Abstract
BACKGROUND & AIMS
Activation of the transcription factor NFκB has been associated with development of inflammatory bowel disease (IBD). COMMD1, a regulator of various transport pathways, has been shown to limit NFκB activation. We investigated the roles of COMMD1 in the pathogenesis of colitis in mice and IBD in humans.
METHODS
We created mice with specific disruption of Commd1 in myeloid cells (Mye-K/O mice); we analyzed immune cell populations and functions and expression of genes regulated by NFκB. Sepsis was induced in Mye-K/O and wild-type mice by cecal ligation and puncture or intraperitoneal injection of lipopolysaccharide (LPS), colitis was induced by administration of dextran sodium sulfate (DSS), and colitis-associated cancer was induced by administration of DSS and azoxymethane. We measured levels of COMMD1 mRNA in colon biopsies from 29 patients with IBD and 16 patients without (controls), and validated findings in an independent cohort (17 patients with IBD and 22 controls). We searched for polymorphisms in or near COMMD1 that were associated with IBD using data from the International IBD Genetics Consortium and performed quantitative trait locus analysis.
RESULTS
In comparing gene expression patterns between myeloid cells from Mye-K/O and wild-type mice, we found that COMMD1 represses expression of genes induced by LPS. Mye-K/O mice had more intense inflammatory responses to LPS and developed more severe sepsis and colitis, with greater mortality. More Mye-K/O mice with colitis developed colon dysplasia and tumors than wild-type mice. We observed reduced expression of COMMD1 in colon biopsies and circulating leukocytes from patients with IBD. We associated single nucleotide variants near COMMD1 with reduced expression of the gene and linked them with increased risk for ulcerative colitis.
CONCLUSIONS
Expression of COMMD1 by myeloid cells has anti-inflammatory effects. Reduced expression or function of COMMD1 could be involved in the pathogenesis of IBD.
Keywords: mouse model, gene regulation, CD, UC
INTRODUCTION
Persistent inflammation is a common maladaptive component in the pathogenesis of human diseases. A prime example of this paradigm is inflammatory bowel disease (IBD), a chronic inflammatory process of the intestinal tract that clinically presents as two phenotypic entities, ulcerative colitis (UC) and Crohn’s disease (CD). This disorder involves an interaction between environmental factors and inherited susceptibility, and is associated with an increased risk for colorectal cancer. 1
The regulation of the inflammatory cascade is a complex process in which the transcription factor NF-κB plays a master regulatory role. 2 Consequently, this factor has also been linked to the pathogenesis of several chronic inflammatory conditions in humans, 2 including IBD. 3, 4 Canonical NF-κB activity is mediated primarily by NF-κB complexes containing the RelA subunit (also known as p65) or its paralog c-Rel. Under basal conditions, these complexes are kept in the cytosol through interactions with the inhibitory IκB proteins. Their activation requires IκB degradation, an event triggered by a critical kinase complex known as IKK that sits at the crossroads of numerous signaling pathways. After signaling inputs abate, homeostatic mechanisms that restore basal NF-κB activity are essential for the physiologic function of this pathway. The induction of IκB gene expression, 5 or the expression of IKK inhibitory proteins, such as A20 or CYLD 6, 7 participate in the timely termination of NF-κB activity. The expression of these factors is under the control of NF-κB itself, thus providing negative feedback loops in the pathway. In addition, it has been recognized that ubiquitination and proteasomal degradation of RelA is critical to terminate transcription of a variety of genes. 8–13 One ligase responsible for these effects contains the scaffold protein Cul2 in association with COMMD1, 10, 12 a prototypical member of the COMMD (Copper metabolism MURR1 domain containing) protein family. 14
In addition to its role in NF-κB regulation, 12, 15 COMMD1 has been implicated in a variety of cellular processes, including copper transport, 16 electrolyte balance, 17–19 and hypoxia responses. 20 Given these pleotropic functions, it has remained unclear whether this factor plays a physiologically important role in the control of inflammation in vivo and whether it could play a role in chronic inflammatory diseases. Here we report that myeloid-specific deficiency of Commd1 leads to more intense activation of LPS-inducible genes and is associated with more severe inflammation. In addition, we present genetic evidence linking gene variants associated with reduced COMMD1 expression to risk for UC in humans, highlighting the physiologic importance of this gene in immunity and IBD pathogenesis.
RESULTS
Commd1 represses pro-inflammatory gene expression in myeloid cells
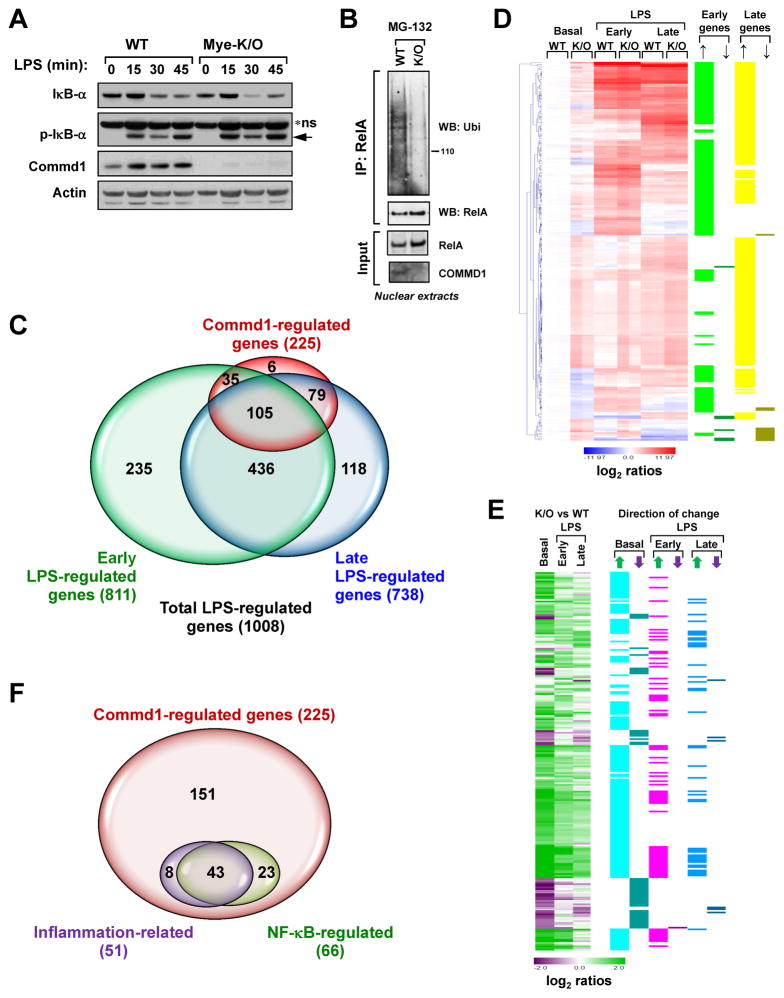
In order to evaluate the potential role of COMMD1 in inflammation, and given the known embryonic lethality that results from complete Commd1 deficiency in mice, 21 we generated a tissue-specific mouse model of Commd1 deficiency. 22 First, Commd1 was selectively deleted in myeloid cells (Mye-K/O), a critical lineage in innate immunity, leading to the expected loss of Commd1 expression in macrophages (Figure S1A). Mye-K/O mice were healthy and B lymphocyte (B220+) and T lymphocyte populations (CD3+ and CD4/CD8) were not significantly different in the spleen or mesenteric lymph nodes (Figures S1B and S1C). Similarly, myeloid populations, including granulocytes (Ly6G+), monocytes and macrophages (CD11b+, Ly6C+ and F4/80), and dendritic cells (CD11chigh, and CD11cintermediate) were not significantly different in Mye-K/O mice (Figures S1B, S1C, and S1D). In line with previous observations, 12, 23, 24 Commd1 deficiency did not alter substantially the phosphorylation or turnover of IκB (Figure 1A), but had a profound effect on RelA ubiquitination (Figure 1B).
