Abstract
Spermatogonial stem cells (SSCs, also called germline stem cells) are self-renewing unipotent stem cells that produce differentiating germ cells in the testis. SSCs can be isolated from the testis and cultured in vitro for long-term periods in the presence of feeder cells (often mouse embryonic fibroblasts). However, the maintenance of SSC feeder culture systems is tedious because preparation of feeder cells is needed at each subculture. In this study, we developed a Matrigel-based feeder-free culture system for long-term propagation of SSCs. Although several in vitro SSC culture systems without feeder cells have been previously described, our Matrigel-based feeder-free culture system is time- and cost- effective, and preserves self-renewability of SSCs. In addition, the growth rate of SSCs cultured using our newly developed system is equivalent to that in feeder cultures. We confirmed that the feeder-free cultured SSCs expressed germ cell markers both at the mRNA and protein levels. Furthermore, the functionality of feeder-free cultured SSCs was confirmed by their transplantation into germ cell-depleted mice. These results suggest that our newly developed feeder-free culture system provides a simple approach to maintaining SSCs in vitro and studying the basic biology of SSCs, including determination of their fate.
Keywords: cell culture, feeder-free, matrigel, spermatogonial stem cells
INTRODUCTION
Spermatogonial stem cells (SSCs), also called germline stem cells, are unipotent precursor cells that self-renew and contribute to spermatogenesis in the testis. Although SSCs represent an extremely rare population (0.02–0.03%) in the testis (Tegelenbosch and de Rooij, 1993), they can be isolated and propagated in vitro from neonatal or adult testes (Kanatsu-Shinohara et al., 2003; Ko et al., 2009; Kubota et al., 2004). Recent study demonstrated that derivation of SSCs is possible even from small biopsied testicular tubules (Ko et al., 2012). Established SSCs are useful stem cell lines that allow not only to study basic reproductive biology but also to develop an in vitro model for applications in assisted reproductive medicine.
SSCs require a specific culture system for successful long-term expansion in vitro. In vitro propagation of SSCs requires glial cell line-derived neurotrophic factor (GDNF) (Kubota et al., 2004; Ryu et al., 2005), which is secreted from Sertoli cells for maintenance of SSCs in the testis (Meng et al., 2000), as well as other growth factors, such as basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) are required for in vitro propagation of SSCs (Kubota et al., 2004; Rastegar et al., 2013). In general, physical interaction of SSCs with the basal epithelial membrane is required to provide a microniche for self-renewing (Oatley and Brinster, 2012; Phillips et al., 2010; Ryu et al., 2006; Silvan et al., 2013). When primary SSCs are derived from the testis and proliferated in vitro, mouse embryonic fibroblasts (MEFs) are the most commonly used feeder cells that play a role of such microniche by allowing secure attachment and proliferation of SSCs. MEFs used as feeder cells may also secrete certain components into the media that support SSC proliferation. However, preparation of MEFs is a multi-step and tedious procedure that includes culturing fibroblasts from mouse embryos and mitotic inactivation by mitomycin C or irradiation at each subculture (Bodnar et al., 2004). Another disadvantage is variability in the quality of prepared MEFs between different batches and laboratories (Mallon et al., 2006). Coculture of SSCs with MEFs results in a requirement for removal of the feeder cells from SSCs. Therefore, feeder-dependent culture systems for maintenance of SSCs in vitro are time-consuming and cost-ineffective. Thus, the availability of feeder-free culture systems suitable for culturing SSCs in vitro would allow large-scale propagation of SSCs.
Matrigel, extracted from the Engelbreth-Holm-Swarm mouse tumors, contains several extracellular matrix (ECM) molecules, such as laminin, collagen, entactin, heparan sulfate proteoglycan, and also growth factors, such as fibroblast growth factor (FGF), EGF, insulin-like growth factor 1 (IGF-1), transforming growth factor beta (TGF-β), and platelet-derived growth factor (PDGF) (Braam et al., 2008; Vukicevic et al., 1992). Matrigel is widely used as the feeder-free substrate to mimic the ECM for cell culture, presumably by replicating cell-ECM interactions. Matrigel has been shown to provide an optimal microenvironment for stem cell culture, especially for embryonic stem cells (ESCs), because of its ability to maintain self-renewality and pluripotency of ESCs (Mallon et al., 2006). In the present study, we evaluated the ability of Matrigel to support the attachment of SSCs and their long-term maintenance in vitro without feeder layers.
