Abstract
Objectives: This study investigated the effect of Muslim prayer (salat) on the α relative power (RPα) of electroencephalography (EEG) and autonomic nervous activity and the relationship between them by using spectral analysis of EEG and heart rate variability (HRV).
Methods: Thirty healthy Muslim men participated in the study. Their electrocardiograms and EEGs were continuously recorded before, during, and after salat practice with a computer-based data acquisition system (MP150, BIOPAC Systems Inc., Camino Goleta, California). Power spectral analysis was conducted to extract the RPα and HRV components.
Results: During salat, a significant increase (p<.05) was observed in the mean RPα in the occipital and parietal regions and in the normalized unit of high-frequency (nuHF) power of HRV (as a parasympathetic index). Meanwhile, the normalized unit of low-frequency (nuLF) power and LF/HF of HRV (as sympathetic indices) decreased according to HRV analyses. RPα showed a significant positive correlation in the occipital and parietal electrodes with nuHF and significant negative correlations with nuLF and LF/HF.
Conclusions: During salat, parasympathetic activity increased and sympathetic activity decreased. Therefore, regular salat practices may help promote relaxation, minimize anxiety, and reduce cardiovascular risk.
Introduction
Islamic prayer, commonly represented by the Arabic term salat, is a form of meditation,1 and it is obligatory for Muslims to perform the prayers five times daily at specific prescribed times of the day. It is a religious physical activity that involves various Quran recitations and the performance of specific postural positions, namely standing, bowing, prostration, and sitting.2
Various meditation forms can influence not only the autonomic nervous system (ANS),3,4 but also the central nervous system (CNS).5,6 For instance, Arambula and colleagues showed an increase in α and θ electroencephalography (EEG) activities during Kundalini yoga meditation, and Peressutti and colleagues showed variations in heart rate variability (HRV) and respiratory rate during meditation.7 Lee and colleagues found that heart rate, respiratory rate, and systolic blood pressure significantly decrease during Qi-training,8 and Raichur and associates described the effect of meditation training on the variation in respiration.9
Power spectral analysis is commonly applied to electrocardiography (ECG) signals to assess HRV as an indicator of the autonomic nervous system.10,11 Many cardiovascular disorder states are hypothesized to be associated with typical variations in HRV.12 The cardiovascular system is usually controlled by autonomic regulation through the activity of the sympathetic and parasympathetic branches of the ANS.13 The sympathetic branch, in a simplified sense, is responsible for stimulating activities associated with the fight-or-flight response, and the parasympathetic branch is responsible for the calming-down response.14 The three common frequency bands in the HRV spectrum are the very-low-frequency (VLF) component (0.001–0.04 Hz), the low-frequency (LF) component (0.04–0.15 Hz), and the high-frequency (HF) component (0.15–0.4 Hz). VLF mainly reflects thermoregulatory cycles, LF is usually considered a marker of mixed sympathetic-parasympathetic nervous activities, and HF reflects parasympathetic (vagal) nervous activities.11,12,15 In addition, the ratio of LF/HF represents the sympathovagal balance, which is essential for good health.12,16 Recently, HRV information has been obtained from individuals before and during meditation to understand ANS response induced by the meditative state.15,17 Meditation affects ANS by increasing and decreasing parasympathetic and sympathetic activities, respectively. EEG can also be investigated with power spectral analysis. The five spectral frequency bands are δ (0.5–4 Hz), θ (4–8 Hz), α (8–13 Hz), β (13–30 Hz), and γ (30–70 Hz), which correspond to the classification of brain waves. The α wave is one of the most dominant brain waves in meditation state. The α wave activity can be measured in all regions of the brain. However, the highest α wave amplitude was observed in the occipital and parietal regions.18 The increasing of α band frequency in meditation was hypothesized to be promoted by changes in ANS, which induce relaxation response in humans.19,20 The generation of α waves is generally associated with stimulation of parasympathetic activity and reduction of the sympathetic activity of ANS.21 High levels of α activities were correlated with low levels of anxiety and feelings of calm and positive affect.22,23
Many studies describe the relationship between the CNS and ANS during meditation. For example, Takahashi and colleagues performed aspectral analysis for EEG band frequencies and HRV components during Zen meditation and discovered that the α EEG power was negatively correlated with normalized unit of LF (nuLF) power as well as in LF/HF and that θ EEG power was positively correlated with normalized unit of HF (nuHF) power.21 Travis demonstrated reduced breath rate, increased respiratory sinus arrhythmia amplitudes, and increased EEG α amplitude compared with EEG and autonomic patterns during Transcendental Meditation.24 Tang and colleagues reported a positive correlation between θ and HF component of HRV during short-term meditation.17 No studies have assessed Muslim prayer (salat), and limited information is available on the functionality of ANS in the interaction between EEG and HRV during salat. This study aims to evaluate the possible correlations between the spectral power of the α band frequency of EEG and HF or LF bands of HRV during salat and to elucidate the physiologic mechanisms between salat and the CNS and ANS.
