an index episode of depression, 90% of patients who participated in the Canadian National Population Health Survey visited their GP at least once, and one-third had at least 6 visits to their primary care physician, indicating a high level of contact.32 This high presence of depression in primary care may be due to several factors, including ease of access to a GP, compared with a specialist, lack of specialists in a patient’s vicinity, or lengthy wait-list times to see a specialist. Indeed, effective MDD treatment can be offered through primary care if interventions are evidence-based.33 In addition, evaluating TRD in primary care provides an opportunity to assess how patients are managed, thereby highlighting whether there is a need for increased dissemination of guidelines to ensure appropriate care.
The aim of the InSight study was 3-fold: to assess the prevalence of TRD in primary care; to extend existing research about the differences in clinical characteristics of TRD, compared to nonresistant, patients; and to assess how patients with TRD are managed in primary care.
Methods
Subjects and Study Criteria
This was a multicentre, retrospective chart review of patients, aged 18 to 75, with a documented primary diagnosis of MDD, based on physician report of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria, who were receiving AD treatment from their primary care physician. Patients with psychiatric or medical comorbidity were not excluded from the study. Patients in this study were considered treatment-resistant if they were observed by the treating physician to have no or minimal improvement following 2 or more AD trials that were a minimum of 6 weeks in duration. While cognitive-behavioural therapy would have been an acceptable treatment alternative, the lack of consistent availability across sites precluded its inclusion as a treatment option.
A total of 135 primary care physicians from across Canada agreed to participate in the InSight Registry: British Columbia (n = 34), Alberta (n = 10), Saskatchewan (n = 1), Manitoba (n = 4), Ontario (n = 39), Quebec (n = 31), and the Atlantic provinces (n = 16). Physicians were contacted based on a registry of primary care physicians through the CHRC. Each physician was asked to review the charts from 10 consecutive patients meeting criteria for MDD, and to complete the CRFs, which were sent via facsimile to the InSight data coordinating centre.
This study was approved by the central Research Ethics Board in each province. Data collection took place between October 2008 and August 2009.
Procedures
The data collection form was developed to capture information regarding sex, age, employment status, BMI, vital signs, medication history, and side effects associated with current medication regimen. Depression severity, and the scale used for its evaluation, were recorded, if completed. Questions to the primary care physician regarding future management were also included: “When will you assess if you have to change treatment?” (reported in weeks); “How will you decide when treatment needs to be altered” (question patient, use a formal scale, wait until patient complains about no response or side effects occur); “How might you alter the treatment?” (refer to psychiatrist or psychologist, refer to other mental health care provider, increase dose of AD, combine with another agent, switch to another agent, do nothing further); “If combining medications, which agent would you use?” (any psychotropic captured); and “If switching meds, which agent would you use?” (any psychotropic captured).
CRFs were scanned using an optical recognition software program (Cardiff TeleForm, New England Survey Systems, Brookline, MA). The data were stored in an electronic database at the CHRC.
Statistical Analysis
Continuous variables are summarized as median and interquartile range. Discrete variables are reported as counts and percentages. For continuous variables, differences between TRD and non-TRD patients were compared using the Kruskal–Wallis test. Categorical variables were tested using Pearson chi-square or Fisher exact tests if the number of patients was fewer than 5. Analyses were performed using SAS software version 9.2 (SAS Institute Inc, Cary, NC) and tested using 2-sided tests at a significance level of 5%.
Results
Treatment-Resistant Depression Prevalence
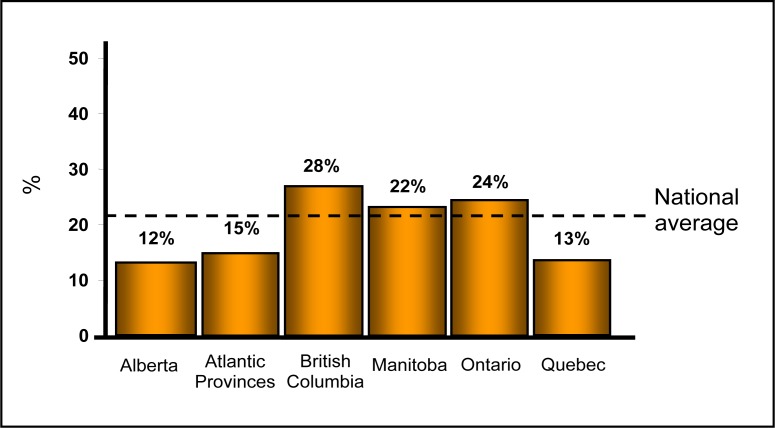
A total of 1282 charts of treatment-seeking depressed patients from 135 physicians were reviewed. Complete data were available for 1212 patients, which formed the full analysis set. Among these patients, 263 were classified as TRD, resulting in a prevalence of 21.7% across Canada (Figure 1). The prevalence across provinces varied (P < 0.001), with British Columbia having the highest rate (28.7%) and Alberta the lowest rate (12.8%). Owing to the small sample size, the TRD prevalence in Saskatchewan was not included (2 patients with TRD in a sample of 4).