We found that feeder-free cultured SSCs proliferated for at least 5 months at a rate comparable to that of SSCs cultured on MEFs. During this time, SSCs retained their cellular properties and functionality. Our feeder-free culture systems have a potential to enable studies of regulatory mechanisms that determine the SSC fate in an efficient and cost-effective way.
MATERIALS AND METHODS
SSC cultures
SSCs were established from Oct4-GFP and Oct4-GFP/LacZ transgenic mice (C57BL/6 background) as previously described (Ko et al., 2009; 2010; 2012). After a two-step digestion of testicular tubules, testicular cells were plated onto gelatin-coated culture dishes (2 × 105 cells/3.8 cm2) with SSC culture medium. SSC colonies were observed under the microscope within 7 days. SSC colonies were collected by gentle pipetting and re-plated on mitomycin C-treated MEFs for expansion (Ko et al., 2009). SSCs were maintained on MEFs or feeder-free (Matrigel-coated) plates and passaged every 5 days. Cell numbers were counted at each passage; cells were replated (5 × 105 cells/well) in 12-well plates. Experiments were conducted under protocols approved by the Konkuk University Animal Care and Use Committee.
SSC medium was composed of StemPro-34 SFM (Invitrogen) with the following supplements: StemPro supplement (Invitrogen), 1× N2 supplement (Invitrogen), 6 mg/ml d-(+)-glucose (Invitrogen), 30 mg/ml pyruvic acid (Invitrogen), 1 μl/ml DL-lactic acid (Sigma), 5 mg/ml bovine serum albumin (BSA; Invitrogen), 1% fetal bovine serum (Invitrogen), 2 mM L-glutamine (Invitrogen), 50 μM β-mercaptoethanol (Invitrogen), 1 × penicillin/streptomycin (Welgene), 1× minimal essential medium (MEM) non-essential amino acids (Invitrogen), 1× MEM vitamins (Invitrogen), 30 ng/ml β-estradiol (Sigma), 60 ng/ml progesterone (Sigma), 20 ng/ml human EGF (Peprotech), 20 ng/ml human bFGF (Peprotech), 20 ng/ml human GDNF (Peprotech), and 103 U/ml murine leukemia inhibitory factor (Prospec).
Preparation of extracellular matrix-coated plates
Culture plates were coated with ECM, Matrigel (BD Biosciences) or laminin (Sigma) by the following procedure. A Matrigel bottle was thawed on ice in a 4°C refrigerator overnight until Matrigel liquified. Matrigel was divided into 300 μl aliquots and stored at −20°C until use. For preparation of Matrigel-coated plates, working Matrigel solution was prepared by diluting 300 μl of Matrigel with 29 ml of DMEM/F12 medium (Invitrogen) and thorough mixing. This solution was added to 12-well plates (0.5 ml per well) or 6-well plates (1 ml per well) to cover the whole surface of the wells. The plates were allowed to sit for 1 h at room temperature or overnight at 4°C. Excess Matrigel solution was then removed, and the plates were washed once with DMEM/F12. For laminin coating, laminin stock solution (1 mg/ml) was divided into 50 μl aliquots and stored at −20°C. Laminin working solutions (20 μg/ml) were prepared by diluting the stock solution with Dulbecco's phosphate-buffered saline (DPBS), and added to 12-well plates (0.5 ml per well) or 6-well plates (1 ml per well). Plates were incubated with laminin solution for at least 2 h in a 37°C in a cell culture incubator, then excess laminin solution was removed and the plates were washed twice with DPBS.
RT-PCR analysis
Total RNA was isolated by using the miRNeasy Mini Kit according to the manufacturer’s instructions (QIAGEN). Total RNA (500 ng) was reverse-transcribed by using the Omniscript RT Kit (QIAGEN) in a total volume of 20 μl. PCR analysis was performed with gene-specific primers (Supplementary Table 1) and Takara Ex Taq DNA polymerase (Takara) according to the manufacturer’s instructions. The PCR conditions were as follows: 32 cycles at 94°C for 30 s, 50–65°C for 30 s, and 72°C for 30 s. The RT-PCR products were analyzed by electrophoresis in 1% agarose gels.