Figure 1. Commd1 regulates the LPS transcriptional program in myeloid cells.
(A) Commd1 does not affect IκB turnover in BMDMs. IκB-α phosphorylation (p-IκB-α) and degradation after LPS treatment were examined by western blotting. * ns, non-specific band (arrow points to pIκB-α) (B)Commd1 deficiency reduces RelA ubiquitination. Commd1 deficient mouse embryo fibroblasts (K/O) or control cells (WT) were used to detect ubiquitinated RelA by ubiquitin immunoblotting of immunoprecipitated RelA. (C) Gene expression differences resulting from Commd1 deficiency substantially overlap with the LPS response. BMDMs from WT and Mye-K/O mice were utilized in duplicate microarray analysis to ascertain genes regulated by Commd1 or by LPS (100ng/mL) at early (3h) or late (24h) time points. The degree of overlapping genes is presented in Venn diagram form. (D) Hierarchical cluster analysis of Commd1-regulated genes during the LPS response. Expression levels normalized to basal expression in WT samples is displayed as color-coded log2-transformed ratios. The gene induction pattern, either early (>3- fold induction at 3h) or late LPS response (>3-fold induction at 24h), is also indicated. The specific effect of Commd1 deficiency is noted by up or down arrows. (E) The genes analyzed in (D) are reanalyzed by calculating ratios between K/O and WT BMDMs, displayed as color-coded log2-transformed manner. The direction of change is indicated in adjacent columns by up or down arrows. (F) Venn diagram of Commd1-regulated genes, indicating those involved in inflammatory responses or regulated by NF-κB.
Next, using bone marrow derived myeloid cells (BMDMs) from the Mye-K/O mice, we assessed the impact of Commd1 on the LPS transcriptional response at a genome-wide level. High density microarray experiments indicated that 1008 genes were regulated by LPS at least 3-fold in two independent series of experiments (Figure 1C, and Tables S1–S3). In addition, the expression of 225 genes was found to be regulated by Commd1 (Tables S1 and S2). Notably, the vast majority of Commd1-regulated genes (219 of 225) was also regulated by LPS, and only few Commd1 target genes (6 out of 225) were outside the LPS transcriptional response (Tables S2 and S3). Hierarchical clustering of these 225 genes was used to visualize the pattern of deregulated expression in Commd1-deficient myeloid cells. Both early and late LPS-inducible genes were affected by Commd1 deficiency (Figure 1D). In most instances, the changes observed consisted of increased gene expression, which were often noted even at basal levels (Figure 1E).
In order to further understand the effect of Commd1 on gene expression in myeloid cells, we performed a functional analysis using the KEGG database. This revealed that only 51 genes (23%) participate in immune regulation (as defined by any of a number of KEGG entries, Table S4), indicating that a large number of Commd1 gene targets in myeloid cells are not known inflammatory regulators (Figure 1F). Next, we assessed the contribution of NF-κB regulation to the effects noted in Commd1-deficient myeloid cells. To that end, NF-κB regulation of genes of interest was ascertained through two overlapping approaches: first, using a curated database of published NF-κB gene targets (publicly available at www.NF-κB.org), and second, using ChIP seq data for RelA in LPS-stimulated BMDMs. 25 This indicated that only 66 genes regulated by Commd1 (29%, Table S4) are known NF-κB or RelA targets, suggesting that a substantial proportion of the effects of Commd1 are either secondary or mediated through other transcriptional regulators.
Commd1 exerts gene selective effects among NF-κB regulated targets
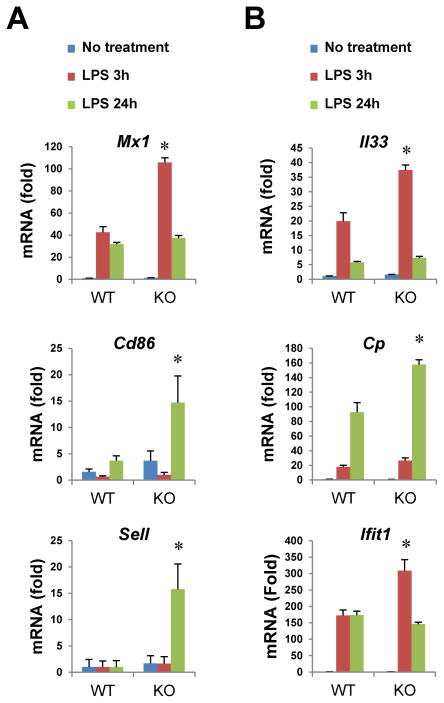
Altogether, these findings suggest a substantial repressive role for Commd1 on the LPS response in myeloid cells and confirmed a negative regulatory role for Commd1 on selected NF-κB regulated genes. Indeed, qRT-PCR analysis corroborated that certain NF-κB target genes were selectively regulated (for example, Mx1, Cd86 and Sell, Figure 2A), while others were relatively insensitive to Commd1 deficiency (such as Il6, Tnfaip3 and Mmp13, Figure S2A). These findings are in line with previous reports of gene specific effects for COMMD1 in cancer cell lines. 12 Enhanced expression of selected genes that are not directly regulated by NF-κB was confirmed as well (Figure 2B). Interestingly, in contrast to these effects, several hypoxia-induced genes were not affected by Commd1 deficiency in BMDMs (Figure S2B), despite its reported role in hypoxia-dependent gene expression. 20, 21 In aggregate the data indicate that Commd1 primarily exerts a gene selective repressive function on the LPS response.