Figure 1.
Prevalence of treatment-resistant depression across Canada
Treatment-Resistant Depression Characteristics
Sex and ethnicity did not differ between non-TRD and TRD groups (Table 1). However, higher age (50, compared with 47, years, P = 0.01), duration of current episode (36 months, compared with 12 months, P < 0.001), and greater work impairment were observed in TRD, compared with non-TRD, patients, with 17.6% of patients with TRD on long-term disability, compared with 10.1% in the non-TRD group (P = 0.002).
Table 1.
Patient demographics
| Variable | Non-TRD group n = 949 | TRD group n = 263 | P |
|---|---|---|---|
| Sex, n (%) | 0.65 | ||
| Male | 303 (32.3) | 81 (30.8) | |
| Female | 635 (67.7) | 182 (69.2) | |
| Age, yearsa | 47 (37–57) | 50 (42–58) | 0.01 |
| Ethnicity, n (%) | 0.34 | ||
| Caucasian | 846 (90.0) | 240 (91.9) | |
| Others | 94 (10.0) | 21 (8.1) | |
| Working status, n (%) | 0.002 | ||
| On disability | 92 (10.1) | 44 (17.6) | |
| Unemployed | 161 (17.8) | 46 (18.4) | |
| In school or homemaker | 178 (19.6) | 49 (19.6) | |
| Employed | 476 (52.5) | 111 (44.4) | |
| Among those employed | 0.02 | ||
| Missed work | 174/473 (36.8) | 26/106 (24.5) | |
| Did not miss work | 299/473 (63.2) | 80/106 (75.5) | |
| Duration of current episode, monthsa | 12 (6–36) | 36 (12–108) | <0.001 |
TRD = treatment-resistant depression
Median, interquartile range
Axes I, II, and III comorbidities were also higher in the TRD, compared with the non-TRD, group (online eFigure 2). Axis I comorbidity in the TRD group was primarily accounted for by anxiety and substance use disorders. The most prevalent Axis II disorder was in the Cluster C category (anxious–fearful) in the TRD, compared with the non-TRD, group (35.0% and 23.6%, respectively, P < 0.001), followed by Cluster B and A disorders in smaller proportions. Among Axis III comorbidities, cardiovascular disease (21.3%), chronic pain (21.7%), primary insomnia (16.4%), and type II diabetes (14.8%) were the most prevalent (Table 2). Further analyses regarding weight revealed that patients with TRD had a higher BMI (28.3 kg/m2 and 26.3 kg/m2, respectively, P < 0.001), and obesity (BMI > 30 kg/m2) was associated with treatment resistance (60.1% and 44.8%, respectively, P < 0.001). Patients with TRD were also more likely to be on lipid-lowering, hypoglycemic and (or) insulin, or antiinflammatory drugs.
Table 2.
Axis III comorbidities screened
| Variable | Non-TRD group n = 949 | TRD group n = 263 | |
|---|---|---|---|
| n (%) | n (%) | P | |
| Cardiovascular disease | 151 (15.9) | 56 (21.3) | 0.04 |
| Chronic pain | 137 (14.4) | 57 (21.7) | 0.005 |
| Sleep disorder | 74 (7.8) | 43 (16.4) | <0.001 |
| Type II diabetes | 88 (9.3) | 39 (14.8) | 0.009 |
| Arthritis | 96 (10.1) | 32 (12.2) | 0.34 |
| Asthma | 68 (7.2) | 22 (8.4) | 0.51 |
| Chronic obstructive pulmonary disease | 22 (2.3) | 15 (5.7) | 0.005 |
| Cancer | 29 (3.1) | 13 (4.9) | 0.14 |
| Osteoporosis | 38 (4.0) | 10 (3.8) | 0.88 |
| Chronic kidney disease | 18 (1.9) | 7 (2.7) | 0.44 |
| Hepatitis | 5 (0.5) | 5 (1.9) | 0.045 |
| Peripheral vascular disease | 12 (1.3) | 4 (1.5) | 0.76 |
TRD = treatment-resistant depression
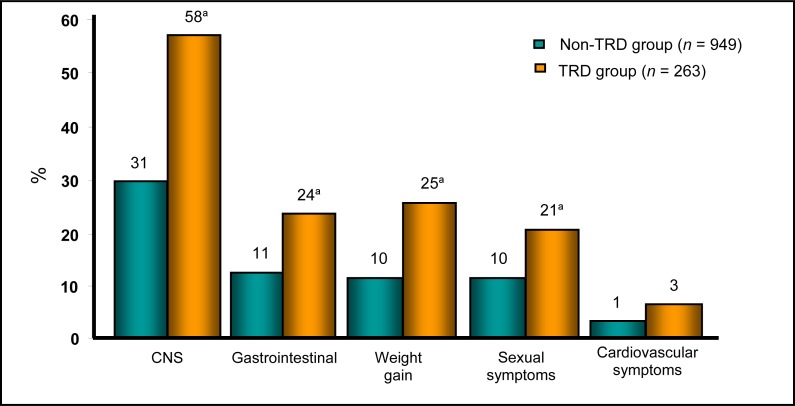
Patients with TRD also reported a greater number of side effects attributed to their current AD regimen: central nervous sysem (for example, drowsiness, dizziness, dry mouth, confusion, headache, tremors, and blurred vision), gastrointestinal (for example, nausea, constipation, and diarrhea), cardiovascular (for example, rapid heart beat), as well as weight gain and sexual dysfunction across the domains of desire, arousal, and orgasm (Figure 3).