Flow-cytometric analysis
SSCs were resuspended in ice-cold DPBS containing 0.5% BSA at 1 × 106 cells/ml, and incubated for 20 min on ice with the following antibodies: CD326 (EpCAM), CD49f (Intergrin α6), fluorescein isothiocyanate (FITC)-conjugated CD29 (Intergrin β1) (all from BD Biosciences), or phycoerythrin-conjugated CD117 (c-kit) (Biolegend). Cells were then washed with 0.5% BSA in PBS and incubated with secondary antibodies: anti-mouse IgM-FITC (Sigma) for CD326 (EpCAM), anti-rat IgGFITC (BD Biosciences) for CD49f (Intergrin α6). Analysis was performed by flow cytometry (BD Biosciences) using the CellQuest software (BD Biosciences).
DNA methylation analysis
Cells were washed with PBS, and genomic DNA was isolated using the Total DNA Extraction kit (iNtRON) according to the manufacturer’s protocol. Genomic DNA was treated with EpiTech Bisulfite (QIAGEN) according to the manufacturer’s recommendation, and used for PCR amplification. The PCR products were subcloned using the PCR Cloning kit (QIAGEN). Primers for bisulfite sequencing are listed in Supplementary Table 1.
Testicular transplantation
Transplantation was performed as described (Ko et al., 2009). Six-week-old C57BL/6 male mice were treated with 40 mg/kg busulfan by intraperitoneal injection and used as recipients 1 month after busulfan treatment. SSC medium (approximately 10 μl) containing 5 × 105 cells and 0.04% trypan blue was injected with a micropipette (40–60 μm diameter tips) into the seminiferous tubules of the testes of recipient mice through the efferent duct (which connects the testis to the epididymis) (Ko et al., 2012). The mice were sacrificed 2 months later, and their testicular tubules were stained for LacZ to reveal positive cells corresponding to colonization by transplanted SSCs.
RESULTS
Long term-expansion of SSCs on Matrigel-coated dishes
SSCs colonies have a typical grape-like morphology when cultured on mitomycin C-treated MEFs (Figs. 1A and 1B). For expansion of the SSCs cultured on feeder cells, feeders have to be prepared for every subculture. This approach has considerable drawbacks, as it involves several tedious and time-consuming procedures, including culturing MEFs and their mitotic inactivation with mitomycin C. Therefore, a feeder-free culture system would allow maintaining and propagating SSCs in a labor- and cost-effective manner.
Fig. 1.
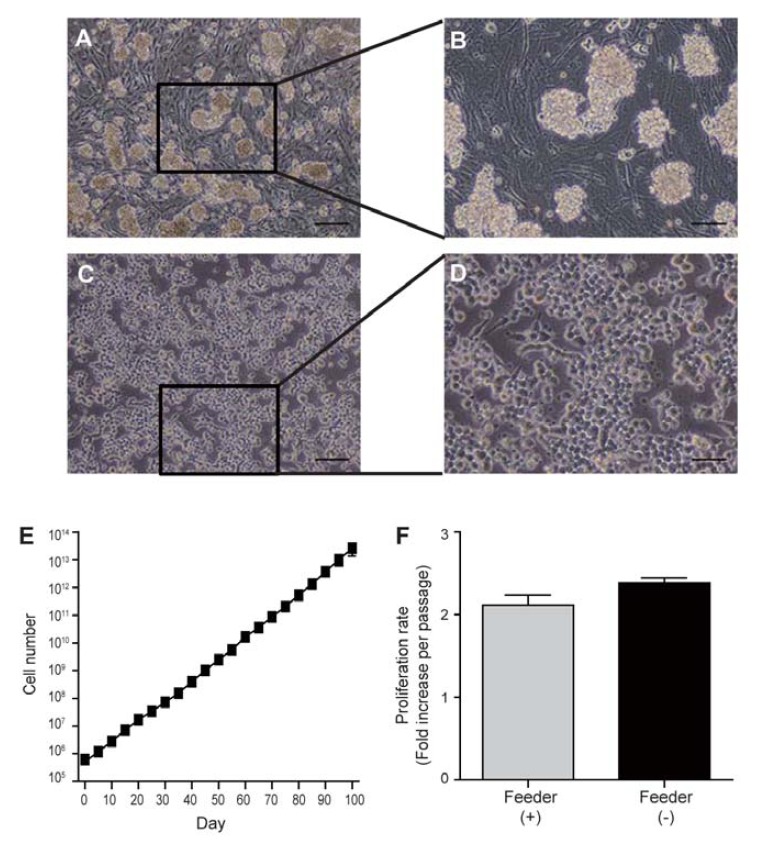
Cultivation of SSCs on Matrigel in vitro. (A) and (B) Fully established SSCs on mitomycin C-treated MEFs. (C, D) SSCs cultured on Matrigel. (E) Cell number increase in Matrigel-based cultures. SSCs were seeded at a density of 5 × 105 cells/well in a 12-well plate, and cell numbers were counted for 100 days. (F) Cell proliferation rate in feeder- and Matrigel-based cultures. Scale bars, 100 μm (A, C) or 50 μm (B, D).
