Abstract
A vaccine that can prevent the transmission of HIV-1 at the site of exposure to the host is one of the best hopes to control the HIV-1 pandemic. The trimeric envelope spike consisting of heterodimers, gp120 and gp41, is essential for virus entry and thus has been a key target for HIV-1 vaccine development. However, it has been extremely difficult to identify the types of antibodies required to block the transmission of various HIV-1 strains and the immunogens that can elicit such antibodies due to the high genetic diversity of the HIV-1 envelope. The modest efficacy of the gp120 HIV-1 vaccine used in the RV144 Thai trial, including the studies on the immune correlates of protection, and the discovery of vaccine-induced immune responses to certain signature regions of the envelope have shown that the gp120 variable loop 2 (V2) is an important region. Since there is evidence that the V2 region interacts with the integrin α4β7 receptor of the host cell, and that this interaction might be important for virus capture, induction of antibodies against V2 loop could be postulated as one of the mechanisms to prevent the acquisition of HIV-1. Immunogens that can induce these antibodies should therefore be taken into consideration when designing HIV-1 vaccine formulations.
1. Introduction
Developing an efficacious prophylactic HIV vaccine that can prevent acquisition of HIV-1 at the initial site of viral entry remains as the best hope to control the spread of the disease and also as one of the biggest challenges of our time. The genetic diversity of HIV-1 and the extraordinary evolution of the viral envelope to evade host immune responses pose a major challenge to the development of globally effective vaccines [1-4]. Vaccine development has focused on six HIV-1 subtypes that collectively account for the majority of HIV-1 infections worldwide.
A number of broadly neutralizing monoclonal antibodies have been generated that target different regions of gp120, including quaternary antibodies that recognize epitopes in the V2 and V3 loops of gp120 [5-14]. However the variable regions of gp120 have not been generally considered as suitable vaccine targets because of their sequence variability. This reasoning has now changed based on the results of the RV144 vaccine trial, which showed an inverse correlation between the antibody response to the V1V2 region [15] and risk of infection [16]. The V2 region may therefore be a site of HIV-1 vulnerability and should be taken into consideration in the design of future HIV-1 vaccines. In this review, we discuss the variable regions of gp120 and in particular the V2 region, the participation of the V2 region in the infection process, the importance of V2-specific antibodies, the lessons learned based on the RV144 trial, and finally the possible path to a successful HIV-1 vaccine.
2. HIV-1 Envelope protein
Comparison of genes in the HIV-1 genome among various isolates reveals that envelope is the most variable in nucleotide sequence. The HIV-1 env gene encodes a single glycosylated protein, gp160, which is subsequently cleaved intracellularly by furin protease during the process of maturation. The product of this cleavage is a heterodimer consisting of non-covalently associated gp120 and gp41 glycoproteins embedded in the viral lipid bilayer [17]. These glycoproteins play a prominent role in the fusion of the viral particles to the target cells [18]. On the surface of the virions, 3 copies of the heterodimer assemble to form a functional trimer spike that is visible in appearance as a characteristic three-bladed propeller (3 gp120 blades connected both at the apex and at the base, corresponding to the gp120/gp41 interface) in electron micrographs of purified viruses and trimmers [19-21].
2.1 Viral entry
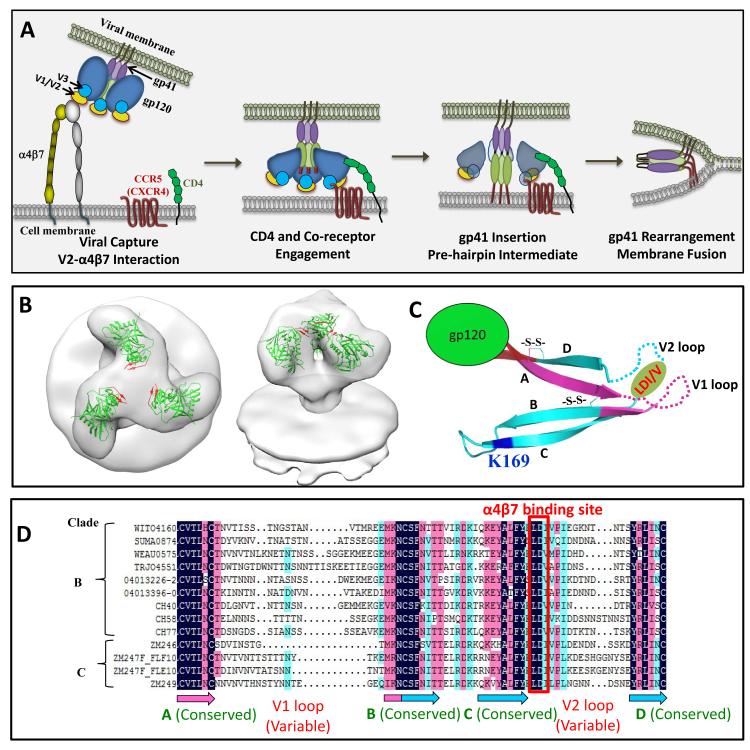
The entry mechanism of HIV-1 into a CD4+ T cell is shown in Figure 1A. The trimeric envelope exists in a highly dynamic state and undergoes a series of well-orchestrated conformational changes upon binding to the primary CD4 receptor on the cell membrane followed by binding to the coreceptor CCR5 or CXCR4 leading to membrane fusion and release of the nucleocapsid core into the cytosol [22]. Analyses by cryoelectron tomography of the trimeric envelope bound to soluble CD4 or to monoclonal antibodies have predicted various conformational changes [19, 23-25]. However, which one of these conformations occurs during the virus-cell interaction is unknown at the present time. The initial weak interactions (Figure 1A) between V1V2 loop of gp120 which is located at the tip of the envelope spike, ~150 Å from the virus envelope [21, 26] and the integrin receptor α4β7 [27-29] (the integrin receptor is discussed in greater detail below), and its effect on the high affinity interactions [30] between gp120 and the CD4 molecule are not well understood. Whether the trimeric spikes of founder viruses exist in a particular conformation that are highly conducive for infection compared to the so-called “non-infectious viruses” that are also present and whether a particular conformation is better suited for entry at the mucosal site compared to entry through the systemic site are all interesting and highly relevant questions that would have a bearing on vaccine design.
Figure 1.
HIV-1 entry pathway. A. HIV-1 is captured through relatively weak interactions between gp120 V1V2 loop and surface molecules such as α4β7 integrin. This leads to high affinity interactions between gp120 and CD4 molecule, the primary receptor on CD4+ T cell. The engagement of CD4 receptor causes a conformational change in the envelope glycoprotein exposing the V3 loop-binding site for the chemokine co-receptor, CCR5 or CXCR4 [19, 138]. Further conformational changes occur after co-receptor interaction leading to the opening up of the two long helices HR1 and HR2 of gp41 and insertion of the N-terminal fusion peptide into the host cell membrane [139, 140]. A gp41 prehairpin intermediate [141], a three-stranded coiled coil stabilized by inter-molecular interactions between HR1 helices, is then formed, followed by the disassociation of gp120 subunits from gp41, allowing the HR2 helices to fold back and interact with the HR1 helices. The trimeric HR1 core, the interacting HR2 helices and the intervening loops in between form a hairpin structure of gp41 that is referred to as the six-helix bundle (6HB). This structure brings the host and the viral membranes in close proximity, facilitating membrane fusion and release of the nucleocapsid core into the cytosol [142-144]. B. CryoEM structure of the envelope trimer with the crystal structure of monomeric gp120 (PDB: 3DNN) fitted into the density map (kindly provided by Alan Merk and Sriram Subramaniam, NCI, NIH). The top and side views are shown; gp120 structure is shown in green. The structure lacks V1V2 domain, but the β-strands to which the V1V2 sequence would be linked are shown in red. C. Structural features of the V1V2 domain (PDB: 3U2S). V1 region is shown in cyan and V2 region is shown in magenta. The four β strands are labeled A to D. Disulfide bonds (-S-S-) are shown as sticks. The variable loop region in V1 and V2 are disordered in the structure and shown as dashed lines. The positions of the α4β7 binding tripeptide, LDI/V, and of the signature residue K169 in V2 loop are shown. C. Sequence alignment of V1V2 domain. Several founder V1V2 sequences from clades B and C viruses that were obtained from the Los Alamos National Laboratory HIV-1 database are aligned. The amino acids highlighted in black are identical, in magenta have ≥75% identity, and in cyan have ≥50% identity. The positions of β strands A-D are indicated at the bottom of the aligned sequences. The colors of the β strands correspond to the colors shown in the structure in (C).