Figure 2. Gene selective effects of Commd1 on NF-κB regulated targets.
BMDMs (2 mice per group, with triplicate cultures per mouse) were stimulated with LPS and gene expression was determined by qRT-PCR. Selected NF-κB target genes are shown (A), while other LPS-inducible genes that are not NF-κB targets are also shown (B).
Myeloid deficiency of Commd1 leads to exaggerated inflammatory responses in vivo
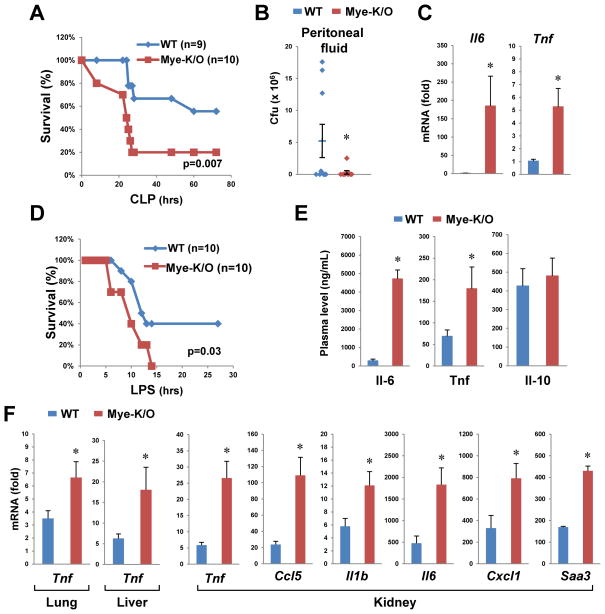
In view of the effects of Commd1 on LPS-induced gene expression, we utilized two models to contrast the inflammatory response of Mye-K/O mice and wild-type (WT) littermate controls. First, we used the cecal ligation and puncture (CLP) model of polymicrobial sepsis. Animal survival was compromised in Mye-K/O mice compared to WT controls (Figure 3A). In contrast, cultures of peritoneal fluid demonstrated lower bacterial counts in the Mye-K/O mice (Figure 3B), indicating that their increased mortality was not due to greater bacterial load. Rather, after accounting for bacterial invasion (by examining animals with negative bacterial cultures), we found that Mye-K/O mice had increased cytokine expression (Figure 3C), suggesting that exaggerated pro-inflammatory gene expression was responsible for their increased susceptibility to sepsis. Next, animals were directly challenged with intraperitoneal LPS injection. Once more, Mye-K/O mice demonstrated greater mortality (Figure 3D) and increased morbidity (Figure S3A). Tissue injury, manifested by LDH release in plasma, was also more pronounced in Mye-K/O mice (Figure S3B), which also displayed greater plasma elevations of Il-6 and Tnf (Figure 3E). In contrast, plasma levels of the anti-inflammatory cytokine Il-10 were comparable to WT. Tissue expression of pro-inflammatory genes was more pronounced in Mye-K/O mice, as exemplified by Tnf, as well as other pro-inflammatory genes (Figure 3F and S4). Altogether, these data indicate that Commd1 deficiency in the myeloid lineage leads to more profound acute inflammatory responses.
Figure 3. Mye-K/O mice have a more severe sepsis response.
(A) CLP leads to higher mortality rate in Mye-K/O mice. Mye-K/O animals (Commd1 F/F, LysM-Cre) were compared to wild-type (WT) littermate controls (Commd1 F/F). Survival without need for euthanasia was plotted and the Kaplan-Meier estimator was calculated. (B) Peritoneal cultures yield lower bacterial counts in Mye-K/O mice (9 mice in each group). Colony forming units (Cfu) in blood agar were determined by progressive dilution. (C) Induction of pro-inflammatory gene expression is greater in Mye-K/O mice. Tissue RNA was analyzed by qRT-PCR (in kidney). To normalize for bacterial load, animals with no detectable bacterial counts were used for this analysis (WT=4, Mye-K/O=6 mice). (D) Mye-K/O mice have greater mortality after LPS administration. Survival was analyzed as in (A). (E) Plasma levels of Il-6, Tnf and Il-10 were determined in Mye-K/O mice (n=9) and WT controls (n=10) at the time of euthanasia in the LPS model. (F) LPS induction of pro-inflammatory genes was determined by qRT-PCR in tissues of Mye-K/O mice (n=10) and WT controls (n=10). * p < 0.05.
Reduced expression of COMMD1 is a common feature of IBD
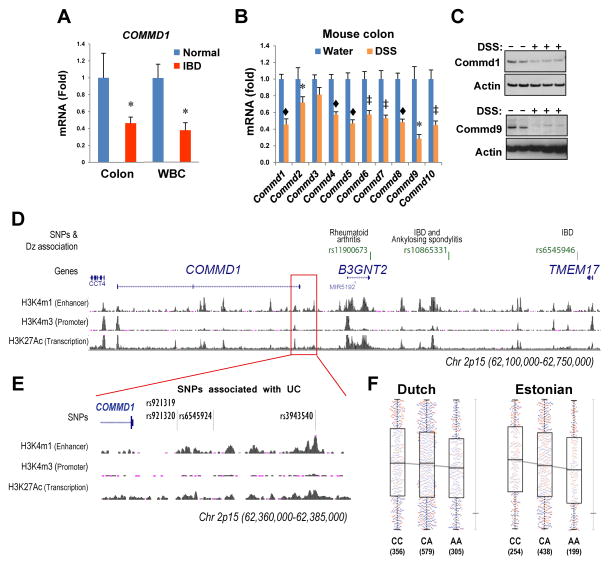
Given the findings made in the Mye-K/O mouse model, we decided to explore whether COMMD1 could play a role in the pathophysiology of inflammatory disorders in humans, choosing IBD as a disease model. To this end, we evaluated the expression of COMMD1 in IBD patients. We found that COMMD1 mRNA levels in colonic endoscopic biopsies were reduced in an Israeli cohort of patients with colitis (n=29, 18 with Crohn’s disease and 11 with ulcerative colitis) compared to unaffected controls (n=16) (p=0.04, Figure 4A, left). This finding was replicated in an independent patient cohort in the USA (p= 0.04, 22 normal controls and 17 patients with IBD, 9 of which had CD and 8 with UC, Figure S5). Similarly, we found that COMMD1 mRNA was reduced in circulating leukocytes from IBD patients with active disease (n=12, 9 with CD and 3 with UC) compared to normal individuals (n=14) (p=0.001, Figure 4A, right). Moreover, in a murine model of colitis induced by Dextran sodium sulfate (DSS) administration, we also observed decreased mRNA and protein expression of Commd1 and other Commd genes in the colon (Figures 4B and 4C). In aggregate, the data indicate that mucosal inflammation is associated with decreased COMMD1 gene expression in clinical and experimental situations.