SSCs in the testis are located and maintained on the basal membrane of testicular tubules. This implies that the in vitro maintenance of SSCs would require environments similar to that in vivo, such as feeder layers. Indeed, we found that SSCs were not able to attach to and propagate on gelatin-coated dishes without feeder cells (Supplementary Fig. 1). Since Matrigel is known to be highly biologically active and closely resembles the basal membrane (because it contains the ECM molecules), we examined the feasibility of using Matrigel-coated plates for the attachment and expansion of SSCs. First, SSCs were plated at different densities (2–7 × 105 cells per well) into Matrigel-coated 12-well plates (Supplementary Fig. 2A). We found that Matrigel could support the attachment of SSCs and their proliferation. We compared the morphology of SSCs grown on Matrigel-coated plates with that of SSCs cultured on feeder cells (Figs. 1A–1D). On feeders, SSCs formed grape-like colonies, whereas on Matrigel they grew as a monolayer. SSCs proliferated on Matrigel in a cell number-dependent manner; their growth was optimal when 5 × 105 cells per well of a 12-well plate were initially plated on Matrigel (Supplementary Fig. 2B). Therefore, we used 5 × 105 cells per well of a 12 well plate for all other experiments.
To examine the expandability of SSCs cultured on Matrigel, we replated them at 5 × 105 cells per well of a 12 well plate every 5 days. The proliferation ability was maintained for more than 100 days at rates of approximately 2.5-fold per 5 days, which was similar to proliferation rates in the feeder culture system (Figs. 1E and 1F). This suggests that Matrigel can support unlimited in vitro proliferation of SSCs for long time periods. However, we found that proliferation ability was lost at passage 3 in SSCs cultured on laminin-coated plates (Supplementary Fig. 1), and that little or no proliferation was observed on gelatin (Supplementary Fig. 1).
Characterization of SSCs under feeder-free conditions
To characterize SSCs cultured in Matrigel-based feeder-free conditions, we performed RT-PCR and FACS analysis to examine SSC-specific gene expression at the mRNA (MEF and testis samples used as negative control and positive control, respectively) and surface protein levels, respectively. As shown in Fig. 2A, SSCs cultured under feeder-free conditions expressed the SSC marker genes Oct4, Rex1, Esg1, Tex18, Vasa, Dazl, Piwil2 and Zfp 145, but not Nanog. FACS analysis showed that expression of SSC-specific surface proteins was similar under feeder and feeder-free conditions (Fig. 2B). Integrin α6 (CD49f), integrin β1 (CD29), and EpCAM (CD326) were strongly expressed, whereas c-kit (CD117) was weakly expressed under both conditions. However, the mESC-specific marker SSEA-1 (CD15) was not expressed in either feeder- or feeder-free-cultured SSCs (data not shown). Thus, the levels of SSC marker gene mRNA and surface proteins in feeder-free-cultured SSCs were similar to those in feeder-cultured SSCs. These results indicate that the feeder-free culture system maintains typical for SSCs levels of expression of both SSC marker gene mRNA and surface proteins.
Fig. 2.