2.2 Constant and variable regions of gp120
HIV-1 gp120 contains numerous potential N-linked glycosylation sites, with more than 20 N-linked glycans that account for about half of the molecular mass of gp120 [31]. HIV-1 gp120 is comprised of 5 variable regions (V1-V5) based on sequence variation among various isolates, and 5 constant regions (C1-C5), which are interspersed between the variable regions. Although, by definition, ‘variable’ regions vary in amino acid sequence, there are several residues in the variable regions of gp120 that are conserved or tolerate only conservative changes [14].
Very few studies have been directed towards the constant regions of gp120 and these studies demonstrate a structure-function interaction between the constant and variable regions. Several discontinuous residues in the constant regions of gp120 are involved in binding to the CD4 receptor, while regions of C4 along with β2 and β3 strands of the V1V2 loop and residues in the V3 loop form the chemokine-receptor binding site of gp120 [32, 33]. It has been shown that amino acid changes in C4 affect the ability of V1V2 to be recognized by conformation-dependent neutralizing monoclonal antibodies [34]. Similarly, infection and syncytia formation can be rescued by an amino acid change in 418 of C4 region in gp120 of chimeric viruses with diverse envelope proteins (specifically V1V2 domains) from different isolates [35]. Similar results have been observed in revertant viruses that contained single amino acid mutations in not only C4 but also in C1, when mutations were present in the V1V2 region [36]. These data suggest not only a structure-function relationship between the constant and variable regions of gp120, but also suggest that the role of the constant regions might be to provide the structural core as well as to compensate for variations in the variable loops which contain the receptor binding sites.
The functional significance of V1 region still remains poorly understood. Early work showed that mutations in the carboxy terminus of V1 resulted in less efficient formation of syncytium by the virus compared to wild type envelope [37]. V1V2 regions are two of the most variable regions of HIV-1. This region is localized to a membrane-distal cap of gp120 (Figure 1 B) as demonstrated by electron microscopy studies [21, 23, 38]. V1V2 region is not essential for virus entry and varies in sequence, length, and in N-linked glycosylation. However, deletion of the V1V2 region renders the virus highly susceptible to neutralization [15, 39-41] as this region is involved in a number of important functions. V1V2 loop is also involved in the formation of quaternary epitopes [9, 13, 42] that are associated with many neutralizing antibodies as also was demonstrated by the crystal structure of the V1V2 domain of HIV-1 gp120 from two clade C strains, complexed with the Fab fragment of monoclonal antibody PG9 [43]. V2 loop plays a role in CD4-binding [33, 37], contributes to the env trimer formation [44], is involved in binding to the gut homing integrin molecule α4β7 (described below) [27], and forms part of the chemokine binding region. This domain has been shown to influence cell tropism, through its interaction with the V3 domain and modulate its conformation [45, 46]. As with the other variable regions, V2 contains N-linked glycosylation sites, which dictate utilization of the CCR5 or CXCR4 co-receptor [47].
A potent neutralizing monoclonal antibody 2909, which targets both the V2 and V3 regions of gp120 was the first quaternary-specific monoclonal antibody to be described [9]. However, it did not exhibit broad neutralizing activity. Several broadly neutralizing quaternary-specific human monoclonal antibodies that target the V1V2 region have since been identified. These include the somatic variant antibodies PG9 and PG16 [13], antibodies CH01-CH04 [48], and antibodies PGT141-145 [49], which respectively neutralize 70-80%, 40-50%, and 40-80% of circulating HIV-1 isolates. These antibodies preferentially bind to the gp120 trimeric form and share specificity for an N-linked glycan at position 160 (Asp) in the V2 loop. Removal of this glycosylation site abolishes the neutralization activity of the antibodies [13, 50]. In addition to these broadly neutralizing antibodies, four V2-specific monoclonal antibodies have been isolated from RV144 vaccinees that neutralize laboratory adapted HIV-1. Two of the monoclonal antibodies CH58 and CH59 bind at or near the site in V2 to which PG9 and CH01 bind, inhibit interactions of V2 peptide with α4β7, and capture infectious AE.92TH023 strain virions [51].
The V3 loop of gp120 is involved in binding to the chemokine receptor and although the length of V3 loop is fairly constant at 34-35 amino acids, its amino acid sequence is highly variable, except in clade C viruses where the V3 sequence is generally conserved [52-54]. V3 is functionally essential for infectivity as deletion of V3 completely abrogates virus infectivity. Several monoclonal and polyclonal V3-specific antibodies have been described, some exhibiting cross clade neutralizing activity [55-57]. However, the V3 monoclonal antibodies unlike the V1V2 broadly neutralizing monoclonal antibodies, at best neutralize 10-20% of tier 2 viruses [56, 57]. Furthermore, V3 is not easily accessible on all viruses [58]. It has also been demonstrated that neutralizing antibodies to HIV-1 arise several weeks after infection [59] and are mainly directed towards the V3 loop [60, 61], with certain individuals developing cross clade broadly neutralizing activity in their serum [62, 63]. Thus, the early studies regarding the function of V3 and the crystal structure demonstrating the presence of conserved secondary structural elements in the crown of V3 [64] indicate that V3 is a possible target for vaccine development [14].
Although, V4-specific neutralizing antibodies have been described in immunized rabbits and V4 has been shown to be a target for early autologous neutralizing antibodies [65, 66], a well-defined function has yet to be attributed to the V4 loop. The V5 region forms part of the CD4 binding site of gp120 and is the only variable region that does not form disulfide-linked loops. So far, no monoclonal or polyclonal neutralizing antibodies directed against the V5 region have been described.
3. Mucosal transmission of HIV-1 and α4β7 integrin receptor
Although the transmission of HIV-1 through heterosexual means is inefficient, it remains the primary route of HIV-1 infection. The early events during HIV-1 sexual transmission and infection are not completely understood. However, it is believed that the virus binds to integrin α1 receptors on the genital epithelial cells triggering a cascade of signaling mechanisms resulting in the release of cytokines including TNF-α. This in turn, causes a breach in the tight junctions, allowing the virus to pass through. Both human and an SIV /macaque model indicate that low levels of viral replication initially occur during the first days of infection in partially activated memory CD4+ T cells and then in fully activated memory CD4+ cells in the genital mucosa. This is a localized event which in theory provides a small window of time to stop the HIV-1 infection before systemic dissemination occurs.
Within days following sexual transmission, infected lymphocytes migrate from the genital mucosa to Peyer’s patches, mesenteric lymph nodes, and lamina propria, which constitute the gut-associated lymphoid tissues (GALT). The migration of lymphocytes is facilitated by the gut mucosal homing integrin receptor α4β7 [67, 68]. Following increased viral replication and massive depletion of CCR5+ α4β7+CD4+ T cells including Th17 α4β7 high CD4+ T cells in both HIV and SIV infections [69-71], the virus then spreads from the GALT to other areas and becomes systemic [72, 73]. In addition, CD4−γδ T cells expressing α4β7 are also deleted as a result of bystander apoptosis [74]. Blocking of α4β7 gut-homing integrin receptor during acute infection leads to decreased plasma and gastrointestinal tissue viral loads in simian immunodeficiency virus-infected rhesus macaques, further supporting an important role for this molecule in HIV and SIV pathogenesis [75].
The α4β7 integrin receptor on activated CD4+ T cells [76] provides a link between the earliest site of HIV-1 infection, the mucosa, and the gut inductive sites where T cells are depleted during infection. The natural ligand for α4β7 receptor is the mucosal addressin cell adhesion molecule-1, MAdCAM1, as well as VCAM1 and fibronectin [77]. The HIV-1 envelope protein gp120 also binds to the active form of α4β7, which is thought to account for the concentration of HIV-1 virus in the GALT [78, 79]. The binding of α4β7 (Figure 1C, D) to the conserved tripeptide, LDI(V) present at the tip of the gp120 V2 loop [27, 72] is facilitated by the extended form of α4β7 from the cell surface and might be instrumental for “permanent” establishment of HIV-1 positive state [27, 28, 72]. This interaction has been shown to mediate the activation of LFA-1 and assist in the formation of virological synapse thus facilitating cell-to-cell spread of HIV-1 [80, 81]. However, unlike CD4 and CCR5, which are required for viral entry, interaction with α4β7 receptor is probably not essential for viral replication. The in vitro conditions do not necessarily simulate in vivo transmission of HIV-1. For instance, during in vivo transmission, the mucosal cells are presumably exposed to a heterogeneous mixture of HIV-1 variants at relatively low concentrations. Thus, transmission fitness might be critical for establishing infection. On the other hand, the cells in vitro are exposed to high concentration of a single HIV-1 variant, which may mimic a different transmission dynamics between the host and the virus.