Figure 4. Lower COMMD1expression is linked to IBD.
(A) COMMD1 gene expression is suppressed in IBD patients. COMMD1 mRNA expression was determined by qRT-PCR in endoscopic colonic biopsies from IBD patients with colitis (n=29, 18 with CD and 11 with UC) and normal controls (n=17). Similarly, COMMD1 mRNA levels in white blood cells (WBC) were determined in IBD patients (n=12, 9 with CD and 3 with UC) and unaffected controls (n=14). (B) and (C) Commd gene expression at the mRNA level (B) was similarly decreased in colonic tissue after DSS-induced acute colitis (7 mice in the DSS group compared to 8 mice in the water group), and this was demonstrated at the protein level for Commd1 and Commd9 (C). * p < 0.05, ‡ p < 0.001, ◆ p < 0.0001. (D) Polymorphisms near COMMD1 are associated with UC risk. A map of the 2p15 locus, including SNPs previously implicated in inflammatory diseases, is shown. The region located 3′ of the COMMD1 gene, where SNPs associated to UC were found, is marked by a red box. (E) A closer view of the location of the SNPs of interest downstream of COMMD1 is shown, including chromatin modifications such as histone 3 K4 monomethylation (H3K4m1), trymethylation (H3K4m3) and histone 3 K27 acetylation (H3K27Ac). (F) cis-eQTL analysis for rs921320 in two independent cohorts. COMMD1 gene expression (in the Y axis) is shown in a box plot for all three genotypes for this SNP (CC, CA or AA). The horizontal bar in the box is the median, the top and bottom of the box show the Inter-quartile range (IQR; Q3 – Q1); the whiskers show the quartile + 1.5x the IQR. The number of individuals in each group is indicated (under the genotype); males and females are denoted by blue and red circles, respectively.
Genetic variants associated with decreased COMMD1 expression are associated with UC risk
In view of the reduced expression of COMMD1 in IBD patients, we investigated whether genetic polymorphisms of this gene might affect IBD risk in humans. Interestingly, genome-wide association studies (GWAS) have linked an intergenic region near COMMD1 to the risk for various inflammatory conditions including IBD (Figure 4D) 3, 26–30, but the precise gene responsible for these effects remains unknown.
We began by interrogating the most recent meta-analysis of GWAS for CD and UC 3 by the International IBD Genetics Consortium (IIBDGC), which is currently available at the Ricopili server (www.broadinstitute.org/mpg/ricopili/). These data indicated a suggestive association signal with UC for SNPs located just downstream of COMMD1 including rs3943540 (p=7.89 × 10−5) and rs921319 (p=7.47 × 10−5) (Figure 4E). Interestingly, both SNPs are in linkage disequilibrium (LD) with each other (r2=1, D′=1). Additional analysis of the 1000 Genomes imputed IIBDGC UC and CD meta-analyses also revealed a suggestive association with UC for a SNP located in the same region, rs6545924 (p=2.27 × 10−5, OR=1.104, 95% confidence interval of the OR between 1.06–1.15).
Epigenetic analysis in myeloid cells performed by the ENCODE project, 31 demonstrated that this genomic locus displays features of a gene regulatory region (Figure 4D and 4E), such as Histone 3 lysine 4 monomethylation (H3K4me1) and lysine 27 acetylation (H3K27Ac), both associated with active enhancers. 32 In agreement with this notion, the SCAN database 33 indicates that there is a significant eQTL effect of rs921319 on COMMD1 gene expression. To further examine the possibility that these variants have an effect on COMMD1 expression, we conducted cis-eQTL mapping on whole peripheral blood of two cohorts (1,240 samples previously studied 34 and 891 samples from the Estonian Biobank). Since the SNPs in question were not present in our eQTL dataset, we tested rs921320, located in very close proximity to rs921319 and a perfect proxy SNP for rs6545924 (r2=0.9, D′=1). We observed a significant eQTL effect of rs921320 on COMMD1 expression, with the same allelic direction for the Dutch and Estonian cohorts (the p values for individual cohorts were p= 0.057 and p=0.0099, respectively; weighted Z-method meta-analysis P = 0.0033). The A–allele of rs921320, which is linked to the risk allele for rs6545924, was associated with reduced expression of COMMD1 (Figure 4F). On the other hand, we did not observe any eQTL effects of rs921320 on the two other neighboring genes, B3GNT2 or TMEM17. Altogether, these findings suggest that genetic variation in the 3′ region of COMMD1, which has regulatory effects on gene expression, is linked to risk for UC.
Myeloid cell deficiency of Commd1 leads to more severe colitis
In order to further examine the possible involvement of COMMD1 in IBD pathogenesis, we begun by assessing the in situ expression of this gene in the colon. Immunofluorescence staining of mouse colon tissue demonstrated staining in the epithelium as well as in mononuclear cells in the lamina propria (Figure S6). Furthermore, confocal microscopy images confirmed that resident lamina propria macrophages (F4/80+) are also COMMD1-positive, in agreement with western blot analysis of primary myeloid cells (Figure 1A). With this in mind and in view of the known contributions of both epithelial and myeloid cells in the pathogenesis of human and murine colitis, 35, 36 we evaluated the role of this gene in this disease process using both epithelial and myeloid cell specific knockout mice.