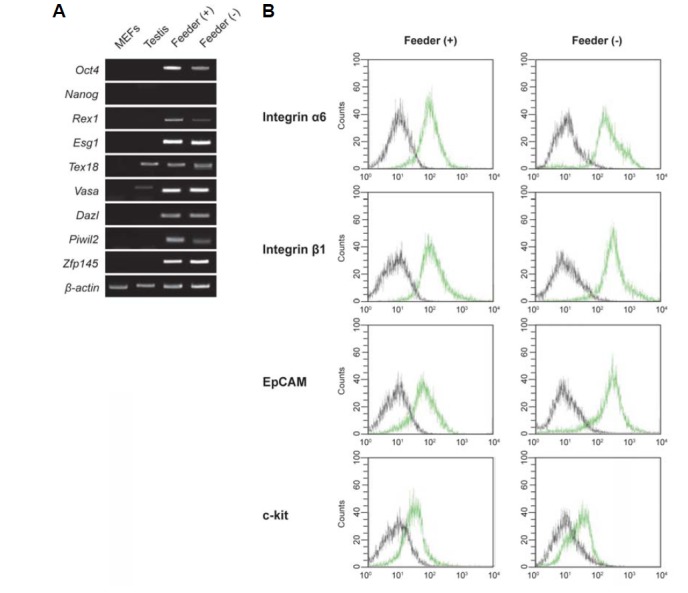
Cellular and molecular characterization of SSCs cultured on Matrigel. (A) RT-PCR analysis of SSCs-specific gene expression. Lane 1, MEFs; lane 2, testis; lane 3, SSCs cultured on mitomycin C-treated MEFs, lane 4; SSCs cultured on Matrigel. (B) FACS analysis of the expression of SSC surface proteins: integrin α6 (CD49f), integrin β1 (CD29), EpCAM (CD326), and c-kit (CD117). Black lines, isotype controls; green lines, SSC surface proteins. SSCs are positive for the SSC markers integrin α6 (CD49f), integrin β1 (CD29), EpCAM (CD326) and c-kit (CD117).
Stability of genomic imprinting patterns in SSCs cultured in the feeder-free system
To investigate the epigenetic stability of SSCs cultured in the feeder-free system, we conducted bisulfite DNA sequencing analysis to determine the genomic imprinting pattern in these SSCs. DNA methylation patterns of the differentially methylated regions (DMRs) of the paternally imprinted gene H19 and maternally imprinted genes Peg1, insulin-like growth factor receptor (Igf2r), and Snrpn were examined in the SSCs harvested from feeder-free cultures. Bisulfite sequencing analysis showed androgenetic patterns in the DNA methylation status of the imprinted genes: H19 was hypermethylated, whereas Peg1, Igf2r and Snrpn were hypomethylated (Fig. 3). These patterns were the same as in the feeder-cultured SSCs. Therefore, our feeder-free culture system is able to maintain stable DNA methylation status of imprinted genes in SSCs.
Fig. 3.
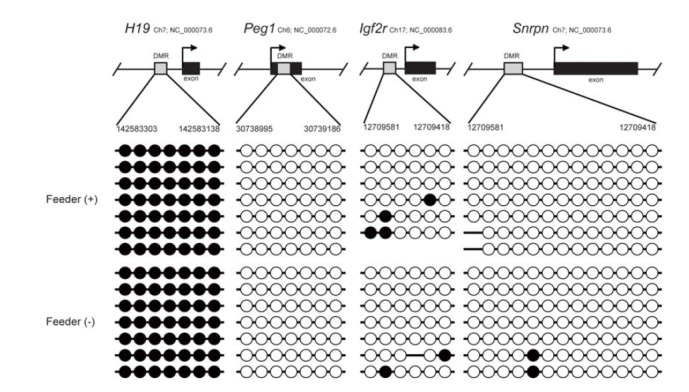
Comparison of the DNA methylation status of paternally and maternally imprinted genes between SSCs in feeder and Matrigel cultures. DNA methylation pattern of DMRs(H19, Peg1, Igf2r and Snrpn) in feeder- or Matrigel-cultured SSCs was analyzed by bisulfite sequencing. Each line represents a separate clone. Black and white circles represent methylated and unmethylated CpGs, respectively.
Determination of stem cell activity by spermatogonial transplantation
To determine the stem cell activity of cultured SSCs, we performed the spermatogonial transplantation assay. Because there is no clear functional in vitro assay for SSCs, the only reliable assay is to examine the recolonization ability of transplanted SSCs in the seminiferous tubules of infertile animals to restore spermatogenesis (Brinster and Zimmermann, 1994; Schlatt, 2002; Tang et al., 2012). Feeder-free cultured Oct4-GFP/LacZ SSCs were harvested (Figs. 4A and 4B), single-cell suspended, and transplanted into the seminiferous tubules of busulfan-treated adult mice through the efferent ducts. Two months after transplantation, the recipients were sacrificed, and their testes were examined for colonization by transplanted SSC by detecting the expression of Oct4-GFP by fluorescence and β-galactosidase expression by LacZ staining. Oct4-GFP/LacZ cells were found in the recipient testicular tubules (Figs. 4C and 4D), indicating that our feeder-free culture system maintains the SSC ability to colonize testicular tubules.