Figure 3.
Percentage of side effects reported
CNS = central nervous system; TRD = treatment-resistant depression
a P < 0.05
Depression Management
Diagnosis and Screening
Patients with TRD, compared with no TRD, were more likely to have ever been referred to a psychiatrist (71.7% and 31%, respectively, P < 0.001). They were also more likely than non-TRD patients to have been questioned about BD symptoms (52.1% and 40.1%, respectively, P < 0.001), although this only occurred in about one-half of all patients.
Only 25% of physicians indicated that they conducted standardized assessments of depression severity (23.9% in the non-TRD group and 31.1% in the TRD group, nonsignificant). The scales used were the HAMD-7,34 the Patient Health Questionnaire-9,35 the Montgomery– Åsberg Depression Rating Scale,36 or the Beck Depression Inventory,37 with the HAMD-7 being the most frequently used.
Psychotropics.
Overall, venlafaxine XR, citalopram, bupropion XL, and escitalopram were the most frequently prescribed (> 10% of sample in each case) ADs (23.8%, 19.3%, 17.7%, and 12.8%, respectively), with minimal use of tricyclic ADs or monoamine oxidase inhibitors. Bupropion XL was more often prescribed for patients with TRD (23.6% and 16.1%, respectively, P = 0.005). Dosing across the most frequently used ADs was only higher for venlafaxine XR and bupropion in the TRD group (Table 3). The TRD group was also more likely to be prescribed at least 2 psychotropics (57% and 25.1%, respectively, P < 0.001), as well as atypical APs (19.8% and 5.6%, respectively, P < 0.001) and benzodiazepines (15.6% and 8.0%, respectively, P = 0.002), compared with the non-TRD group.
Table 3.
Dosing of current therapy
| Variable | Non-TRD group n = 949 | TRD group n = 26 | P |
|---|---|---|---|
| Number of current psychiatric medications, n (%) | <0.001 | ||
| 1 | 711 (74.9) | 113 (43.0) | |
| 2 | 238 (25.1) | 150 (57.0) | |
| Total daily dose, median (interquartile range) | |||
| Venlafaxine XR | 150 (75–225) | 187.5 (75.0–262.5) | 0.02 |
| Citalopram | 20 (20–40) | 30 (20–40) | 0.37 |
| Bipropion XL | 150 (150–300) | 300 (150–300) | 0.002 |
| Escitalopram | 10 (10–20) | 10 (10–20) | 0.21 |
TRD = treatment-resistant depression; XL = long acting; XR = extended release
Subsequent Treatment Strategies.
There were no between-group differences for the duration physicians waited to assess if a change in treatment was necessary (8 and 6 weeks, respectively, P = 0.78). The decision to change treatment was more often based on reported lack of effectiveness than side effects. The intervention differed between groups, where patients with TRD were more likely to be referred to a psychiatrist or psychologist (50.2% and 34.5%, respectively, P < 0.001), and non-TRD patients were more likely to have their dose increased (61.0% and 52.5%, P = 0.01). There were no between-group differences in whether a patient was offered a combination treatment or switched to another AD. When combining medications, patients with TRD were most likely to be prescribed an atypical AP, while non-TRD patients were more likely to receive bupropion XL, adjunctively. When switching medications, venlafaxine XR, escitalopram, bupropion XL, and duloxetine were most frequently selected (28.4%, 14.4%, 13.8%, and 12.6%, respectively). Non-TRD patients were more likely to be switched to venlafaxine XR, with no other group differences across escitalopram, bupropion, and duloxetine.
Discussion
Treatment-Resistant Depression Prevalence
In our study, the Canada-wide prevalence of TRD in primary care was 21.7%, compared with a rate of 30% in the STAR*D trial,38 where patients were recruited both from specialty clinics and primary care settings. These findings emphasize the persistence of depressive symptoms in a large proportion of patients with MDD, despite adequate trials of at least 2 ADs.