In the RV144 study, it was found that antibodies against the HIV-1 Env gp120 V1V2 region were associated with lower risk of infection (see below). A vaccine efficacy of 31.2% in the absence of any difference in the viral loads between vaccinees and placebos, and a correlation with the induction of binding antibodies that recognized the V2 region containing the α4β7-binding site, would suggest that the α4β7 interaction might have played a significant role in transmission competence. However this question, clearly a critical one, requires further investigation using more defined and quantitative assays, which are currently underway in our laboratories.
4. Clinical trials, correlates of risk, and the importance of V2
Most vaccines protect through the induction of antibodies, although cellular immune responses are equally critical in controlling or preventing disease progression. The predominant protective correlates of almost all licensed human vaccines appear to be the induction of sufficient quantities of antibodies [82]. Nevertheless, in almost all diseases, CD4+ T cell help is necessary for B cell development. There are only two vaccines, BCG vaccine against tuberculosis [83, 84] and the herpes zoster vaccine [85, 86], to date, that work predominantly by inducing cellular immune responses. The HIV vaccine field has oscillated between trying to develop a vaccine that would induce either potent cellular or antiviral neutralizing antibody responses against a majority of circulating virus strains. Traditional methods of using live attenuated, chemically inactivated, or whole killed viruses cannot be tested as potential candidate HIV-1 vaccines because it is too dangerous [87]. Very few HIV-1 vaccine clinical trials have made it past the phase I or phase II trials. To date, there have been only four large-scale human HIV vaccine efficacy trials [88-91].
VAX003 and VAX004 Trials
The first two phase III randomized double-blind control HIV vaccine trials, VAX003 and VAX004 utilized monomeric recombinant gp120 (bivalent vaccine containing two genetic variants of the recombinant envelope protein gp120 subtype B and subtype E A244) adsorbed to alum, with the goal of generating neutralizing antibodies that would prevent HIV-1 entry. Although, the cohorts and the geographical location of VAX003 [90] and VAX004 trials [91] were different, they were both conducted in high-risk populations. In both trials, the vaccine did not prevent HIV-1 infection or delay disease progression even though in VAX004 trial, all vaccinees produced neutralizing and CD4 blocking antibodies [92].
STEP and Phambili Trials
The failures of VAX003 and VAX004 human clinical trials prompted the scientific community to design and conduct the first efficacy trial of a vector-based vaccine for inducing cell-mediated immune responses [88]. The precedent for this thinking was the demonstrated role of Gag-specific cellular responses in long-term nonprogressors, elite controllers [93, 94], and in non-human primate models [95-100].
The Merck HIV vaccine was tested in the STEP and Phambili trials [88, 101] as a multicenter randomized double-blind placebo controlled study. The vaccine consisted of three different replication defective adenovirus type 5 vectors, each expressing one of the subtype B HIV-1 genes, Gag, Pol, or Nef [88]. The hypothesis was that the vaccine would induce CD8+ T cell responses that would control viral replication following infection and was supported by the results of the vaccine in non-human primates [96]. Since the vaccine did not contain Env genes, it did not induce Env-specific binding or neutralizing antibodies. Although the vaccine stimulated T cells, it did not protect the volunteers from virus acquisition nor did it reduce the viral loads after infection. The interim analyses showed an increase in HIV infection rates in uncircumcised male vaccinees compared to the controls, resulting in the trials being halted before completion [88, 102]. This was a major setback in the HIV field as this vaccine had been viewed as highly promising.
RV144: The Thai Trial
Since the VaxGen vaccine (AIDSVAX B/E) had previously failed, using this vaccine as a boost along with Sanofi Pasteurs’ recombinant canarypox vector ALVAC-HIV (vCP1521) as a prime in a large phase III trial (RV144) that was ongoing at that time in Thailand and had previously been shown to be safe and immunogenic in a phase I/II trial [103] was met with great skepticism [104]. Nonetheless, the RV144 trial was justified and conducted as planned [105, 106]. When the results were analyzed, the vaccine showed a modest but significant protective efficacy [89].
The RV144 trial, the largest HIV vaccine randomized, multicenter, double blind, placebo controlled efficacy trial conducted so far, enrolled a total of 16,395 men and women in Thailand, at risk for HIV-1 infection via heterosexual exposure. This trial was conducted by the US Military HIV Research Program (USMHRP) in collaboration with the Thai Ministry of Health and the National Institutes of Health [89]. The volunteers were monitored at the end of the 6-month vaccination series and every 6 months thereafter for 3 years for two co-primary endpoints, HIV-1 infection and early HIV-1 viremia. The vaccine regimen consisted of 4 injections at 0, 1, 3, and 6 months of recombinant canarypox vector ALVAC-HIV (vCP1521) expressing CRF01_AE gp120 from strain 92TH023, linked to the transmembrane anchoring portion of gp41 from HIV subtype B strain LAI that was devoid of the entire gp41 ectodomain, and HIV-1 clade B (LAI) Gag and protease as the prime followed by two simultaneous injections of bivalent AIDSVAX B/E CHO produced recombinant gp120 [B and E Env gp120 derived from MN and CRF01_AE strain, CM244 (A244), respectively] at 3 and 6 months. Both A244 and MN recombinant gp120 proteins were modified by an N-terminal 11 amino acid deletion and addition of a herpes simplex virus gD protein-derived tag as part of an early expression and purification strategy [107, 108]. The results of the RV144 vaccine trial for the first time demonstrated a modest but significant protection, with an estimated efficacy of 31.2% from HIV-1 acquisition (p = 0.04) at 42 months. Furthermore, the data through months 12 and 18 showed an even higher efficacy, estimated to be 60% and 44%, respectively [109]. These data renewed the hope that it would indeed be possible to develop an efficacious HIV-1 vaccine.
This trial provided an opportunity to identify immune correlates of protection and a consortium of scientists was put together to conduct a two-stage analysis of vaccine-induced antibody responses, innate, and cellular immune responses [16, 110]. In a series of pilot studies, plasma and PBMCs from uninfected placebos (20%) and vaccinees (80%) at baseline and week 26 (2 weeks after the last boost at week 24) were analyzed for 32 different immunologic assays including binding antibodies, ELIPSOT, and ICS responses. The results of the pilot study demonstrated that binding antibodies primarily to subtype E A244 rather than to subtype B MN gp120 was induced in 95% of the vaccinees at week 26 but waned substantially over the next 6 months. Some of the antibodies induced were to the V2 loop of gp120 from diverse HIV-1 subtypes as assessed by peptide microarray analysis, ELISA, and Biacore studies [111, 112]. The vaccine induced antibodies specific to both linear and conformational regions of V2 loop. The antibodies targeted multiple binding epitopes (Figure 1C, D) in the mid-region of the V2 loop (amino acid positions 163-178). This included the highly conserved amino acids, the integrin-binding motif LDI/V, and also the less conserved regions. The V2 antibodies were cross-reactive with V2 peptides derived from several subtypes and the dominant linear V2 epitope was located in the C β-strand (Figure 1C) of the V1V2 complex [111, 112].
The cellular responses in the RV144 vaccinees were predominantly CD4+ T cell-mediated, preferentially directed to gp120 V2 epitopes, which included the α4β7 integrin binding region, and were of the effector memory phenotype, with majority of the responders producing both IL-2 and IFNγ [113]. The CD8+ T cell responses were barely measurable in the ICS assay and the frequency of the responses was equivalent to the frequency of responses seen in the placebo recipients.
Based on the results of the pilot studies and several criteria, 17 assay types were selected for the case-control study that included plasma and PBMCs from 41 HIV-1 infected vaccinees and 205 frequency-matched uninfected vaccinees (controls). These assays generated results for 158 variables, and from these 6 were selected as primary variables for assessment of infection risk. Two of the six primary variables showed significant correlation with infection among vaccine recipients. The level of plasma IgG antibody binding to gp70-V1V2 (a recombinant fusion protein containing the first and second variable regions of gp120 from subtype B strain, Case A2 fused to murine leukemia virus gp70) 15] correlated inversely with the risk of infection and the level of plasma IgA antibody binding to a panel of 14 Env glycoproteins correlated directly with risk of infection [16, 51].