Fig. 4.
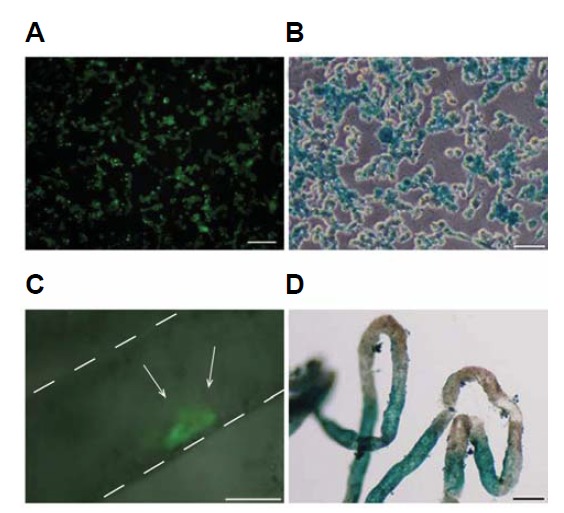
SSC transplantation into testicular tubules. (A) Oct4-GFP fluorescence (green) and (B) LacZ-positive staining (blue) in SSCs cultured on Matrigel. (C, D) Approximately 5 × 105 SSCs cultured on Matrigel were injected with a micropipette into the seminiferous tubules of the testes of busulfan (40 mg/kg) treated male mice. (C) A recipient testis that received Oct4-GFP expressing donor SSCs (green). White arrows indicate colonization by transplanted SSCs in the recipient testicular tubules. (D) LacZ staining of the recipient testicular tubules, indicating the presence of the transplanted LacZ SSCs. Blue stretches of the tubules indicate the presence of donor cell-derived germ cell colonies. Scale bars, 50 μm.
DISCUSSION
SSCs generally require the basement membrane to maintain their stem cell properties both in vivo and in vitro (Matsui et al., 1992; Resnick et al., 1992; Spradling et al., 2001). A long-term survival of SSCs in vitro on feeder layers was initially reported, but they disappear within 1 week when cultured directly on a tissue culture dish without feeders (Nagano et al., 1998), which is consistent with our finding that gelatin-coated dishes could not support SSC culture. This suggests that a feeder layer is essential for maintaining SSCs in vitro. However, feeder cells require cumbersome procedures for preparation of mitotically arrested MEFs, and batch-to-batch and lab-to-lab variability in prepared MEFs contributes to possible variability and contamination in the experimental results (Mallon et al., 2006). Thus, development of feeder-free culture systems would provide more reproducible SSC cultures and facilitate scale-up in a time- and cost-effective manner.
Several previous attempts have been made to culture SSCs under feeder-free conditions using laminin (Kanatsu-Shinohara et al., 2005; 2011), but in this case new SSC culture medium components were required. However, our feeder-free culture system uses the same SSC medium as the feeder culture systems, so no new components need to be prepared. Furthermore, we found an obvious difference in the SSC proliferation rate between laminin and Matrigel (Supplementary Fig. 1). Although it was not significantly different during the first 3 passages, SSC proliferation on laminin was dramatically reduced after 4 passages. This suggests that Matrigel is more suitable for maintaining proliferating SSC cultures in vitro without feeder layers.
It should be noted that efficient propagation of SSCs on Matrigel requires more than 5 × 105 cells per well of a 12 well plate. Our feeder-free culture is cell number-dependent with the optimal cell density of approximately 1.3 × 105 cells/cm2. It is possible that the cell number-dependent growth of SSCs under feeder-free conditions may be related to the action of survival-enhancing autocrine factors, which is well described in ESCs (Mittal and Voldman, 2011).