Treatment-Resistant Depression Clinical Characteristics
Published reports indicate TRD is associated with early age at onset, a more complex illness course (for example, high frequency of prior episodes), as well as psychiatric and medical comorbidity (for example, anxiety or personality disorders, and cardiac diseases).18,39 While we did not capture episode recurrence and age of onset, our findings are consistent with differences in age, duration of episode, and cardiovascular disease. We also report increased presence of additional comorbidities, medication use, side effects, and decreased work function in patients with TRD.
Over 50% of TRD patients had an Axis I or III diagnosis, and over 30% had a comorbid personality disorder. Consequently, for a subgroup of depressed patients with comorbidities, existing medications may be inadequate to relieve the additional symptoms. Importantly, comorbidity contributes to a higher all-cause mortality rate for patients with TRD, which is reported to be about 13% during 4 to 8 years40 and 32% during 7 years.41 These findings also emphasize the importance of different treatment strategies to target multiple psychiatric and medical conditions. However, the resulting increase in medication use may also play a role in decreased AD efficacy through drug interactions, as well as increased side effect burden. The higher number of adverse events in the patients with TRD observed in this study may be due to an increase in medication use, compared with the non-TRD group. In addition, considering this was a cross-sectional study, it is also difficult to disambiguate treatment-emergent adverse events and worsening depression symptoms.
Notably, patients with TRD had higher BMI and presence of obesity, which is reflective of the increased rates of cardiovascular disease and diabetes in MDD, and potentially in TRD.19 Additionally, increased use of atypical APs can also lead to increased body weight.42 One proposed consequence of increased body weight is reduced AD efficacy, especially where BMIs exceed 25 kg/m2.43–45 Several explanations have been suggested for this change in AD efficacy with higher BMI, including increased inflammatory activity, effects on the hypothalamic–pituitary–adrenal axis, increased neurovegetative symptoms, such as disturbed sleep and appetite, as well as pharmacokinetic alterations that occur with greater body fat, resulting in reduced drug bioavailability.45–47
The negative impact of depression on work function (for example, presenteeism, absenteeism, unemployment, and long-term disability) is well documented.48–51 However, the effects of TRD on work status is less clear. In our study, we report a higher rate of long-term disability in the TRD group, which contributes to the increased economic burden in this subpopulation. While employed non-TRD patients missed more work than employed patients with TRD, this may be driven by the finding that non-TRD patients are more likely to remain employed, and consequently miss more work days.
Depression Management
It is important to note that there can be several barriers to effective physician treatment of depressed patients, including physician attitudes and reluctance to initiate therapy, which should be further evaluated.52,53 However, clinical practice guidelines are also a useful tool to aid psychiatric management. According to current treatment guidelines, psychiatric management should begin with a thorough evaluation of symptoms and their impact on function, with an emphasis on suicidality, followed by a clarification of polarity to rule out BD, as well as additional details on comorbidity and concomitant medications.54 In our study, only 50% of patients are screened for bipolarity. Importantly, a significant proportion of patients who were unresponsive to ADs may have undetected BD.55,56 Further, the use of validated depression scales should be used to monitor patient progress, which will help to determine improvement (more than a 20% decrease in depression scores after 2 weeks), response (more than a 50% decrease in depression scores after adequate treatment duration), as well as to identify treatment-emergent adverse effects and unresolved symptoms that would warrant other treatment strategies.57 As only 25% of clinicians used a formal scale in our study to evaluate symptoms and the decision to change treatment was primarily driven by patient questioning, further education on the benefits of tracking symptoms quantitatively is warranted.
Regarding treatment, primary care physicians tended to follow the prevailing guidelines. While pharmacotherapy was the mainstay for treatment, referral for neurostimulation therapies for TRD patients, in particular, would be indicated.57 All of the commonly prescribed medications reported (that is, bupropion XL, citalopram, escitalopram, and venlafaxine XR) were considered first-line agents at the time of the study. However, the duration of time before treatment adjustment was longer than recommended: 6 weeks for the TRD group and 8 weeks for the non-TRD group. Current evidence suggests making a change if there is no improvement after 4 to 6 weeks.58 This is due to findings that lack of early improvement is predictive of nonresponse.59,60
Switching to an agent with demonstrated superiority (for example, duloxetine, escitalopram, sertraline, or venlafaxine XR) or prescribing adjunctive therapy (for example, aripiprazole, olanzapine, risperidone, or lithium) are considered first-line recommendations for nonresponse.58 In our study, while the switch strategies adhered to the guidelines, it should be noted that switching to bupropion XL is considered a second-line treatment strategy, owing to lack of evidence of superiority over other agents, as well as increased risk for drug interactions.58 However, treatment should be individualized based on symptom profile, in addition to other factors, including tolerability, comorbidity, and previous response. Therefore, in cases where patients are having difficulty with sexual side effects, for example, bupropion would be an appropriate medication.61 The use of atypical APs was more likely to be used in the TRD group, which is also consistent with MDD guidelines. However, a greater proportion of patients with TRD received a benzodiazepine, compared with non-TRD patients. While duration of use was not specified, there is a consensus across guidelines that benzodiazepines should only be used in the short term (no more than 4 weeks), owing to the lack of efficacy data beyond this time point,62,63 as well as the increased risk of abuse, cognitive impairment, and falls in the elderly.58,64,65
A primary limitation of our study is the retrospective data collection method. It should be noted that this may not be a representative sample of primary care patients, owing to a lack of random sampling and potential selection biases. Other limitations include physician report of psychiatric and medical diagnoses instead of a structured interview, no correction for multiple comparisons, and no accounting for any strata or clustering of physicians across sites. In addition, it is generally accepted that TRD reflects a failure of at least 2 adequate AD trials; however, treatment compliance was not assessed. It is also unclear whether true treatment resistance may require further nonresponse.