The vaccine-induced responses were associated with two signatures in the V2 loop that were sites of immune pressure. Amino acids K (lysine) 169 and I (isoleucine) 181 (numbering based on HXB2 sequence; see Figure 1C) were critical for antibody binding and sieve analysis demonstrated that viruses in vaccine breakthrough infections were mismatched at residue K169 and matched at residue I181. The vaccine efficacy against viruses matching the vaccine at position 169 and mismatching the vaccine at position 181 were 48% (p = 0.0036) and 78% (p = 0.0028), respectively when the sequences of breakthrough infections in vaccinees and placebo recipients were compared [114]. Furthermore, four monoclonal antibodies specific to epitopes within the V2 region were isolated from RV144 vaccine recipients. The epitopes for two of the monoclonal antibodies contained the amino acid K169 [51]. In addition to functional binding antibodies, weak Tier 1 virus neutralizing antibody responses mediated by antibodies specific to the V3 loop of gp120 were also present in the sera of vaccinated individuals. Although, the titers waned over a period of time, low titers of neutralizing antibodies persisted in a subset of RV144 vaccinees for at least 3 years [115]. The presence of additional signature sequences associated with vaccine-induced responses or neutralizing antibodies in variable regions other than V2 or in constant regions of gp120 are yet to be explored.
The importance of V2-specific binding antibodies and a critical requirement for Env protein for blocking SIV acquisition was also demonstrated in two separate NHP studies [116, 117]. The first study assessed the protective efficacy of Ad26 and MVA containing SIVsm543 inserts expressing Gag-Pol and Env immunogens against repetitive, heterologous, intrarectal SIVMac251challenge. This study showed a strong vaccine-induced protection against a diverse SIV challenge strain and a correlation between multiple cellular and humoral immune responses with virological control, specifically with vaccine elicited V2-specific antibodies [116]. In the second study by Pegu et al., [117], macaques were immunized with a regimen intended to mimic the RV 144 trial and then challenged with SIVmac251 through the intrarectal route. Macaques were primed with ALVAC-SIV expressing Gag, Pol, and Env followed by subsequent injections with SIV-gp120 protein formulated in alum. Sera from protected animals had antibodies with higher avidity to gp120 with specificity to the V1V2 region and these antibodies reduced the infectivity of SIVmac251 in cells that expressed high levels of α4β7 integrins, suggesting a functional role for V2 antibodies [117].
The similarity of the results obtained in both NHP studies with RV144 is encouraging but one has to be cautious in drawing firm correlations between the human and animal model studies. Two other studies in rhesus macaques with an ALVAC-SIV prime and recombinant gp120 boosts showed complete protection in 25% of the animals, with good ADCVI responses in 2 of the 3 protected animals [118, 119]. Taken together, the evidence supports the idea that antibodies can prevent transmission of HIV-1, leading to the hypothesis that even higher vaccine efficacy in humans and animal models could be achieved if cross-clade neutralizing antibodies and/ or higher titers of antibodies that block the initial site of transmission can be induced.
Although the RV144 trial allowed us to study the immune correlates and generated hypothesis, the underlying mechanism of protection in the trial is yet to be elucidated. The direct correlation of plasma IgA antibodies with risk of infection is perplexing. It is possible that IgA may be blocking ADCC, ADCVI, virus aggregation, or phagocytosis and thus interfering with protective IgG effector functions as has been shown in studies with other pathogens [120, 121]. The role of mucosal IgA, which is primarily dimeric could not be evaluated in the RV144 study as mucosal samples were not collected. The mechanism to explain the functional role of V2 binding Abs and the inverse correlation with risk of infection is not understood, although it can be speculated that the antibodies specific to V1V2 region prevent the binding of V1V2 to the coreceptor CCR5 or to α4β7 and thus prevent viral entry [27, 73, 122, 123].
Closing Thoughts
The failure of the VAX003 and VAX004 trials and the success of the RV144 trial suggest a priming effect by the RV144 vaccine for both binding and neutralizing antibodies. The priming vector vCP1521 carried the env gene of CRF01_AE whose V2 region is nearly identical to that of the V2 of A244 gp120 protein boost but vastly different from the V2 carried by the MN gp120 boost. The peak neutralizing antibody responses seen after the final boost in the RV144 trial was stronger than the response seen after the two protein injections of gp120 alone in VAX003 [115]. In the RV144 trial, the antibody titers significantly decreased 1 year after the final boost. Therefore, in future trials, the aim would be to induce high titer and long-lasting V2 specific binding and neutralizing antibodies. To achieve this, one would also have to consider formulating the vaccine with adjuvants other than alum [124].
Induction of long-lasting antibodies will be addressed in three separate trials (RV305, RV306, and RV328) being conducted by USMHRP in Thailand that are either ongoing or are scheduled to start soon. The vaccine regimen in RV305 (n=162) consists of administering two additional boosts of either AIDSVAX B/E CHO produced recombinant gp120 alone, or simultaneous injections of ALVAC and AIDSVAX B/E recombinant gp120, or two injections of ALVAC alone. Each arm will include 45 previously vaccinated RV144 individuals and 9 placebos. A repeat of the vaccines used in the RV144 trial will be tested in a separate 4-arm clinical trial (RV306; n= 360). The volunteers will receive a total of 4 or 5 injections consisting of two injections of ALVAC followed by two (similar to the RV144 trial) or three simultaneous injections of AIDSVAX vaccine or the fifth injection will consist of AIDSVAX in the absence of ALVAC or ALVAC in the absence of AIDSVAX. In addition, there is yet another trial RV328 (n = 40), a repeat of VAX003 with addition boosts. These trials will address several unanswered questions such as (i) the role of additional protein boosts; (ii) the effect of priming with ALVAC on the immune response; and (iii) the role of mucosal antibodies in preventing infection. In a subset of volunteers, gut and bone marrow biopsies (these were not available in the previous trials) will also be collected in addition to PBMCs to address the role of cellular immune responses.
Another unknown factor is the role of neutralizing antibodies and the titers required to induce protection or reduce the risk of infection. Although several neutralizing antibodies have been isolated from HIV-1 infected patients, only a fraction (20%) of patients chronically infected with HIV-1 make broadly neutralizing antibodies and only after 3-4 years of infection [125]. These antibodies are generally not elicited by immunization with envelope proteins [126-128]. Induction of broadly neutralizing antibodies may require precise engineering of the envelope protein or the use of certain immunization strategies or vectors to stimulate germline B cell receptors of these specific antibodies to initiate the maturation process [48, 128-130]. Other factors to consider are the induction of long-lived high affinity broadly neutralizing antibodies and better assay systems to measure neutralization that would mimic the in vivo neutralization of HIV-1 in the host.
Simultaneously with strategies to optimize the required B cell responses, various approaches to induce effective CD4 and CD8 T cell responses are paramount for developing a successful vaccine. It is known that during a natural HIV-1 infection, induction of CD8+ T cell responses results in the suppression of HIV replication [131]. Because of the immune pressure, escape mutants in the target epitopes would be rapidly selected [132-134]. It is also known that during acute HIV-1 infection, the viral epitopes that are targeted by T cells early in the infection differ from those targeted later in infection and help control viral replication [135, 136]. An efficacious vaccine should probably induce cellular responses against both the early and late T cell epitopes. An ideal vaccine would be one that is not skewed towards generating a particular response but would generate a combination of humoral, cellular, and mucosal immune responses [137], which could prevent HIV-1 entry into the cell either by binding, neutralizing, or by killing the virus-infected cell. The immunogens should in addition be capable of eliciting and maintaining sufficient effector memory T and B cells to be able to promptly mount the required immune response at the site of entry (mucosal or humoral). Another aspect to consider while designing a vaccine is the ability of the vaccine to enhance innate immune responses or prevent the virus from dampening this response.
The results of the three failed and one successful trial have highlighted the importance of the vaccine platform, the adjuvants used, and the mode of HIV-1 transmission in the cohorts tested. Several trials are in the pipeline to meaningfully address these issues as well as the need for testing the efficacy of the vaccine in geographical areas where the HIV-1 incidence is high. The design and development of an efficacious preventative HIV-1 vaccine is challenging and daunting, but RV144 has given a glimmer of hope that such a vaccine is probable in the foreseeable future.
Funding acknowledgement
This work was supported by a cooperative agreement (W81XWH-11-2-0174) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense (DoD) and a grant to VBR from the NIAID, NIH (AI102725).
Footnotes
DoD disclaimer: The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army or the Department of Defense.