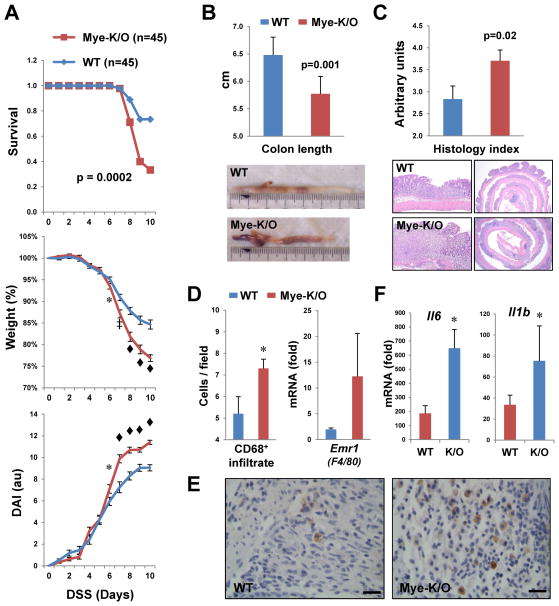
First, we used the DSS model, which triggers colonocyte injury and an innate immune response to luminal bacteria that result in acute colitis. Mye-K/O mice demonstrated more severe disease that WT animals, with excess mortality, more profound weight loss, and worse disease activity (Figure 5A). In agreement with this, colon shortening (a macroscopic marker of tissue injury) and histologic evaluation of the colonic mucosa both indicated more damage in Mye-K/O mice (Figures 5B and 5C). In contrast to these results, Commd1 inactivation in the intestinal epithelium did not result in any appreciable alteration of DSS-induced colitis (Figure S7). The more severe inflammatory response in Mye-K/O mice was correlated with greater numbers of CD68+ myeloid cells in the colon as seen by immunohistochemistry (Figures 5D, left panel and 5E), and a consistent trend was observed at the mRNA level for the macrophage-specific gene Emr1 (F4/80, Figure 5D, right panel). In addition, Mye-K/O mice displayed increased expression of pro-inflammatory genes, such as Il6 and Il1b (Figure 5F). However, myeloid and lymphoid populations in the MLN (Figure S8) and the spleen (not shown), were not significantly different between the groups.
Figure 5. Myeloid deficiency of Commd1 predisposes to more severe colitis.
(A) Worse survival, weight loss and disease activity index (DAI) were noted in Mye-K/O mice compared to wild-type littermate controls during acute DSS-induced colitis. * p < 0.05, ‡ p < 0.001, ◆ p < 0.0001. (B) Colon length was significantly shorter in Mye-K/O mice (n=26) after acute colitis compared to WT controls (n=20). Representative images are shown. (C) Worse tissue injury was also evident histologically. Using a severity score, colonic sections were examined (11 Mye-K/O and 9 WT mice). Representative histologic sections are shown. (D) and (E) Expansion of macrophage populations in the colonic mucosa of Mye-K/O mice. In the left panel, the number of CD68+ cells present in the mucosa was assessed by immunohistochemistry in a tissue microarray of DSS colitis samples (Mye-K/O=13, WT=12 samples). In the right panel, the colonic expression of the macrophage marker gene Emr1 (F4/80) was determined by qRT-PCR (Mye-K/O=18, WT=23 samples). Representative images of the CD68 immunohistochemistry are shown in (E), magnification bar = 20μm. (F) RNA extracted from colonic tissue was utilized for qRT-PCR analysis for the genes indicated (Mye-K/O=18, WT=23 samples).
Myeloid cell deficiency of Commd1 promotes colitis-dysplasia progression
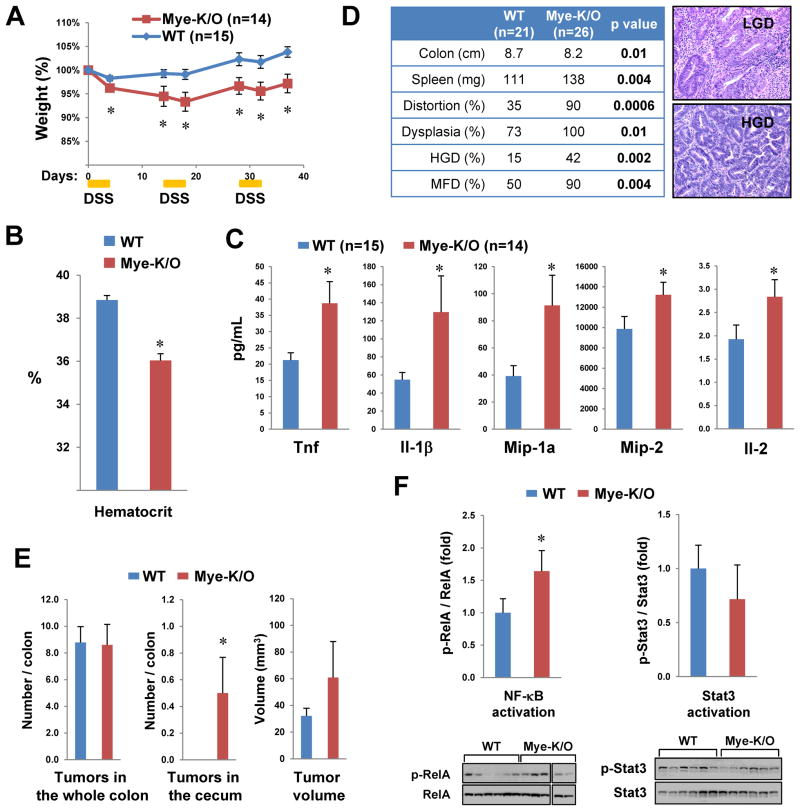
Further analysis indicated that recurrent DSS administration, which mimics the chronic nature of IBD, also resulted in more severe disease in Mye-K/O mice, manifested as greater weight loss (Figure 6A) and more severe anemia (Figure 6B). Colonic tissue from these animals also produced greater amounts of several pro-inflammatory factors such as Tnf (Figure 6C and S9). In addition, azoxymethane (AOM) followed by recurrent DSS treatments was utilized to examine progression to dysplasia. In this model, Mye-K/O mice also displayed more severe disease, evidenced by more severe colonic shortening, splenomegaly, and histologic distortion of the mucosal architecture (Figure 6D). Moreover, after 7 weeks of treatment, Mye-K/O mice had a greater propensity to develop dysplasia, high-grade dysplasia (HGD) or multifocal dysplasia (MFD) as assessed histologically (Figure 6D). By 10 weeks, small adenomas could be visualized, but the total number of lesions was not increased in Mye-K/O mice (Figure 6E). Nevertheless, only Mye-K/O mice developed proximal colonic tumors and there was a tendency for the tumor burden to be greater in these animals as a result of greater adenoma volume (Figure 6E). In colonic tissue, Mye-K/O animals displayed greater NF-κB activation as evidenced by the ratio of the transcriptionally active form of RelA (phosphorylated at Ser536) to total RelA. However, STAT3 activation, another pathway known to promote cancer in the context of chronic tissue inflammation, 37 was not altered in these mice (Figure 6F). Altogether, these data highlight a role for myeloid cell expression of Commd1 in chronic colonic inflammation and progression to dysplasia.