We chose Matrigel as a matrix in our feeder-free culture for SSCs. Matrigel is the manufacturer’s trademark for ECM extracted from the Engelbreth-Holm-Swarm tumors (Vukicevic et al., 1992). In our initial experiments on feeder-free SSC culture, we used standard Matrigel that contains a mixture of ECM molecules and growth factors, such as EGF, IGF-1, TGF-β and PDGF. Later, we switched to growth factor–reduced (GFR) Matrigel for use in the feeder-free cultures. We found no difference between standard Matrigel and GFR-Matrigel in their ability to support SSC proliferation (Supplementary Fig. 3). This suggests that the growth factors in standard Matrigel may not influence SSC proliferation under feeder-free culture conditions, which was also confirmed by the results that adding IGF-1, TGF-β, and PDGF did not support proliferation of SSCs on gelatin-coated dishes. Thus, a combination of the major ECM components, such as laminin, collagen, entactin, and heparan sulfate proteoglycan, likely plays a role in creating proper basement membrane-like environment for SSCs in our feeder-free culture.
Our expression analysis of genes for intracellular and cell surface SSCs markers revealed no differences between our feeder-free culture system and a feeder culture. DNA imprinting patterns of SSCs were also preserved in the feeder-free culture: the methylation status was androgenetic in both culture types. Furthermore, we found that SSCs transplanted to the male mouse testicular tubes were able to colonize the recipient testis. Therefore, the feeder-free culture system can maintain the cellular and molecular phenotypes as well as functionality of SSCs.
Our feeder-free culture system for expansion of SSCs is simple and efficient, because it uses the same SSC medium as the feeder cultures. Moreover, in comparison with the laminin-based culture, our Matrigel-based feeder-free culture system is superior as it allows long-term cell proliferation, whereas laminin cannot support SSC proliferation after 3 passages.
In this context, the Matrigel-based culture system provides time- and cost-effective unified approaches for feeder-free expansion of SSCs. Moreover, as it excludes variability caused by the presence of feeder cells, this system should be suitable for more definitive experiments to study the molecular mechanisms regulating SSCs, which is important in basic reproductive and stem cell biology, and also for therapeutic applications of germ cells in infertility treatment.
In this study, we developed a novel feeder-free culture system to support long-term maintenance of SSCs. We have demonstrated that our culture system preserves SSC properties at the molecular and cellular levels and their functionality. The advantage of our feeder-free culture system is that it provides a time- and cost-effective method to maintain SSCs, because it does not require feeders and uses the same medium as feeder cultures. Thus, our culture protocol will make culturing SSCs simple and reproducible, facilitate scale-up, and allow definitive experiments to evaluate individual factors affecting SSC stemness, which is important both in basic and applied reproductive biology.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (2013031170) and by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare (HI12C0337), Republic of Korea.
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Bodnar M.S., Meneses J.J., Rodriguez R.T., Firpo M.T. Propagation and maintenance of undifferentiated human embryonic stem cells. Stem Cells Dev. 2004;13:243–253. doi: 10.1089/154732804323099172. [DOI] [PubMed] [Google Scholar]
- Braam S.R., Zeinstra L., Litjens S., Ward-van Oostwaard D., van den Brink S., van Laake L., Lebrin F., Kats P., Hochstenbach R., Passier R., et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells. 2008;26:2257–2265. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- Brinster R.L., Zimmermann J.W. Spermatogenesis following male germ-cell transplantation. Proc. Natl. Acad. Sci. USA. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Ogonuki N., Inoue K., Miki H., Ogura A., Toyokuni S., Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol. Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Miki H., Inoue K., Ogonuki N., Toyokuni S., Ogura A., Shinohara T. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol. Reprod. 2005;72:985–991. doi: 10.1095/biolreprod.104.036400. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Inoue K., Ogonuki N., Morimoto H., Ogura A., Shinohara T. Serum- and feeder-free culture of mouse germline stem cells. Biol. Reprod. 2011;84:97–105. doi: 10.1095/biolreprod.110.086462. [DOI] [PubMed] [Google Scholar]