Nevertheless, this report provides a valuable snapshot of physician-reported management of one of the most prevalent psychiatric disorders encountered by primary care physicians in Canada. Further longitudinal characterization of TRD is necessary, and future research should examine the prevalence of TRD in different settings and evaluate biomarkers that could aid in treatment selection.
In summary, TRD is prevalent, posing a significant issue, owing to its association with functional and symptom burden. The management of patients within a primary care sample from across Canada mostly followed clinical guidelines regarding AD choice, duration, and treatment strategies. However, further dissemination of recommended guidelines, including earlier treatment adjustments and training on the use of formal scales, would improve current treatment practices.
Acknowledgments
The authors thank Anna Cyriac for statistical support during the analysis of these data. This study was sponsored by the CHRC and Biovail.
Ms Rizvi has received travel funding from Eli Lilly and St Jude Medical. Mr Grima and Ms Tan are employees of the CHRC. Dr Rotzinger is a shareholder of Protagenic Therapeutics, Inc, and is named on patents for “Teneurin C-terminal associated peptides (TCAP) and uses thereof.” Dr Lin is an employee of the CHRC, and has received speaking honoraria from Sanofi-Aventis, AstraZeneca and Pfizer. Dr McIntyre is on speaker and advisory boards for, or has received research funds from, 13 CME, AstraZeneca, Bristol-Myers Squibb, CME Outfitters, France Foundation, GlaxoSmithKline, Eli Lilly, Janssen-Ortho, Organon, Lundbeck, Pfizer, National Alliance for Research on Schizophrenia and Depression, National Institutes of Mental Health, Optum Health, Physicians’ Postgraduate Press, Stanley Medical Research Institute, Shire, and Merck. Dr Kennedy has received grant and research support from Bristol-Myers Squibb, Canadian Institutes of Health Research, Clera Inc, Eli Lilly, GlaxoSmithKline, Janssen Ortho, Lundbeck, Ontario Brain Institute, Servier, and St Jude Medical. He is a consultant to AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Lundbeck, Pfizer, Servier, and St Jude Medical.
Abbreviations
- AD
antidepressant
- AP
antipsychotic
- BD
bipolar disorder
- BMI
body mass index
- CRF
case report form
- CHRC
Canadian Heart Research Centre
- GP
general practitioner
- HAMD-7
Hamilton Depression Rating Scale—7 item
- MDD
major depressive disorder
- STAR*D
Sequenced Treatment Alternatives to Relieve Depression
- TRD
treatment-resistant depression
- XL
long acting
- XR
extended release
Footnotes
This paper was previously presented, by Rizvi SJ, Rotzinger S, Casanova A, Tan M, Lin P, McIntyre RS, Grima E, Kennedy SH, Treatment resistant depression in primary care and contributing factors across Canada: results from the InSight study, at the joint annual meetings of the Canadian Association of Neuroscience and the Canadian College of Neuropsychopharmacology, Ottawa, ON, May 14–18, 2010.