Figure 6. Commd1 deficiency leads to worse chronic colitis and transition to dysplasia.
(A) Recurrent administration of DSS caused more weight loss in Mye-K/O mice. (B) Chronic colitis led to more severe anemia in Mye-K/O mice (5 mice per group). (C) Pro-inflammatory cytokine production is elevated in colonic tissue of Mye-K/O mice. Colonic full-thickness tissue harvested from colitic mice was cultured overnight and the secretion of cytokines into the growth media was determined. (D) Progression to dysplasia was worse in Mye-K/O animals. AOM was administered prior to chronic colitis induction and dysplasia was assessed histologically at 7 weeks. Representative images are displayed. (E) Tumor number and volume in mice at 10 wks of colitis/dysplasia induction is depicted. * p < 0.05. (F) Greater NF-κB activation in inflamed colonic tissue of Mye-K/O mice. Activation of NF-κB/RelA and Stat3 was assessed in inflamed colonic tissue of animals treated with AOM/DSS. Western blot analysis was performed with tissue lysates and the active phosphorylated form was quantitated and normalized to expression of the respective target.
DISCUSSION
COMMD1 plays an essential role to restrain the activity of NF-κB, a master regulator of inflammation. Specifically, COMMD1 acts to terminate NF-κB transcriptional activation through the ubiquitin-mediated degradation of NF-κB subunits. A detailed molecular mechanism by which COMMD1 restrains NF-κB has been previously examined and reported. 8, 10, 12, 13 The studies presented here confirm the negative regulatory role of this factor on the NF-κB pathway, 10, 12, 13, 15 and also underscore the gene selective nature of these effects. Moreover, the studies in this model corroborate that key aspects of the reported mechanism are evident in the knockout mice, namely normal IκB phosphorylation and turnover, but diminished ability to terminate NF-κB transcriptional activity (ie, reduced RelA/p65 ubiquitination). Importantly, this study investigates for the first time the physiological relevance of these events in disease pathogenesis. The data indicate that COMMD1 expression in the myeloid compartment plays an important role in the regulation of inflammatory responses in vivo. Given that diverse functions have been reported for this gene, 17, 38–40 this report fills a critical void by underscoring that COMMD1 participates in immune regulation. In addition, the results also suggest that COMMD1 has broader effects on pro-inflammatory gene expression programs that might extend beyond the NF-κB pathway. Whether these effects are the result of secondary perturbations or are the result of direct regulation of transcription factor(s) other than NF-κB, should be further examined in the future.
In the context of intestinal inflammation and progression to cancer, previous studies have underscored the importance of epithelial and myeloid cells in these processes. 14, 36, 37 In particular, epithelial intrinsic NF-κB activation has been shown to be a protective response that promotes cell survival and barrier function,41 while myeloid cell NF-κB activation is important in sustaining the inflammatory response.35 The increased propensity to inflammation and colitis observed as a result of myeloid cell deficiency of Commd1 is in agreement with this paradigm and suggests that the immune regulatory roles of this gene are physiologically. In addition, the increased progression to dysplasia in Mye-K/O mice is in agreement with the role of myeloid cells in promoting tumor growth through various pro-inflammatory cytokines. 42 In this regard, our model displays increased production of Tnf and greater tissue activation of NF-κB, both of which have been shown to promote cancer progression in the setting of colitis. 35, 43 In addition, we also found no appreciable phenotype of enterocyte-specific deficiency of Commd1, which stands in contrast to other models of disrupted NF-κB activity in the epithelium.35, 41 This may reflect the fact that unlike other models, cell survival genes are not regulated by COMMD1 in the transcriptome analysis presented here. Furthermore, it suggests that other reported roles of Commd1, such as the regulation of various transport mechanisms, may be more relevant in these cells.
These observations in the animal model, coupled with the finding that genetic variants linked to lower COMMD1 expression are also associated with increased risk for UC, suggest that this gene participates in the pathogenesis of this disorder in humans. In this context, it is important to note a recent report of a risk association for COMMD7, another member of the COMMD gene family. 44 Moreover, COMMD1 acts in concert with Cul2 to promote RelA ubiquitination, 10, 12, 13 and the CUL2 gene has been linked to IBD susceptibility. 45 Additional studies, including ongoing resequencing efforts, will be required to validate the genetic association seen here with greater statistical confidence. However, the aggregate of our data and published reports suggest the possibility that homeostatic mechanisms that restore NF-κB to its basal state, when decreased, may enhance the risk for IBD.
Our studies also identified that inflammation can lead to suppressed expression of COMMD1, as seen in patients with colitis as well as in animal models. Our data suggest that this is likely the consequence rather than the cause of inflammation in human disease, because this suppression can be induced by inflammation itself in animal studies. The decrease in COMMD1 expression probably plays a physiologic role in the initiation of an appropriate inflammatory response, but in view of the more severe inflammatory responses seen when this gene is deleted in myeloid cells, persistent COMMD1 suppression is probably maladaptive during chronic inflammation. Therefore, this work highlights the need to gain a refined understanding of the molecular events that control COMMD1 gene expression in the setting of inflammation. With this knowledge, we may begin to envision potential interventions that might restore gene expression, which could be tested for their therapeutic potential in chronic inflammatory disorders.
MATERIALS AND METHODS
Human studies
All procedures involving human subjects were reviewed and approved by the respective Institutional Review Boards (at UT Southwestern Medical Center, The Mayo Clinic and Tel-Aviv Sourasky Medical Center). Circulating leukocytes and intestinal biopsies were obtained at the time of endoscopy as part of the patients’ ongoing medical care.