- Ko K., Tapia N., Wu G., Kim J.B., Bravo M.J., Sasse P., Glaser T., Ruau D., Han D.W., Greber B., et al. Induction of pluripotency in adult unipotent germline stem cells. Cell Stem Cell. 2009;5:87–96. doi: 10.1016/j.stem.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Ko K., Arauzo-Bravo M.J., Kim J., Stehling M., Scholer H.R. Conversion of adult mouse unipotent germline stem cells into pluripotent stem cells. Nat. Protoc. 2010;5:921–928. doi: 10.1038/nprot.2010.44. [DOI] [PubMed] [Google Scholar]
- Ko K., Wu G., Arauzo-Bravo M.J., Kim J., Francine J., Greber B., Muhlisch J., Joo J.Y., Sabour D., Fruhwald M.C., et al. Autologous pluripotent stem cells generated from adult mouse testicular biopsy. Stem Cell Rev. 2012;8:435–444. doi: 10.1007/s12015-011-9307-x. [DOI] [PubMed] [Google Scholar]
- Kubota H., Avarbock M.R., Brinster R.L. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc. Natl. Acad. Sci USA. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallon B.S., Park K.Y., Chen K.G., Hamilton R.S., McKay R.D. Toward xeno-free culture of human embryonic stem cells. Int. J. Biochem. Cell Biol. 2006;38:1063–1075. doi: 10.1016/j.biocel.2005.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y., Zsebo K., Hogan B.L. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992;70:841–847. doi: 10.1016/0092-8674(92)90317-6. [DOI] [PubMed] [Google Scholar]
- Meng X., Lindahl M., Hyvonen M.E., Parvinen M., de Rooij D.G., Hess M.W., Raatikainen-Ahokas A., Sainio K., Rauvala H., Lakso M., et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- Mittal N., Voldman J. Nonmitogenic survival-enhancing autocrine factors including cyclophilin A contribute to density-dependent mouse embryonic stem cell growth. Stem Cell. Res. 2011;6:168–176. doi: 10.1016/j.scr.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M., Avarbock M.R., Leonida E.B., Brinster C.J., Brinster R.L. Culture of mouse spermatogonial stem cells. Tissue Cell. 1998;30:389–397. doi: 10.1016/s0040-8166(98)80053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley J.M., Brinster R.L. The germline stem cell niche unit in mammalian testes. Physiol. Rev. 2012;92:577–595. doi: 10.1152/physrev.00025.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips B.T., Gassei K., Orwig K.E. Spermatogonial stem cell regulation and spermatogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010;365:1663–1678. doi: 10.1098/rstb.2010.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastegar T., Minaee M.B., Habibi Roudkenar M., Raghardi Kashani I., Amidi F., Abolhasani F., Barbarestani M. Improvement of expression of alpha6 and beta1 Integrins by the co-culture of adult mouse spermatogonial stem cells with SIM mouse embryonic fibroblast cells (STO) and growth factors. Iran. J. Basic Med. Sci. 2013;16:134–139. [PMC free article] [PubMed] [Google Scholar]
- Resnick J.L., Bixler L.S., Cheng L., Donovan P.J. Long-term proliferation of mouse primordial germ cells in culture. Nature. 1992;359:550–551. doi: 10.1038/359550a0. [DOI] [PubMed] [Google Scholar]
- Ryu B.Y., Kubota H., Avarbock M.R., Brinster R.L. Conservation of spermatogonial stem cell self-renewal signaling between mouse and rat. Proc. Natl. Acad. Sci. USA. 2005;102:14302–14307. doi: 10.1073/pnas.0506970102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu B.Y., Orwig K.E., Oatley J.M., Avarbock M.R., Brinster R.L. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells. 2006;24:1505–1511. doi: 10.1634/stemcells.2005-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlatt S. Germ cell transplantation. Mol. Cell. Endocrinol. 2002;186:163–167. doi: 10.1016/s0303-7207(01)00661-x. [DOI] [PubMed] [Google Scholar]
- Silvan U., Diez-Torre A., Moreno P., Arluzea J., Andrade R., Silio M., Arechaga J. The spermatogonial stem cell niche in testicular germ cell tumors. Int. J. Dev. Biol. 2013;57:185–195. doi: 10.1387/ijdb.130068ja. [DOI] [PubMed] [Google Scholar]
- Spradling A., Drummond-Barbosa D., Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- Tang L., Rodriguez-Sosa J.R., Dobrinski I. Germ cell transplantation and testis tissue xenografting in mice. J. Vis. Exp. 2012;6:pii: 3545. doi: 10.3791/3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegelenbosch R.A., de Rooij D.G. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat. Res. 1993;290:193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- Vukicevic S., Kleinman H.K., Luyten F.P., Roberts A.B., Roche N.S., Reddi A.H. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp. Cell Res. 1992;202:1–8. doi: 10.1016/0014-4827(92)90397-q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