References
- 1.McCutchan FE. Understanding the genetic diversity of HIV-1. AIDS. 2000;14(Suppl 3):S31–44. [PubMed] [Google Scholar]
- 2.Kijak GH, McCutchan FE. HIV diversity, molecular epidemiology, and the role of recombination. Curr Infect Dis Rep. 2005 Nov;7(6):480–8. doi: 10.1007/s11908-005-0051-8. [DOI] [PubMed] [Google Scholar]
- 3.Letvin NL. Progress and obstacles in the development of an AIDS vaccine. Nat Rev Immunol. 2006 Dec;6(12):930–9. doi: 10.1038/nri1959. [DOI] [PubMed] [Google Scholar]
- 4.Girard MP, Osmanov S, Assossou OM, Kieny MP. Human immunodeficiency virus (HIV) immunopathogenesis and vaccine development: a review. Vaccine. 2011 Aug 26;29(37):6191–218. doi: 10.1016/j.vaccine.2011.06.085. [DOI] [PubMed] [Google Scholar]
- 5.Gorny MK, Conley AJ, Karwowska S, Buchbinder A, Xu JY, Emini EA, et al. Neutralization of diverse human immunodeficiency virus type 1 variants by an anti-V3 human monoclonal antibody. J Virol. 1992 Dec;66(12):7538–42. doi: 10.1128/jvi.66.12.7538-7542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorny MK, Xu JY, Karwowska S, Buchbinder A, Zolla-Pazner S. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J Immunol. 1993 Jan 15;150(2):635–43. [PubMed] [Google Scholar]
- 7.Conley AJ, Gorny MK, Kessler JA, 2nd, Boots LJ, Ossorio-Castro M, Koenig S, et al. Neutralization of primary human immunodeficiency virus type 1 isolates by the broadly reactive anti-V3 monoclonal antibody, 447-52D. J Virol. 1994 Nov;68(11):6994–7000. doi: 10.1128/jvi.68.11.6994-7000.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanfield RL, Gorny MK, Williams C, Zolla-Pazner S, Wilson IA. Structural rationale for the broad neutralization of HIV-1 by human monoclonal antibody 447-52D. Structure. 2004 Feb;12(2):193–204. doi: 10.1016/j.str.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Gorny MK, Stamatatos L, Volsky B, Revesz K, Williams C, Wang XH, et al. Identification of a new quaternary neutralizing epitope on human immunodeficiency virus type 1 virus particles. J Virol. 2005 Apr;79(8):5232–7. doi: 10.1128/JVI.79.8.5232-5237.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krachmarov CP, Honnen WJ, Kayman SC, Gorny MK, Zolla-Pazner S, Pinter A. Factors determining the breadth and potency of neutralization by V3-specific human monoclonal antibodies derived from subjects infected with clade A or clade B strains of human immunodeficiency virus type 1. J Virol. 2006 Jul;80(14):7127–35. doi: 10.1128/JVI.02619-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pantophlet R, Wrin T, Cavacini LA, Robinson JE, Burton DR. Neutralizing activity of antibodies to the V3 loop region of HIV-1 gp120 relative to their epitope fine specificity. Virology. 2008 Nov;381(2):251–60. doi: 10.1016/j.virol.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burke V, Williams C, Sukumaran M, Kim SS, Li H, Wang XH, et al. Structural basis of the cross-reactivity of genetically related human anti-HIV-1 mAbs: implications for design of V3-based immunogens. Structure. 2009 Nov 11;17(11):1538–46. doi: 10.1016/j.str.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009 Oct 9;326(5950):285–9. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zolla-Pazner S, Cardozo T. Structure-function relationships of HIV-1 envelope sequence-variable regions refocus vaccine design. Nat Rev Immunol. 2010 Jul;10(7):527–35. doi: 10.1038/nri2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinter A, Honnen WJ, He Y, Gorny MK, Zolla-Pazner S, Kayman SC. The V1/V2 domain of gp120 is a global regulator of the sensitivity of primary human immunodeficiency virus type 1 isolates to neutralization by antibodies commonly induced upon infection. J Virol. 2004 May;78(10):5205–15. doi: 10.1128/JVI.78.10.5205-5215.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012 Apr 5;366(14):1275–86. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wayne CK, Berkley SF. The renaissance in HIV vaccine development--future directions. N Engl J Med. 2010 Jul 29;363(5):e7. doi: 10.1056/NEJMp1007629. [DOI] [PubMed] [Google Scholar]
- 18.McCune JM, Rabin LB, Feinberg MB, Lieberman M, Kosek JC, Reyes GR, et al. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell. 1988 Apr 8;53(1):55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- 19.Harris A, Borgnia MJ, Shi D, Bartesaghi A, He H, Pejchal R, et al. Trimeric HIV-1 glycoprotein gp140 immunogens and native HIV-1 envelope glycoproteins display the same closed and open quaternary molecular architectures. Proc Natl Acad Sci U S A. 2011 Jul 12;108(28):11440–5. doi: 10.1073/pnas.1101414108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyerson JR, Tran EE, Kuybeda O, Chen W, Dimitrov DS, Gorlani A, et al. Molecular structures of trimeric HIV-1 Env in complex with small antibody derivatives. Proc Natl Acad Sci U S A. 2013 Jan 8;110(2):513–8. doi: 10.1073/pnas.1214810110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Bartesaghi A, Borgnia MJ, Sapiro G, Subramaniam S. Molecular architecture of native HIV-1 gp120 trimers. Nature. 2008 Sep 4;455(7209):109–13. doi: 10.1038/nature07159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallo SA, Finnegan CM, Viard M, Raviv Y, Dimitrov A, Rawat SS, et al. The HIV Env-mediated fusion reaction. Biochim Biophys Acta. 2003 Jul 11;1614(1):36–50. doi: 10.1016/s0005-2736(03)00161-5. [DOI] [PubMed] [Google Scholar]
- 23.White TA, Bartesaghi A, Borgnia MJ, Meyerson JR, de la Cruz MJ, Bess JW, et al. Molecular architectures of trimeric SIV and HIV-1 envelope glycoproteins on intact viruses: strain-dependent variation in quaternary structure. PLoS Pathog. 2010;6(12):e1001249. doi: 10.1371/journal.ppat.1001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White TA, Bartesaghi A, Borgnia MJ, de la Cruz MJ, Nandwani R, Hoxie JA, et al. Three-dimensional structures of soluble CD4-bound states of trimeric simian immunodeficiency virus envelope glycoproteins determined by using cryo-electron tomography. J Virol. 2011 Dec;85(23):12114–23. doi: 10.1128/JVI.05297-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran EE, Borgnia MJ, Kuybeda O, Schauder DM, Bartesaghi A, Frank GA, et al. Structural mechanism of trimeric HIV-1 envelope glycoprotein activation. PLoS Pathog. 2012;8(7):e1002797. doi: 10.1371/journal.ppat.1002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu SR, Loving R, Lindqvist B, Hebert H, Koeck PJ, Sjoberg M, et al. Single-particle cryoelectron microscopy analysis reveals the HIV-1 spike as a tripod structure. Proc Natl Acad Sci U S A. Nov 2;107(44):18844–9. doi: 10.1073/pnas.1007227107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arthos J, Cicala C, Martinelli E, Macleod K, Van Ryk D, Wei D, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008 Mar;9(3):301–9. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 28.Cicala C, Martinelli E, McNally JP, Goode DJ, Gopaul R, Hiatt J, et al. The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc Natl Acad Sci U S A. 2009 Dec 8;106(49):20877–82. doi: 10.1073/pnas.0911796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geijtenbeek TB, Krooshoop DJ, Bleijs DA, van Vliet SJ, van Duijnhoven GC, Grabovsky V, et al. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat Immunol. 2000 Oct;1(4):353–7. doi: 10.1038/79815. [DOI] [PubMed] [Google Scholar]
- 30.Dalgleish AG, Beverley PC, Clapham PR, Crawford DH, Greaves MF, Weiss RA. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 1985 Dec 20 2;Jan 20 2;312(5996):763–7. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 31.Yoon V, Fridkis-Hareli M, Munisamy S, Lee J, Anastasiades D, Stevceva L. The GP120 molecule of HIV-1 and its interaction with T cells. Curr Med Chem. 2010;17(8):741–9. doi: 10.2174/092986710790514499. [DOI] [PubMed] [Google Scholar]