References
- 1.Ustun TB, Ayuso-Mateos JL, Chatterji S. Global burden of unipolar depressive disorders in the year 2000. Br J Psychiatry. 2004;184:386–392. doi: 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- 2.Lerner D, Henke RM. What does research tell us about depression, job performance, and work productivity? J Occ Environ Med. 2008;50:401–410. doi: 10.1097/JOM.0b013e31816bae50. [DOI] [PubMed] [Google Scholar]
- 3.Olchanski N, McInnis Myers M, et al. The economic burden of treatment-resistant depression. Clin Ther. 2013;35:512–522. doi: 10.1016/j.clinthera.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Malhi GS, Parker GB, Crawford J, et al. Treatment-resistant depression: resistant to definition? Acta Psychiatr Scand. 2005;112:302–309. doi: 10.1111/j.1600-0447.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- 5.Gibson TB, Jing Y, Smith Carls G, et al. Cost burden of treatment resistance in patients with depression. Am J Manag Care. 2010;16:370–377. [PubMed] [Google Scholar]
- 6.Ivanova JI, Birnbaum HG, Kidolezi Y, et al. Direct and indirect costs of employees with treatment-resistant and non-treatment-resistant major depressive disorder. Curr Med Res Opin. 2010;26:2475–2484. doi: 10.1185/03007995.2010.517716. [DOI] [PubMed] [Google Scholar]
- 7.Souery D, Amsterdam J, de Montigny C, et al. Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol. 1999;9:83–91. doi: 10.1016/s0924-977x(98)00004-2. [DOI] [PubMed] [Google Scholar]
- 8.Thase ME. Treatment-resistant depression: prevalence, risk factors, and treatment strategies. J Clin Psychiatry. 2011;72:e18. doi: 10.4088/JCP.8133tx4c. [DOI] [PubMed] [Google Scholar]
- 9.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 10.Shelton RC, Osuntokun O, Heinloth AN, et al. Therapeutic options for treatment-resistant depression. CNS Drugs. 2010;24:131–161. doi: 10.2165/11530280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Thase ME, Corya SA, Osuntokun O, et al. A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, and fluoxetine in treatment-resistant major depressive disorder. J Clin Psychiatry. 2007;68:224–236. doi: 10.4088/jcp.v68n0207. [DOI] [PubMed] [Google Scholar]
- 12.Papakostas GI, Shelton RC. Use of atypical antipsychotics for treatment-resistant major depressive disorder. Curr Psychiatry Rep. 2008;10:481–486. doi: 10.1007/s11920-008-0077-3. [DOI] [PubMed] [Google Scholar]
- 13.Berman RM, Fava M, Thase ME, et al. Aripiprazole augmentation in major depressive disorder: a double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr. 2009;14:197–206. doi: 10.1017/s1092852900020216. [DOI] [PubMed] [Google Scholar]
- 14.El-Khalili N, Joyce M, Atkinson S, et al. Extended-release quetiapine fumarate (quetiapine XR) as adjunctive therapy in major depressive disorder (MDD) in patients with an inadequate response to ongoing antidepressant treatment: a multicentre, randomized, double-blind, placebo-controlled study. Int J Neuropsychopharmacol. 2010;23:1–16. doi: 10.1017/S1461145710000015. [DOI] [PubMed] [Google Scholar]
- 15.Rizvi SJ, Donovan M, Giacobbe P, et al. Neurostimulation therapies for treatment resistant depression: a focus on vagus nerve stimulation and deep brain stimulation. Int Rev Psychiatry. 2011;23:424–436. doi: 10.3109/09540261.2011.630993. [DOI] [PubMed] [Google Scholar]
- 16.Fagiolini A, Kupfer DJ. Is treatment-resistant depression a unique subtype of depression? Biol Psychiatry. 2003;53:640–648. doi: 10.1016/s0006-3223(02)01670-0. [DOI] [PubMed] [Google Scholar]
- 17.Fekadu A, Wooderson SC, Markopoulo K, et al. What happens to patients with treatment-resistant depression? A systematic review of medium to long term outcome studies. J Affect Disord. 2009;116:4–11. doi: 10.1016/j.jad.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Souery D, Oswald P, Massat I, et al. Clinical factors associated with treatment resistance in major depressive disorder: results from a European multicenter study. J Clin Psychiatry. 2007;68:1062–1070. doi: 10.4088/jcp.v68n0713. [DOI] [PubMed] [Google Scholar]
- 19.Carney RM, Freedland KE. Treatment-resistant depression and mortality after acute coronary syndrome. Am J Psychiatry. 2009;166:410–417. doi: 10.1176/appi.ajp.2008.08081239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duhameau B, Ferré JC, Jannin P, et al. Chronic and treatment-resistant depression: a study using arterial spin labeling perfusion MRI at 3Tesla. Psychiatry Res. 2010;182:111–116. doi: 10.1016/j.pscychresns.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Lambert G, Johansson M, Agren H, et al. Reduced brain norepinephrine and dopamine release in treatment-refractory depressive illness: evidence in support of the catecholamine hypothesis of mood disorders. Arch Gen Psychiatry. 2000;57:787–793. doi: 10.1001/archpsyc.57.8.787. [DOI] [PubMed] [Google Scholar]