GWAS and QTL analysis
Genetic association data were obtained from the 1000 genomes imputed meta-analysis from 15 genome-wide association studies of CD and/or UC conducted by the IIBDGC. 3 We conducted cis-eQTL mapping on whole peripheral blood of two cohorts; 1,240 samples from a previously published study 34 and 891 samples from the Estonian Biobank, both hybridized to Illumina HT12-v3 oligonucleotide arrays using methodology as described in detail elsewhere. 46
Statistical analysis
In all graphs, the mean is presented and the error bars correspond to the standard error of the mean (SEM). Statistical comparisons between mean values were performed using one-tailed, heteroscedastic, Student’s t test. For non-parametric variables, the Chi-square test was employed. Survival curves were examined using the Kaplan-Meier analysis.
Supplementary materials and methods
All other materials and methods are described in the supplementary section.
Supplementary Material
Acknowledgments
Grant support: The work of E.B. was supported by a National Institutes of Health grant (R01 DK073639), a CCFA Senior Research Award (SRA # 2737), a Cancer Prevention & Research Institute of Texas grant (CPRIT RP130409), and the Disease Oriented Clinical Scholars’ Program at UT Southwestern. R.K.W was supported by supported by a VIDI grant (91713308) from the Netherlands Organization for Scientific Research (NWO). The work of N.G. was supported by a start-up grant from the U.S.-Israel Binational Science Foundation (BSF #2009339) and a grant from the German Israeli Foundation. The Estonian Genome Center at the University of Tartu (T.E. and A.P.) received financing from FP7 programs (ENGAGE, OPENGENE), targeted financing from the Estonian Government (SF0180142s08), Estonian Research Roadmap through the Estonian Ministry of Education and Research, Center of Excellence in Genomics (EXCEGEN) and University of Tartu (SP1GVARENG).
We are grateful to Eric Fearon for kindly providing reagents utilized here. We are also grateful to John Shelton, Christine Komarck and Baozhi Chen for technical support for this project.
Abbreviations used in this paper
- AOM
azoxymethane
- BMDM
bone marrow derived myeloid cells
- Cfu
colony forming units
- ChIP
chromatin immunoprecipitation
- cis-eQTL
cis-expression quantitative trait locus
- CLP
cecal ligation and puncture
- COMMD1
Copper metabolism MURR1 domain containing 1
- DAI
disease activity index
- DSS
dextran sodium sulfate
- GWAS
genome wide association studies
- H3K4m1
histone 3 lysine 4 monomethylation
- H3K4m3
histone 3 lysine 4 trimethylation
- H3K27Ac
histone 3 lysine 27 acetylation
- HGD
high-grade dysplasia
- IIBDGC
International IBD genetic consortium
- IKK
IκB kinase
- IQR
inter-quartile range
- KEGG
Kyoto encyclopedia of genes and genomes
- K/O
knockout
- LD
linkage disequilibrium
- LDH
lactate dehydrogenase
- LGD
low-grade dysplasia
- LPS
lipopolysaccharide
- MFD
multifocal dysplasia
- MLN
mesenteric lymph node
- Mye-K/O
myeloid-specific Commd1 knockout
- NF-κB
nuclear factor-κB
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- SEM
standard error of the mean
- SNP
single nucleotide polymorphism
- WT
wild-type
Footnotes
Disclosures: None of the authors has a conflict of interest in connection to this work.
Author contributions: HL was responsible for the bulk of the experimental animal data performed here, including experiment execution and data analysis. LC, PB, RW, XM and PS participated in the colitis and colitis-associated cancer mouse models. SDM performed the pathological analysis of the tissue samples. AW, HS and MK performed the microarray experiments and analysis of myeloid cell LPS responses. SB-S, CV, WAF, VK and NG performed the gene expression analysis in human subjects. MM, MR, FY and VA participated in the CLP experiments. SVS and RKW performed the GWAS analysis. H-JW, TE, AM and LF performed the cis-eQTL analysis. MH, CW and BVDS were responsible for generating the mouse model and participated in specific portions of experimental design and interpretation. EB was responsible for the overall study design, data interpretation, and manuscript writing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayden MS, Ghosh S. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–34. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–24. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lees CW, Barrett JC, Parkes M, et al. New IBD genetics: common pathways with other diseases. Gut. 2011;60:1739–1753. doi: 10.1136/gut.2009.199679. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann A, Levchenko A, Scott ML, et al. The IκB-NF-κB signaling module: temporal control and selective gene activation. Science. 2002;298:11241–1245. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 6.Kovalenko A, Chable-Bessia C, Cantarella G, et al. The tumour suppressor CYLD negatively regulates NF-κB signalling by deubiquitination. Nature. 2003;424:801–805. doi: 10.1038/nature01802. [DOI] [PubMed] [Google Scholar]
- 7.Wertz IE, O’Rourke KM, Zhou H, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 8.Natoli G, Chiocca S. Nuclear ubiquitin ligases, NF-κB degradation, and the control of inflammation. Sci Signal. 2008;1:pe1. doi: 10.1126/stke.11pe1. [DOI] [PubMed] [Google Scholar]
- 9.Saccani S, Marazzi I, Beg AA, et al. Degradation of promoter-bound p65/RelA is essential for the prompt termination of the nuclear factor κB response. J Exp Med. 2004;200:107–113. doi: 10.1084/jem.20040196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mao X, Gluck N, Li D, et al. GCN5 is a required cofactor for a ubiquitin ligase that targets NF-κB/RelA. Genes Dev. 2009;23:849–861. doi: 10.1101/gad.1748409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryo A, Suizu F, Yoshida Y, et al. Regulation of NF-κB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol Cell. 2003;12:1413–1426. doi: 10.1016/s1097-2765(03)00490-8. [DOI] [PubMed] [Google Scholar]
- 12.Maine GN, Mao X, Komarck CM, et al. COMMD1 promotes the ubiquitination of NF-κB subunits through a Cullin-containing ubiquitin ligase. EMBO Journal. 2007;26:436–447. doi: 10.1038/sj.emboj.7601489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geng H, Wittwer T, Dittrich-Breiholz O, et al. Phosphorylation of NF-κB p65 at Ser468 controls its COMMD1-dependent ubiquitination and target gene-specific proteasomal elimination. EMBO Rep. 2009;10:381–386. doi: 10.1038/embor.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burstein E, Hoberg JE, Wilkinson AS, et al. COMMD proteins: A novel family of structural and functional homologs of MURR1. J Biol Chem. 2005;280:22222–22232. doi: 10.1074/jbc.M501928200. [DOI] [PubMed] [Google Scholar]