- 32.Rizzuto CD, Wyatt R, Hernandez-Ramos N, Sun Y, Kwong PD, Hendrickson WA, et al. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998 Jun 19;280(5371):1949–53. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 33.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998 Jun 18;393(6686):648–59. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKeating JA, Shotton C, Cordell J, Graham S, Balfe P, Sullivan N, et al. Characterization of neutralizing monoclonal antibodies to linear and conformation-dependent epitopes within the first and second variable domains of human immunodeficiency virus type 1 gp120. J Virol. 1993 Aug;67(8):4932–44. doi: 10.1128/jvi.67.8.4932-4944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freed EO, Martin MA. Evidence for a functional interaction between the V1/V2 and C4 domains of human immunodeficiency virus type 1 envelope glycoprotein gp120. J Virol. 1994 Apr;68(4):2503–12. doi: 10.1128/jvi.68.4.2503-2512.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang WK, Essex M, Lee TH. Single amino acid substitution in constant region 1 or 4 of gp120 causes the phenotype of a human immunodeficiency virus type 1 variant with mutations in hypervariable regions 1 and 2 to revert. J Virol. 1996 Jan;70(1):607–11. doi: 10.1128/jvi.70.1.607-611.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullivan N, Thali M, Furman C, Ho DD, Sodroski J. Effect of amino acid changes in the V1/V2 region of the human immunodeficiency virus type 1 gp120 glycoprotein on subunit association, syncytium formation, and recognition by a neutralizing antibody. J Virol. 1993 Jun;67(6):3674–9. doi: 10.1128/jvi.67.6.3674-3679.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu SR, Loving R, Lindqvist B, Hebert H, Koeck PJ, Sjoberg M, et al. Single-particle cryoelectron microscopy analysis reveals the HIV-1 spike as a tripod structure. Proc Natl Acad Sci U S A. 2010 Nov 2;107(44):18844–9. doi: 10.1073/pnas.1007227107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stamatatos L, Cheng-Mayer C. An envelope modification that renders a primary, neutralization-resistant clade B human immunodeficiency virus type 1 isolate highly susceptible to neutralization by sera from other clades. J Virol. 1998 Oct;72(10):7840–5. doi: 10.1128/jvi.72.10.7840-7845.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao J, Sullivan N, Desjardin E, Parolin C, Robinson J, Wyatt R, et al. Replication and neutralization of human immunodeficiency virus type 1 lacking the V1 and V2 variable loops of the gp120 envelope glycoprotein. J Virol. 1997 Dec;71(12):9808–12. doi: 10.1128/jvi.71.12.9808-9812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rusert P, Krarup A, Magnus C, Brandenberg OF, Weber J, Ehlert AK, et al. Interaction of the gp120 V1V2 loop with a neighboring gp120 unit shields the HIV envelope trimer against cross-neutralizing antibodies. J Exp Med. 2011 Jul 4;208(7):1419–33. doi: 10.1084/jem.20110196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Changela A, Wu X, Yang Y, Zhang B, Zhu J, Nardone GA, et al. Crystal structure of human antibody 2909 reveals conserved features of quaternary structure-specific antibodies that potently neutralize HIV-1. J Virol. 2011 Mar;85(6):2524–35. doi: 10.1128/JVI.02335-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011 Dec 15;480(7377):336–43. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen B, Vogan EM, Gong H, Skehel JJ, Wiley DC, Harrison SC. Structure of an unliganded simian immunodeficiency virus gp120 core. Nature. 2005 Feb 24;433(7028):834–41. doi: 10.1038/nature03327. [DOI] [PubMed] [Google Scholar]
- 45.Koito A, Stamatatos L, Cheng-Mayer C. Small amino acid sequence changes within the V2 domain can affect the function of a T-cell line-tropic human immunodeficiency virus type 1 envelope gp120. Virology. 1995 Feb 1;206(2):878–84. doi: 10.1006/viro.1995.1010. [DOI] [PubMed] [Google Scholar]
- 46.Koito A, Harrowe G, Levy JA, Cheng-Mayer C. Functional role of the V1/V2 region of human immunodeficiency virus type 1 envelope glycoprotein gp120 in infection of primary macrophages and soluble CD4 neutralization. J Virol. 1994 Apr;68(4):2253–9. doi: 10.1128/jvi.68.4.2253-2259.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pollakis G, Kang S, Kliphuis A, Chalaby MI, Goudsmit J, Paxton WA. N-linked glycosylation of the HIV type-1 gp120 envelope glycoprotein as a major determinant of CCR5 and CXCR4 coreceptor utilization. J Biol Chem. 2001 Apr 20;276(16):13433–41. doi: 10.1074/jbc.M009779200. [DOI] [PubMed] [Google Scholar]
- 48.Bonsignori M, Hwang KK, Chen X, Tsao CY, Morris L, Gray E, et al. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J Virol. 2011 Oct;85(19):9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011 Sep 22;477(7365):466–70. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doores KJ, Burton DR. Variable loop glycan dependency of the broad and potent HIV-1-neutralizing antibodies PG9 and PG16. J Virol. 2010 Oct;84(20):10510–21. doi: 10.1128/JVI.00552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, et al. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity. 2013 Jan;38(1):176–86. doi: 10.1016/j.immuni.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trkola A, Dragic T, Arthos J, Binley JM, Olson WC, Allaway GP, et al. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996 Nov 14;384(6605):184–7. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 53.Gaschen B, Taylor J, Yusim K, Foley B, Gao F, Lang D, et al. Diversity considerations in HIV-1 vaccine selection. Science. 2002 Jun 28;296(5577):2354–60. doi: 10.1126/science.1070441. [DOI] [PubMed] [Google Scholar]
- 54.Cardozo T, Kimura T, Philpott S, Weiser B, Burger H, Zolla-Pazner S. Structural basis for coreceptor selectivity by the HIV type 1 V3 loop. AIDS Res Hum Retroviruses. 2007 Mar;23(3):415–26. doi: 10.1089/aid.2006.0130. [DOI] [PubMed] [Google Scholar]
- 55.Gorny MK, Williams C, Volsky B, Revesz K, Wang XH, Burda S, et al. Cross-clade neutralizing activity of human anti-V3 monoclonal antibodies derived from the cells of individuals infected with non-B clades of human immunodeficiency virus type 1. J Virol. 2006 Jul;80(14):6865–72. doi: 10.1128/JVI.02202-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pantophlet R, Aguilar-Sino RO, Wrin T, Cavacini LA, Burton DR. Analysis of the neutralization breadth of the anti-V3 antibody F425-B4e8 and re-assessment of its epitope fine specificity by scanning mutagenesis. Virology. 2007 Aug 1;364(2):441–53. doi: 10.1016/j.virol.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hioe CE, Wrin T, Seaman MS, Yu X, Wood B, Self S, et al. Anti-V3 monoclonal antibodies display broad neutralizing activities against multiple HIV-1 subtypes. PLoS One. 5(4):e10254. doi: 10.1371/journal.pone.0010254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bou-Habib DC, Roderiquez G, Oravecz T, Berman PW, Lusso P, Norcross MA. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J Virol. 1994 Sep;68(9):6006–13. doi: 10.1128/jvi.68.9.6006-6013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, et al. B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol. 2008 Dec;82(24):12449–63. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore JP, Nara PL. The role of the V3 loop of gp120 in HIV infection. AIDS. 1991;5(Suppl 2):S21–33. doi: 10.1097/00002030-199101001-00004. [DOI] [PubMed] [Google Scholar]
- 61.Frost SD, Trkola A, Gunthard HF, Richman DD. Antibody responses in primary HIV-1 infection. Curr Opin HIV AIDS. 2008 Jan;3(1):45–51. doi: 10.1097/COH.0b013e3282f310ae. [DOI] [PubMed] [Google Scholar]
- 62.Binley JM, Lybarger EA, Crooks ET, Seaman MS, Gray E, Davis KL, et al. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J Virol. 2008 Dec;82(23):11651–68. doi: 10.1128/JVI.01762-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doria-Rose NA, Klein RM, Daniels MG, O’Dell S, Nason M, Lapedes A, et al. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J Virol. Feb;84(3):1631–6. doi: 10.1128/JVI.01482-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang X, Burke V, Totrov M, Williams C, Cardozo T, Gorny MK, et al. Conserved structural elements in the V3 crown of HIV-1 gp120. Nat Struct Mol Biol. Aug;17(8):955–61. doi: 10.1038/nsmb.1861. [DOI] [PubMed] [Google Scholar]
- 65.Burke B, Gomez-Roman VR, Lian Y, Sun Y, Kan E, Ulmer J, et al. Neutralizing antibody responses to subtype B and C adjuvanted HIV envelope protein vaccination in rabbits. Virology. 2009 Apr;387(1):147–56. doi: 10.1016/j.virol.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moore PL, Gray ES, Choge IA, Ranchobe N, Mlisana K, Abdool Karim SS, et al. The c3-v4 region is a major target of autologous neutralizing antibodies in human immunodeficiency virus type 1 subtype C infection. J Virol. 2008 Feb;82(4):1860–9. doi: 10.1128/JVI.02187-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wagner N, Lohler J, Kunkel EJ, Ley K, Leung E, Krissansen G, et al. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996 Jul 25;382(6589):366–70. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- 68.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000 Oct 5;343(14):1020–34. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 69.Kader M, Bixler S, Roederer M, Veazey R, Mattapallil JJ. CD4 T cell subsets in the mucosa are CD28+Ki-67-HLA-DR-CD69+ but show differential infection based on alpha4beta7 receptor expression during acute SIV infection. J Med Primatol. 2009 Oct;38(Suppl 1):24–31. doi: 10.1111/j.1600-0684.2009.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kader M, Wang X, Piatak M, Lifson J, Roederer M, Veazey R, et al. Alpha4(+)beta7(hi)CD4(+) memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. 2009 Sep;2(5):439–49. doi: 10.1038/mi.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McKinnon LR, Nyanga B, Chege D, Izulla P, Kimani M, Huibner S, et al. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. J Immunol. 2011 Dec 1;187(11):6032–42. doi: 10.4049/jimmunol.1101836. [DOI] [PubMed] [Google Scholar]