- 22.Price RB, Shungu DC, Mao X, et al. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry. 2009;65:792–800. doi: 10.1016/j.biopsych.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anttila S, Viikki M, Huuhka K, et al. TPH2 polymorphisms may modify clinical picture in treatment-resistant depression. Neurosci Lett. 2009;464:43–46. doi: 10.1016/j.neulet.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 24.Viikki M, Sami A, Olli K, et al. Vascular endothelial growth factor (VEGF) polymorphism is associated with treatment resistant depression. Neurosci Lett. 2010;477:105–108. doi: 10.1016/j.neulet.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 25.Bonvicini C, Minelli A, Scassellati C, et al. Serotonin transporter gene polymorphisms and treatment-resistant depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:934–939. doi: 10.1016/j.pnpbp.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 26.Gaynes BN, Rush AJ, Trivedi MH, et al. Major depression symptoms in primary care and psychiatric care settings: a cross-sectional analysis. Ann Fam Med. 2007;5:126–134. doi: 10.1370/afm.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stegenga BT, Kamphuis MH, King M, et al. The natural course and outcome of major depressive disorder in primary care: the PREDICT-NL study. Soc Psychiatry Psychiatr Epidemiol. 2012;47:87–95. doi: 10.1007/s00127-010-0317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ornstein S, Stuart G, Jenkins R. Depression diagnoses and antidepressant use in primary care practices: a study from the Practice Partner Research Network (PPRNet) J Fam Pract. 2000;49:68–72. [PubMed] [Google Scholar]
- 29.Regier DA, Narrow WE, Rae DS, et al. The de facto US mental and addictive disorders service system. Epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry. 1993;50:85–94. doi: 10.1001/archpsyc.1993.01820140007001. [DOI] [PubMed] [Google Scholar]
- 30.Stafford RS, Ausiello JC, Misra B, et al. National patterns of depression treatment in primary care. Prim Care Companion J Clin Psychiatry. 2000;2:211–216. doi: 10.4088/pcc.v02n0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wittchen HU, Pittrow D. Prevalence, recognition and management of depression in primary care in Germany: the Depression 2000 study. Hum Psychopharmacol. 2002;17(Suppl 1):S1–S11. doi: 10.1002/hup.398. [DOI] [PubMed] [Google Scholar]
- 32.Patten SB, Beck C. Major depression and mental health care utilization in Canada: 1994 to 2000. Can J Psychiatry. 2004;49:303–309. doi: 10.1177/070674370404900505. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy SH. A review of antidepressant therapy in primary care: current practices and future directions. Prim Care Companion CNS Disord. 2013;15:e1–e9. doi: 10.4088/PCC.12r01420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McIntyre RS, Konarski JZ, Mancini DA, et al. Measuring the severity of depression and remission in primary care: validation of the HAMD-7 scale. CMAJ. 2005;173:1327–1334. doi: 10.1503/cmaj.050786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Int Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montgomery SA, Åsberg M. New depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:3829. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 37.Beck AT, Steer RA, Ball R, et al. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 38.Warden D, Trivedi MH, Wisniewski SR, et al. Predictors of attrition during initial (citalopram) treatment for depression: a STAR*D report. Am J Psychiatry. 2007;164:1189–1197. doi: 10.1176/appi.ajp.2007.06071225. [DOI] [PubMed] [Google Scholar]
- 39.Dudek D, Rybakowski JK, Siwek M, et al. Risk factors of treatment resistance in major depression: association with bipolarity. J Affect Disord. 2010;126:268–271. doi: 10.1016/j.jad.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Shergill SS, Robertson MM, Stein G, et al. Outcome in refractory depression. J Affect Disord. 1999;54:287–294. doi: 10.1016/s0165-0327(98)00201-8. [DOI] [PubMed] [Google Scholar]
- 41.O’Leary DA, Lee AS. Seven year prognosis in depression: mortality and readmission risk in the Nottingham ECT cohort. Br J Psychiatry. 1996;169:423–429. doi: 10.1192/bjp.169.4.423. [DOI] [PubMed] [Google Scholar]
- 42.McIntyre RS, McCann SM, Kennedy SH. Antipsychotic metabolic effects: weight gain, diabetes mellitus, and lipid abnormalities. Can J Psychiatry. 2001;46:273–281. doi: 10.1177/070674370104600308. [DOI] [PubMed] [Google Scholar]
- 43.Kloiber S, Ising M, Reppermund S, et al. Overweight and obesity affect treatment response in major depression. Biol Psychiatry. 2007;62:321–326. doi: 10.1016/j.biopsych.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Khan A, Schwartz KA, Kolts RL, et al. BMI, sex and antidepressant response. J Affect Disord. 2007;99:101–106. doi: 10.1016/j.jad.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 45.Uher R, Mors O, Hauser J, et al. Body weight as a predictor of antidepressant efficacy in the GENDEP project. J Affect Disord. 2009;118:147–154. doi: 10.1016/j.jad.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Capuron L, Poitou C, Machaux-Tholliez D, et al. Relationship between adiposity, emotional status and eating behaviour in obese women: role of inflammation. Psychol Med. 2011;41:1517–1528. doi: 10.1017/S0033291710001984. [DOI] [PubMed] [Google Scholar]