Materials and Methods
Participants
This study recruited 30 healthy Muslim men aged 20–35 years. The participants had no neurologic or psychological disorder, and they were asked not to take any heavy meals or to do physical activity at least 4 hours before measurements were taken.
Procedure
The experimental procedure for each study participant was divided into three sessions: prebaseline, Duha salat practice, and postbaseline. Before the first session (prebaseline) recording, the participants lay on a bed in a quiet, semi-darkened room and were asked to relax in the supine position for 20 minutes so that they could adapt to the experimental conditions. EEG and ECG signals were simultaneously recorded. The data were collected with both eyes open for 2 minutes, followed by both eyes closed for another 2 minutes and both eyes open for 2 minutes. Then, the participants were allowed to rest for 5 minutes.
In the Duha salat session, the participants were asked to perform salat. EEG and ECG signals were collected throughout the performance of each of the different postural positions of salat. Figure 1 shows a complete single cycle of prayer movements. Participants were reminded to keep their eyes opened during the salat session. In the postbaseline session, data were recorded, and the procedure was similar to that used for the prebaseline data recording.
FIG. 1.
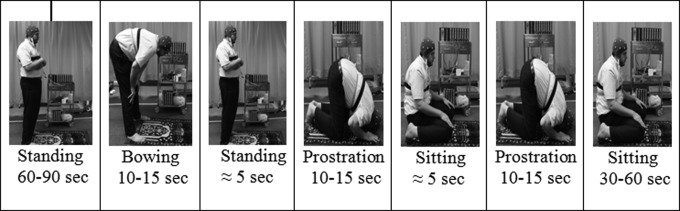
Complete cycle of salat postures and movements.
Data acquisition and signal processing
During the experiment, EEG and ECG signals were continuously recorded with a computer-based data acquisition system (MP150; BIOPAC Systems Inc., Camino Goleta, California). To avoid any artifacts due to physical movements, only four static positions (standing, bowing, sitting, and prostrating) were analyzed; the signals in between movements were excluded.
EEG was recorded with an AgCl electrode cap, with electrodes positioned on the participant's head with the use of the standard 10–20 system. On the basis of previous studies, electrodes were placed at O1, O2, P3, P4, C3, C4, F3, and F4 and referenced to the linked ear lobe electrode during recording. Electrode impedances were brought below 5 K Ω. Unipolar recording technique was used to record the signals. The signals were sampled at a rate of 1000 samples/s and amplified with BIOPAC EEG100C amplifiers. As a preliminary step to estimate power spectral density, all signals were band-pass filtered between 1.0 and 100 Hz with AcqKnowledge 4.0 software (BIOPAC Systems Inc.). Then, a Matlab program (MATLAB R2010a, MathWorks, Natick, Massachusetts) was written to estimate the power spectral density of the EEG signals. This program is based on Welch's averaged periodogram method with a Hanning window that has a 1024-point fast Fourier transformation and 512-point overlapping for each segment length of the signal recorded. The resulting values were normalized into the α relative power (RPα) according to the following equation:
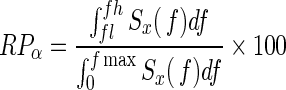 |
where fmax=95 Hz, fl=8 Hz, fh=13 Hz.25
ECG signal was obtained with three electrodes attached to the participant's chest in a standard lead II configuration. The sampling rate of the ECG was 1000 samples/s, and the signal was amplified with a BIOPAC ECG100C differential amplifier. Then, the signal was band-pass filtered between 0.5 and 35 Hz before signal analysis. A Hanning window with a 256-point fast Fourier transformation and 128-point overlapping was used for Welch's method to evaluate the power spectral density in HRV. HRV was calculated from a series of 5-minute epochs of ECG signal according to guidelines. Spectral HRV components were evaluated and obtained in absolute values of power (ms2) based on their frequency to one of the following three bands: VLF, LF, and HF. The HF and LF components of HRV were conventionally observed in normalized units (nuHF and nuLF). The LF/HF ratio, an estimate of the balance between sympathetic and parasympathetic activities, was also calculated from the absolute power of both frequency components.