- 72.Cicala C, Arthos J, Fauci AS. HIV-1 envelope, integrins and co-receptor use in mucosal transmission of HIV. Journal of Translational Medicine. 2010;9(Suppl 1) doi: 10.1186/1479-5876-9-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nawaz F, Cicala C, Van Ryk D, Block KE, Jelicic K, McNally JP, et al. The genotype of early-transmitting HIV gp120s promotes alpha (4) beta(7)-reactivity, revealing alpha (4) beta(7) +/CD4+ T cells as key targets in mucosal transmission. PLoS Pathog. 2011 Feb;7(2):e1001301. doi: 10.1371/journal.ppat.1001301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li H, Pauza CD. HIV envelope-mediated, CCR5/alpha4beta7-dependent killing of CD4-negative gammadelta T cells which are lost during progression to AIDS. Blood. 2011 Nov 24;118(22):5824–31. doi: 10.1182/blood-2011-05-356535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ansari AA, Reimann KA, Mayne AE, Takahashi Y, Stephenson ST, Wang R, et al. Blocking of alpha4beta7 gut-homing integrin during acute infection leads to decreased plasma and gastrointestinal tissue viral loads in simian immunodeficiency virus-infected rhesus macaques. J Immunol. 2011 Jan 15;186(2):1044–59. doi: 10.4049/jimmunol.1003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005 Oct;5(10):783–92. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- 77.Darc M, Hait SH, Soares EA, Cicala C, Seuanez HN, Machado ES, et al. Polymorphisms in the alpha4 integrin of neotropical primates: insights for binding of natural ligands and HIV-1 gp120 to the human alpha4beta7. PLoS One. 2011;6(9):e24461. doi: 10.1371/journal.pone.0024461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998 Apr 17;280(5362):427–31. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 79.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004 Sep 20;200(6):749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jolly C, Kashefi K, Hollinshead M, Sattentau QJ. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J Exp Med. 2004 Jan 19;199(2):283–93. doi: 10.1084/jem.20030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, et al. The immunological synapse. Annu Rev Immunol. 2001;19:375–96. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- 82.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008 Aug 1;47(3):401–9. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 83.Fletcher HA. Correlates of immune protection from tuberculosis. Curr Mol Med. 2007 May;7(3):319–25. doi: 10.2174/156652407780598520. [DOI] [PubMed] [Google Scholar]
- 84.McShane H, Pathan AA, Sander CR, Keating SM, Gilbert SC, Huygen K, et al. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med. 2004 Nov;10(11):1240–4. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- 85.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005 Jun 2;352(22):2271–84. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 86.Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008 Mar 15;197(6):825–35. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Duerr A, Wasserheit JN, Corey L. HIV vaccines: new frontiers in vaccine development. Clin Infect Dis. 2006 Aug;43(4):500–11. doi: 10.1086/505979. [DOI] [PubMed] [Google Scholar]
- 88.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008 Nov 29;372(9653):1881–93. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009 Dec 3;361(23):2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 90.Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006 Dec 15;194(12):1661–71. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 91.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005 Mar 1;191(5):654–65. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 92.Gilbert P, Wang M, Wrin T, Petropoulos C, Gurwith M, Sinangil F, et al. Magnitude and breadth of a nonprotective neutralizing antibody response in an efficacy trial of a candidate HIV-1 gp120 vaccine. J Infect Dis. 2010 Aug 15;202(4):595–605. doi: 10.1086/654816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goulder PJ, Bunce M, Krausa P, McIntyre K, Crowley S, Morgan B, et al. Novel, cross-restricted, conserved, and immunodominant cytotoxic T lymphocyte epitopes in slow progressors in HIV type 1 infection. AIDS Res Hum Retroviruses. 1996 Dec 10;12(18):1691–8. doi: 10.1089/aid.1996.12.1691. [DOI] [PubMed] [Google Scholar]
- 94.Baker BM, Block BL, Rothchild AC, Walker BD. Elite control of HIV infection: implications for vaccine design. Expert Opin Biol Ther. 2009 Jan;9(1):55–69. doi: 10.1517/14712590802571928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Riviere Y. Virus-specific cytotoxic T lymphocyte responses in patients infected with the human immunodeficiency virus, HIV-1. Cell Mol Biol (Noisy-le-grand) 1994;40(Suppl 1):45–8. [PubMed] [Google Scholar]
- 96.Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002 Jan;415(6869):331–5. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 97.Schneidewind A, Brockman MA, Yang R, Adam RI, Li B, Le Gall S, et al. Escape from the dominant HLA-B27-restricted cytotoxic T-lymphocyte response in Gag is associated with a dramatic reduction in human immunodeficiency virus type 1 replication. J Virol. 2007 Nov;81(22):12382–93. doi: 10.1128/JVI.01543-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Letvin NL. Correlates of immune protection and the development of a human immunodeficiency virus vaccine. Immunity. 2007 Sep;27(3):366–9. doi: 10.1016/j.immuni.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 99.Vanderford TH, Bleckwehl C, Engram JC, Dunham RM, Klatt NR, Feinberg MB, et al. Viral CTL escape mutants are generated in lymph nodes and subsequently become fixed in plasma and rectal mucosa during acute SIV infection of macaques. PLoS Pathog. 2011 May;7(5):e1002048. doi: 10.1371/journal.ppat.1002048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kannagi M, Chalifoux LV, Lord CI, Letvin NL. Suppression of simian immunodeficiency virus replication in vitro by CD8+ lymphocytes. J Immunol. 1988 Apr 1;140(7):2237–42. [PubMed] [Google Scholar]
- 101.Gray GE, Allen M, Moodie Z, Churchyard G, Bekker LG, Nchabeleng M, et al. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis. 2011 Jul;11(7):507–15. doi: 10.1016/S1473-3099(11)70098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008 Nov 29;372(9653):1894–905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nitayaphan S, Pitisuttithum P, Karnasuta C, Eamsila C, de Souza M, Morgan P, et al. Safety and immunogenicity of an HIV subtype B and E prime-boost vaccine combination in HIV-negative Thai adults. J Infect Dis. 2004 Aug 15;190(4):702–6. doi: 10.1086/422258. [DOI] [PubMed] [Google Scholar]
- 104.Burton DR, Desrosiers RC, Doms RW, Feinberg MB, Gallo RC, Hahn B, et al. Public health. A sound rationale needed for phase III HIV-1 vaccine trials. Science. 2004 Jan 16;303(5656):316. doi: 10.1126/science.1094620. [DOI] [PubMed] [Google Scholar]
- 105.McNeil JG, Johnston MI, Birx DL, Tramont EC. Policy rebuttal. HIV vaccine trial justified. Science. 2004 Feb 13;303(5660):961. doi: 10.1126/science.1096161. [DOI] [PubMed] [Google Scholar]
- 106.Belshe R, Franchini G, Girard MP, Gotch F, Kaleebu P, Marthas ML, et al. Support for the RV144 HIV vaccine trial. Science. 2004 Jul 9;305(5681):177–80. doi: 10.1126/science.305.5681.177b. author reply -80. [DOI] [PubMed] [Google Scholar]
- 107.Berman PW. Development of bivalent rgp120 vaccines to prevent HIV type 1 infection. AIDS Res Hum Retroviruses. 1998 Oct;14(Suppl 3):S277–89. [PubMed] [Google Scholar]
- 108.Lasky LA, Groopman JE, Fennie CW, Benz PM, Capon DJ, Dowbenko DJ, et al. Neutralization of the AIDS retrovirus by antibodies to a recombinant envelope glycoprotein. Science. 1986 Jul 11;233(4760):209–12. doi: 10.1126/science.3014647. [DOI] [PubMed] [Google Scholar]