- 15.Ganesh L, Burstein E, Guha-Niyogi A, et al. The gene product Murr1 restricts HIV-1 replication in resting CD4+ lymphocytes. Nature. 2003;426:853–857. doi: 10.1038/nature02171. [DOI] [PubMed] [Google Scholar]
- 16.van de Sluis B, Rothuizen J, Pearson PL, et al. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum Mol Genet. 2002;11:165–173. doi: 10.1093/hmg/11.2.165. [DOI] [PubMed] [Google Scholar]
- 17.Biasio W, Chang T, McIntosh CJ, et al. Identification of Murr1 as a regulator of the human δ epithelial sodium channel. J Biol Chem. 2004;279:5429–5434. doi: 10.1074/jbc.M311155200. [DOI] [PubMed] [Google Scholar]
- 18.Drevillon L, Tanguy G, Hinzpeter A, et al. COMMD1-Mediated Ubiquitination Regulates CFTR Trafficking. PLoS One. 2011;6:e18334. doi: 10.1371/journal.pone.0018334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith L, Litman P, Liedtke CM. COMMD1 interacts with the COOH terminus of NKCC1 in Calu-3 airway epithelial cells to modulate NKCC1 ubiquitination. Am J Physiol Cell Physiol. 2013;305:C133–C146. doi: 10.1152/ajpcell.00394.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van de Sluis B, Mao X, Zhai Y, et al. COMMD1 disrupts HIF-1α/β dimerization and inhibits human tumor cell invasion. J Clin Invest. 2010;120:2119–2130. doi: 10.1172/JCI40583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Sluis B, Muller P, Duran K, et al. Increased activity of hypoxia-inducible factor 1 is associated with early embryonic lethality in Commd1 null mice. Mol Cell Biol. 2007;27:4142–4156. doi: 10.1128/MCB.01932-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vonk WI, Bartuzi P, de Bie P, et al. Liver-specific Commd1 knockout mice are susceptible to hepatic copper accumulation. PLoS One. 2011;6:e29183. doi: 10.1371/journal.pone.0029183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao X, Gluck N, Chen B, et al. Copper metabolism MURR1 domain containing 1 (COMMD1) regulates Cullin-RING ligases by preventing Cullin-associated NEDD8-dissociated (CAND1) binding. J Biol Chem. 2011;286:32355–32365. doi: 10.1074/jbc.M111.278408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starokadomskyy P, Gluck N, Li H, et al. CCDC22 deficiency in humans blunts activation of proinflammatory NF-κB signaling. J Clin Invest. 2013;123:2244–2256. doi: 10.1172/JCI66466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barish GD, Yu RT, Karunasiri M, et al. Bcl-6 and NF-κB cistromes mediate opposing regulation of the innate immune response. Genes Dev. 2010;24:2760–5. doi: 10.1101/gad.1998010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reveille JD, Sims AM, Danoy P, et al. Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat Genet. 2010;42:123–7. doi: 10.1038/ng.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okada Y, Terao C, Ikari K, Kochi Y, et al. Meta-analysis identifies nine new loci associated with rheumatoid arthritis in the Japanese population. Nat Genet. 2012;44:511–6. doi: 10.1038/ng.2231. [DOI] [PubMed] [Google Scholar]
- 28.Kenny EE, Pe’er I, Karban A, et al. A genome-wide scan of Ashkenazi Jewish Crohn’s disease suggests novel susceptibility loci. PLoS Genet. 2012;8:e1002559. doi: 10.1371/journal.pgen.1002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson CA, Boucher G, Lees CW, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246–252. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franke A, McGovern DP, Barrett JC, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Consortium TEP. A user’s guide to the encyclopedia of DNA elements (ENCODE) PLoS Biol. 2011;9:e1001046. doi: 10.1371/journal.pbio.1001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaikkonen MU, Spann NJ, Heinz S, et al. Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Mol Cell. 2013;51:310–25. doi: 10.1016/j.molcel.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicolae DL, Gamazon E, Zhang W, et al. Trait-associated SNPs are more likely to be eQTLs: annotation to enhance discovery from GWAS. PLoS Genet. 2010;6:e1000888. doi: 10.1371/journal.pgen.1000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu J, Wolfs MG, Deelen P, et al. Unraveling the regulatory mechanisms underlying tissue-dependent genetic variation of gene expression. PLoS Genet. 2012;8:e1002431. doi: 10.1371/journal.pgen.1002431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greten FR, Eckmann L, Greten TF, et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Bollrath J, Phesse TJ, von Burstin VA, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Bollrath J, Greten FR. IKK/NF-κB and STAT3 pathways: central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep. 2009;10:1314–1319. doi: 10.1038/embor.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss KH, Lozoya JC, Tuma S, et al. Copper-Induced Translocation of the Wilson Disease Protein ATP7B Independent of Murr1/COMMD1 and Rab7. Am J Pathol. 2008;173:1783–1794. doi: 10.2353/ajpath.2008.071134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyayama T, Hiraoka D, Kawaji F, et al. Roles of COMM-domain-containing 1 in stability and recruitment of the copper-transporting ATPase in a mouse hepatoma cell line. Biochemical Journal. 2010;429:53–61. doi: 10.1042/BJ20100223. [DOI] [PubMed] [Google Scholar]
- 40.Chang T, Ke Y, Ly K, et al. COMMD1 regulates the delta epithelial sodium channel (δENaC) through trafficking and ubiquitination. Biochem Biophys Res Commun. 2011;411:506–511. doi: 10.1016/j.bbrc.2011.06.149. [DOI] [PubMed] [Google Scholar]
- 41.Nenci A, Becker C, Wullaert A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 42.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Popivanova BK, Kitamura K, Wu Y, et al. Blocking TNF-α in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kabakchiev B, Silverberg MS. Expression quantitative trait loci analysis identifies associations between genotype and gene expression in human intestine. Gastroenterology. 2013;144:1488–96. 1496e1–3. doi: 10.1053/j.gastro.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rivas MA, Beaudoin M, Gardet A, et al. Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat Genet. 2011;43:1066–1073. doi: 10.1038/ng.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fehrmann RS, Jansen RC, Veldink JH, et al. Trans-eQTLs reveal that independent genetic variants associated with a complex phenotype converge on intermediate genes, with a major role for the HLA. PLoS Genet. 2011;7:e1002197. doi: 10.1371/journal.pgen.1002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.