Statistical analysis
Experimental data were analyzed with SPSS software, version 17 (SPSS Inc., Chicago, Illinois). Analysis of variance (ANOVA) was used to test the changes in the means of the RPα and HRV variables during salat and pre- and postbaseline. Additional comparisons were also conducted with the post hoc test. The Pearson product-moment correlation coefficient was obtained to determine the correlation between the HRV frequency power and RPα of the EEG signals and also between HRV and respiration. A p-value less than .05 was considered to represent a statistically significant difference.
Results
EEG and ECG signals from all participants were analyzed. The average prayer duration performed in this study was 5.23 (standard deviation, 1.46) min. Table 1 shows the means and standard deviations of RPα during pre- and postbaseline and salat practice. ANOVA tests showed that the means of RPα were significantly higher (p<.05) during salat than those of the two baselines before and after salat in most of the eight electrode positions, where O1 (F[2,87]=3.60; p=.031), O2 (F[2,87]=3.39; p=.038), P3 (F[2,87]=3.27; p=.043), P4 (F[2,87]=3.50; p=.034), C3 (F[2,87]=3.22; p=.045), C4 (F[2,87]=3.36; p=.039), F3 (F[2,87]=1.87; p=.159), and F4 (F[2,87]=3.36; p=.039). Furthermore, post hoc analysis showed that there were significant differences between Salat and prebaseline for all electrode positions except F3, but no significant difference between salat and postbaseline condition.
Table 1.
α Relative Power During Pre- and Postbaseline and Salat Practice
| Mean RPα (SD) (μv2/Hz) | |||
|---|---|---|---|
| Scalp position | Prebaseline | During salat | Postbaseline |
| O1 | 23.06 (8.09) | 29.13 (8.59) | 27.02 (9.87) |
| O2 | 22.40 (6.89) | 27.42 (6.78) | 25.36 (8.68) |
| P3 | 24.03 (7.43) | 28.80 (6.93) | 26.72 (7.35) |
| P4 | 23.45 (5.49) | 28.15 (6.83) | 25.23 (8.22) |
| C3 | 15.86 (3.57) | 18.29 (4.42) | 16.92 (3.03) |
| C4 | 15.18 (2,81) | 17.66 (5.13) | 16.47 (2.60) |
| F3 | 10.56 (2.88) | 12.00 (4.03) | 11.59 (1.47) |
| F4 | 10.04 (2.45) | 11.66 (3.04) | 10.58 (1.70) |
RPα, α relative power; SD, standard deviation.
Table 2 presents the changes in HRV components during, before, and after salat practices. The results showed that LF, HF, and nuHF significantly increased whereas nuLF and LF/HF significantly decreased during salat practice. ANOVA test results showed that the differences in means of all HRV parameters between prebaseline, Duha salat practice, and postbaseline were significant at the 5% level (p<.05), where VLF (F [2,87]=5.80; p=.004), LF (F [2,87]=4.23; p=.018), HF (F[2,87]=9.52; p=.000), total power (F[2,87]=7.13; p=.002), nuLF (F[2,87]=4.82; p=.010), nuHF (F[2,87]=4.82; p=.010), LF/HF (F[2,87]=3.72; p=.028), and heart rate (F[2,87]=5.86; p=.004). In addition, post hoc analysis showed significant differences between salat and prebaseline but no significant difference between salat and postbaseline condition.
Table 2.
Heart Rate Variability Parameters During Pre- and Postbaseline and Salat Practice
| HRV parameter | Prebaseline | During salat | Postbaseline |
|---|---|---|---|
| VLF power (ms2) | 217.93 (155.22) | 409.37 (287.24) | 310.39 (188.27) |
| LF power (ms2) | 909.91 (296.82) | 1190.70 (464.67) | 1072.92 (345.13) |
| HF power (ms2) | 538.64 (192.57) | 800.15 (268.31) | 683.11 (230.46) |
| Total HRV power (ms2) | 1666.49 (568.57) | 2400.26 (949.47) | 2066.47 (711.99) |
| nuLF | 62.91 (3.92) | 59.23 (5.62) | 61.11 (4.00) |
| nuHF | 37.08 (3.92) | 40.76 (5.62) | 38.88 (4.00) |
| LF/HF | 1.72 (0.29) | 1.50 (0.35) | 1.60 (0.30) |
Values are mean (standard deviation).
HRV, heart rate variability; VLF, very low frequency; LF, low frequency; HF, high frequency; LF/HF, ratio of LF to HF; nuLF, normalized unit of LF; nuHF, normalized unit of HF.