- 109.Robb ML, Rerks-Ngarm S, Nitayaphan S, Pitisuttithum P, Kaewkungwal J, Kunasol P, et al. Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E: a post-hoc analysis of the Thai phase 3 efficacy trial RV 144. Lancet Infect Dis. 2012 Jul;12(7):531–7. doi: 10.1016/S1473-3099(12)70088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rolland M, Gilbert P. Evaluating immune correlates in HIV type 1 vaccine efficacy trials: what RV144 may provide. AIDS Res Hum Retroviruses. 2012 Apr;28(4):400–4. doi: 10.1089/aid.2011.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Karasavvas N, Billings E, Rao M, Williams C, Zolla-Pazner S, Bailer RT, et al. The Thai Phase III HIV Type 1 Vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. AIDS Res Hum Retroviruses. 2012 Nov;28(11):1444–57. doi: 10.1089/aid.2012.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zolla-Pazner S, deCamp AC, Cardozo T, Karasavvas N, Gottardo R, Williams C, et al. Analysis of V2 antibody responses induced in vaccinees in the ALVAC/AIDSVAX HIV-1 vaccine efficacy trial. PLoS One. 2013;8(1):e53629. doi: 10.1371/journal.pone.0053629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.de Souza MS, Ratto-Kim S, Chuenarom W, Schuetz A, Chantakulkij S, Nuntapinit B, et al. The Thai phase III trial (RV144) vaccine regimen induces T cell responses that preferentially target epitopes within the V2 region of HIV-1 envelope. J Immunol. 2012 May 15;188(10):5166–76. doi: 10.4049/jimmunol.1102756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, Hertz T, et al. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature. 2012 Oct 18;490(7420):417–20. doi: 10.1038/nature11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, de Souza MS, et al. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J Infect Dis. 2012 Aug 1;206(3):431–41. doi: 10.1093/infdis/jis367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012 Feb 2;482(7383):89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pegu P, Vaccari M, Gordon S, Keele BF, Doster M, Guan Y, et al. Antibodies with high avidity to the gp120 envelope protein in protection from simian immunodeficiency virus SIV(mac251) acquisition in an immunization regimen that mimics the RV-144 Thai trial. J Virol. 2013 Feb;87(3):1708–19. doi: 10.1128/JVI.02544-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pal R, Venzon D, Letvin NL, Santra S, Montefiori DC, Miller NR, et al. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J Virol. 2002 Jan;76(1):292–302. doi: 10.1128/JVI.76.1.292-302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pal R, Venzon D, Santra S, Kalyanaraman VS, Montefiori DC, Hocker L, et al. Systemic immunization with an ALVAC-HIV-1/protein boost vaccine strategy protects rhesus macaques from CD4+ T-cell loss and reduces both systemic and mucosal simian-human immunodeficiency virus SHIVKU2 RNA levels. J Virol. 2006 Apr;80(8):3732–42. doi: 10.1128/JVI.80.8.3732-3742.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Griffiss JM, Goroff DK. IgA blocks IgM and IgG-initiated immune lysis by separate molecular mechanisms. J Immunol. 1983 Jun;130(6):2882–5. [PubMed] [Google Scholar]
- 121.Jarvis GA, Griffiss JM. Human IgA1 blockade of IgG-initiated lysis of Neisseria meningitidis is a function of antigen-binding fragment binding to the polysaccharide capsule. J Immunol. 1991 Sep 15;147(6):1962–7. [PubMed] [Google Scholar]
- 122.Nakamura GR, Fonseca DP, O’Rourke SM, Vollrath AL, Berman PW. Monoclonal antibodies to the V2 domain of MN-rgp120: fine mapping of epitopes and inhibition of alpha4beta7 binding. PLoS One. 2012;7(6):e39045. doi: 10.1371/journal.pone.0039045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Parrish NF, Wilen CB, Banks LB, Iyer SS, Pfaff JM, Salazar-Gonzalez JF, et al. Transmitted/founder and chronic subtype C HIV-1 use CD4 and CCR5 receptors with equal efficiency and are not inhibited by blocking the integrin alpha4beta7. PLoS Pathog. 2012;8(5):e1002686. doi: 10.1371/journal.ppat.1002686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alving CR, Peachman KK, Rao M, Reed SG. Adjuvants for human vaccines. Curr Opin Immunol. 2012 Jun;24(3):310–5. doi: 10.1016/j.coi.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL, et al. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J Virol. 2011 May;85(10):4828–40. doi: 10.1128/JVI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Annu Rev Immunol. 2010 Mar;28:413–44. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- 127.Stamatatos L. HIV vaccine design: the neutralizing antibody conundrum. Curr Opin Immunol. 2012 Jun;24(3):316–23. doi: 10.1016/j.coi.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 128.McMichael AJ, Haynes BF. Lessons learned from HIV-1 vaccine trials: new priorities and directions. Nat Immunol. 2012 May;13(5):423–7. doi: 10.1038/ni.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ma BJ, Alam SM, Go EP, Lu X, Desaire H, Tomaras GD, et al. Envelope deglycosylation enhances antigenicity of HIV-1 gp41 epitopes for both broad neutralizing antibodies and their unmutated ancestor antibodies. PLoS Pathog. 2011 Sep;7(9):e1002200. doi: 10.1371/journal.ppat.1002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McGuire AT, Hoot S, Dreyer AM, Lippy A, Stuart A, Cohen KW, et al. Engineering HIV envelope protein to activate germline B cell receptors of broadly neutralizing anti-CD4 binding site antibodies. J Exp Med. 2013 Mar 27; doi: 10.1084/jem.20122824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Markel H. The search for effective HIV vaccines. N Engl J Med. 2005 Aug 25;353(8):753–7. doi: 10.1056/NEJMp058146. [DOI] [PubMed] [Google Scholar]
- 132.Leslie AJ, Pfafferott KJ, Chetty P, Draenert R, Addo MM, Feeney M, et al. HIV evolution: CTL escape mutation and reversion after transmission. Nat Med. 2004 Mar;10(3):282–9. doi: 10.1038/nm992. [DOI] [PubMed] [Google Scholar]
- 133.Feeney ME, Tang Y, Pfafferott K, Roosevelt KA, Draenert R, Trocha A, et al. HIV-1 viral escape in infancy followed by emergence of a variant-specific CTL response. J Immunol. 2005 Jun 15;174(12):7524–30. doi: 10.4049/jimmunol.174.12.7524. [DOI] [PubMed] [Google Scholar]
- 134.Goepfert PA, Lumm W, Farmer P, Matthews P, Prendergast A, Carlson JM, et al. Transmission of HIV-1 Gag immune escape mutations is associated with reduced viral load in linked recipients. J Exp Med. 2008 May 12;205(5):1009–17. doi: 10.1084/jem.20072457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Goulder PJ, Altfeld MA, Rosenberg ES, Nguyen T, Tang Y, Eldridge RL, et al. Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection. J Exp Med. 2001 Jan 15;193(2):181–94. doi: 10.1084/jem.193.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Goonetilleke N, Liu MK, Salazar-Gonzalez JF, Ferrari G, Giorgi E, Ganusov VV, et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med. 2009 Jun 8;206(6):1253–72. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Benmira S, Bhattacharya V, Schmid ML. An effective HIV vaccine: A combination of humoral and cellular immunity? Curr HIV Res. 2010 Sep;8(6):441–9. doi: 10.2174/157016210793499286. [DOI] [PubMed] [Google Scholar]
- 138.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 139.Furuta RA, Wild CT, Weng Y, Weiss CD. Capture of an early fusion-active conformation of HIV-1 gp41. Nat Struct Biol. 1998 Apr;5(4):276–9. doi: 10.1038/nsb0498-276. [DOI] [PubMed] [Google Scholar]
- 140.Colman PM, Lawrence MC. The structural biology of type I viral membrane fusion. Nat Rev Mol Cell Biol. 2003 Apr;4(4):309–19. doi: 10.1038/nrm1076. [DOI] [PubMed] [Google Scholar]
- 141.Gao G, Wieczorek L, Peachman KK, Polonis VR, Alving CR, Rao M, et al. Designing a soluble near full-length HIV-1 gp41 trimer. J Biol Chem. 2013 Jan 4;288(1):234–46. doi: 10.1074/jbc.M112.424432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Melikyan GB, Markosyan RM, Hemmati H, Delmedico MK, Lambert DM, Cohen FS. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J Cell Biol. 2000 Oct 16;151(2):413–23. doi: 10.1083/jcb.151.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chan DC, Kim PS. HIV entry and its inhibition. Cell. 1998 May 29;93(5):681–4. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 144.Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997 May 22;387(6631):426–30. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]