- 47.Vicennati V, Pasquali R. Abnormalities of the hypothalamic– pituitary–adrenal axis in non-depressed women with abdominal obesity and relations with insulin resistance: evidence for a central and a peripheral alteration. J Clin Endocrinol Metab. 2000;85:4093–4098. doi: 10.1210/jcem.85.11.6946. [DOI] [PubMed] [Google Scholar]
- 48.Ahola K, Virtanen M, Honkonen T, et al. Common mental disorders and subsequent work disability: a population-based Health 2000 Study. J Affect Disord. 2011;134:365–372. doi: 10.1016/j.jad.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 49.Birnbaum HG, Kessler RC, Kelley D, et al. Employer burden of mild, moderate, and severe major depressive disorder: mental health services utilization and costs, and work performance. Depress Anxiety. 2010;27:78–89. doi: 10.1002/da.20580. [DOI] [PubMed] [Google Scholar]
- 50.Lerner D, Adler DA, Rogers WH, et al. Work performance of employees with depression: the impact of work stressors. Am J Health Promot. 2010;24:205–213. doi: 10.4278/ajhp.090313-QUAN-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patten SB, Wang JL, Williams JV, et al. Prospective evaluation of the effect of major depression on working status in a population sample. Can J Psychiatry. 2009;54:841–845. doi: 10.1177/070674370905401207. [DOI] [PubMed] [Google Scholar]
- 52.Patten SB, Kennedy SH, Lam RW, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. I. Classification, burden and principles of management. J Affect Disord. 2009;117(Suppl 1):S5–S14. doi: 10.1016/j.jad.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 53.Sharma V, Khan M, Smith A. A closer look at treatment resistant depression: is it due to a bipolar diathesis? J Affect Disord. 2005;84:251–257. doi: 10.1016/j.jad.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 54.Correa R, Akiskal H, Gilmer W, et al. Is unrecognized bipolar disorder a frequent contributor to apparent treatment resistant depression? J Affect Disord. 2010;127:10–18. doi: 10.1016/j.jad.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 55.Lam RW, Kennedy SH, Grigoriadis S, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) Clinical guidelines for the management of major depressive disorder in adults. III. Pharmacotherapy. J Affect Disord. 2009;117(Suppl 1):S26–S43. doi: 10.1016/j.jad.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 56.Kennedy SH, Milev R, Giacobbe P, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) Clinical guidelines for the management of major depressive disorder in adults. IV. Neurostimulation therapies. J Affect Disord. 2009;117(Suppl 1):S44–S53. doi: 10.1016/j.jad.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 57.Szegedi A, Jansen WT, van Willigenburg AP, et al. Early improvement in the first 2 weeks as a predictor of treatment outcome in patients with major depressive disorder: a meta-analysis including 6562 patients. J Clin Psychiatry. 2009;70:344–353. doi: 10.4088/jcp.07m03780. [DOI] [PubMed] [Google Scholar]
- 58.Kudlow PA, Cha DS, McIntyre RS. Predicting treatment response in major depressive disorder: the impact of early symptomatic improvement. Can J Psychiatry. 2012;57:782–788. doi: 10.1177/070674371205701211. [DOI] [PubMed] [Google Scholar]
- 59.Craven MA, Bland R. Depression in primary care: current and future challenges. Can J Psychiatry. 2013;58:442–448. doi: 10.1177/070674371305800802. [DOI] [PubMed] [Google Scholar]
- 60.Gask L. Educating family physicians to recognize and manage depression: where are we now? Can J Psychiatry. 2013;58:449–455. doi: 10.1177/070674371305800803. [DOI] [PubMed] [Google Scholar]
- 61.Zisook S, Rush AJ, Haight BR, et al. Use of bupropion in combination with serotonin reuptake inhibitors. Biol Psychiatry. 2006;59:203–210. doi: 10.1016/j.biopsych.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 62.Davidson JR. Major depressive disorder treatment guidelines in America and Europe. J Clin Psychiatry. 2010;71(Suppl E1):e04. doi: 10.4088/JCP.9058se1c.04gry. [DOI] [PubMed] [Google Scholar]
- 63.Higuchi T. Major depressive disorder treatment guidelines in Japan. J Clin Psychiatry. 2010;71(Suppl E1):e05. doi: 10.4088/JCP.9058se1c.05gry. [DOI] [PubMed] [Google Scholar]
- 64.Barker MJ, Greenwood KM, Jackson M, et al. Cognitive effects of long-term benzodiazepine use: a meta-analysis. CNS Drugs. 2004;18:37–48. doi: 10.2165/00023210-200418010-00004. [DOI] [PubMed] [Google Scholar]
- 65.Lavsa SM, Fabian TJ, Saul MI, et al. Influence of medications and diagnoses on fall risk in psychiatric inpatients. Am J Health Syst Pharm. 2010;67:1274–1280. doi: 10.2146/ajhp090611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.