Table 3 shows the correlation coefficients between the RPα and HRV parameters. The results indicate that RPα was significantly positively correlated with nuHF and significantly negatively correlated with nuLF and LF/HF in the occipital and parietal regions of the brain during salat practice. The pre- and postbaseline conditions showed no significant correlations between RPα and HRV components.
Table 3.
Correlation Coefficients Between α Relative Power and Heart Rate Variability During Pre- and Postbaseline and Salat
| HRV Parameters | HRV Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Scalp position | nuLF | nuHF | LF/HF | Scalp position | nuLF | nuHF | LF/HF |
| Prebaseline | O1 | 0.153 | −0.153 | 0.111 | O2 | −0.087 | 0.087 | −0.090 |
| P3 | −0.170 | 0.170 | −0.142 | P4 | −0. 120 | 0.120 | −0.103 | |
| C3 | −0.080 | 0.113 | −0.113 | C4 | −0.159 | 0.159 | −0.143 | |
| F3 | −0.072 | 0.072 | −0.036 | F4 | −0.024 | 0.024 | −0.017 | |
| During salat | O1 | −0.514** | 0.514** | −0.546** | O2 | −0.626** | 0.626** | −0.689** |
| P3 | −0.392* | 0.392* | −0.479** | P4 | −0.406* | 0.406* | −0.477** | |
| C3 | −0.281 | 0.281 | −0.318 | C4 | −0.314 | 0.314 | −0.365* | |
| F3 | −0.202 | 0.202 | −0.283 | F4 | −0.224 | 0.224 | −0.329 | |
| Postbaseline | O1 | −0.286 | 0.286 | −0.293 | O2 | −0.275 | 0.275 | −0.279 |
| P3 | −0.233 | 0.233 | −0.252 | P4 | −0.222 | 0.222 | −0.239 | |
| C3 | −0.237 | 0.237 | −0.253 | C4 | −0.262 | 0.262 | −0.283 | |
| F3 | −0.164 | 0.164 | −0.133 | F4 | −0.151 | 0.151 | −0.173 | |
Correlation is significant at the 0.05 level (two-tailed).
Correlation is significant at the 0.01 level (two-tailed).
Discussion
This study investigated the EEG and ECG signals of 30 young, healthy Muslim men. It aimed to explain the effect and the possible relationships among the relative power spectra of the α band frequency of EEG and ANS activities represented by the frequency bands of HRV during salat.
The results (Table 1) indicated that RPα was significantly higher (p<.05) during salat than at pre- and postbaseline. A notable increase in α wave activity was observed at the occipital and parietal regions of both brain hemispheres. The production of αwaves is normally promoted by the parasympathetic nervous system with suppression of the sympathetic system.21 These findings strongly suggest that the high levels of α activity during salat are associated with increased relaxation, reduced tension, sustained focus, and a balanced condition of the human mind and body.5,6,26 The results were also in accordance with previous laboratory results that demonstrated increased RPα during prostrate position, particularly during salat positions.27
The novelty of this study is the marked increase in general HRV components power during salat practice. Both the LF and HF components significantly increased at p<.05, as well as the nuHF power when compared with pre- and postbaseline. There was a relatively stronger parasympathetic mobilization. The significant reduction in nuLF substantiates the reduced sympathetic activates because the LF band power of the HRV is mainly related to sympathetic modulation when expressed in normalized units,12 and the parasympathetic (vagal) modulation is a major contributor to HF band power. The ratio of LF/HF represents the sympathovagal balance.28 However, an LF/HF ratio of 1.72 and 1.60 at prebaseline and postbaseline, respectively, versus 1.50 during salat practice indicates a relatively stronger parasympathetic mobilization. These findings were consistent with a previous study that reported an increase in nuHF (as a parasympathetic index) and a decrease in nuLF (as a sympathetic index) during meditation.3,4,15,21
During salat, positive significant correlations were found between RPα in the occipital and parietal regions and that in nuHF (as an index of parasympathetic tone), and negative significant correlations were found with RPα in nuLF and LF/HF (as indices of sympathetic tone) (Table 3). The significant correlations between the power spectral components of HRV and RPα reflect the sympathovagal balance, suggesting that parasympathetic and sympathetic nervous activities increase and decrease during salat, respectively. Furthermore, these results present an important point in the interpretation of the physiologic mechanisms between the CNS and ANS among salat. These findings also agreed with those of a recent study on meditation.21 The aforementioned study reported that the percentage change in α EEG power was positively correlated with nuHF and negatively correlated with nuLF and LF/HF.
In conclusion, in light of these results, the increased EEG occipital and parietal RPα during salat suggests that salat produces positive changes in brain function and human well-being. These changes are associated with an increase in the parasympathetic component and decrease sympathetic component in the ANS. This combination of high parasympathetic activity in nuHF power, low sympathetic activity in nuLF power, and increase in the EEG RPα during salat practice suggest that the interaction between the central nervous system and ANS during salat promotes relaxation and minimizes anxiety for individuals who regularly practice salat.
Acknowledgment
This research was supported and funded by the Prime Minister's Department, Malaysia (project no. 66-02-03-0061/H-00000-3703), and University of Malaya, through a postgraduate grant (PS107-2010A).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Alwasiti HH, Aris I, Jantan A. EEG activity in Muslim prayer: a pilot study. Maejo Int J Sci Technol 2010;4:496–511 [Google Scholar]
- 2.Yucel S. The Effects of Prayer on Muslim Patients' Well-Being [microform]. Boston: Boston University School of Theology, 2007 [Google Scholar]
- 3.Wu SD, Lo PC. Inward-attention meditation increases parasympathetic activity: a study 1based on heart rate variability. Biomed Res 2008;29:245–250 [DOI] [PubMed] [Google Scholar]
- 4.Sarang P, Telles S. Effects of two yoga based relaxation techniques on heart rate variability (HRV). Int J Stress Manage 2006;13:460–475 [Google Scholar]
- 5.Chang KM, Lo PC. F-VEP and Alpha-suppressed EEG-physiological evidence of inner-light perception during Zen meditation. Biomed Eng Appl Basis Communicat 2006;18:1–7 [Google Scholar]
- 6.Kasamatsu A, Hirai T. An electroencephalographic study on the zen meditation (Zazen). Psychiatry Clin Neurosci 1966;20:315–336 [DOI] [PubMed] [Google Scholar]
- 7.Peressutti C, Martín-González JM, M. García-Manso J, et al. Heart rate dynamics in different levels of Zen meditation. Int J Cardiol 2010;145:142–146 [DOI] [PubMed] [Google Scholar]
- 8.Lee MS, Kim BG, Huh HJ, et al. Effect of Qi-training on blood pressure, heart rate and respiration rate. Clin Physiol 2000;20:173–176 [DOI] [PubMed] [Google Scholar]
- 9.Raichur RN, Kulkarni SB, Rahul RR, et al. Effect of meditation training on pulmonary function tests. Recent Res Sci Technol 2010;2:16 [Google Scholar]
- 10.Akselrod S, Gordon D, Ubel F, et al. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 1981;213:220–222 [DOI] [PubMed] [Google Scholar]
- 11.Pomeranz B, Macaulay RJ, Caudill MA, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol 1985;248:H151–153 [DOI] [PubMed] [Google Scholar]
- 12.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Eur Heart J 1996;93:1043–1065 [PubMed] [Google Scholar]
- 13.Aubert AE, Seps B, Beckers F. Heart rate variability in athletes. Sports Med 2003;33:889–919 [DOI] [PubMed] [Google Scholar]
- 14.Humphreys RB, Eagan KP. Autonomic foundations of consciousness and mind-body medicine. In Consciousness Research Abstracts from “Toward a science of consciousness: Tucson 2000.” Thorverton, UK: Journal of Consciousness Studies, 2000. (in press). http://www.mbcring.com/mind-bodymedicine.net/parasympatheticpathways.com/afmbfinsmbk.html, accessed April22, 2014 [Google Scholar]
- 15.Nesvold A, Fagerland MW, Davanger S, et al. Increased heart rate variability during nondirective meditation. Eur J Prev Cardiol 2012;19:773–780 [DOI] [PubMed] [Google Scholar]
- 16.Malliani A, Lombardi F, Pagani M. Power spectrum analysis of heart rate variability: a tool to explore neural regulatory mechanisms. Br Heart J 1994;71:1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang YY, Ma Y, Fan Y, et al. Central and autonomic nervous system interaction is altered by short-term meditation. Proc Natl Acad Sci U S A 2009;106:8865–8870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfurtscheller G, Neuper C, Mohl W. Event-related desynchronization (ERD) during visual processing. Int J Psychophysiol 1994;16:147–153 [DOI] [PubMed] [Google Scholar]
- 19.Delmonte MM. Physiological responses during meditation and rest. Appl Psychophysiol Biofeedback 1984;9:181–200 [DOI] [PubMed] [Google Scholar]
- 20.Ivanovski B, Malhi GS. The psychological and neurophysiological concomitants of mindfulness forms of meditation. Acta Neuropsychiatrica 2007;19:76–91 [DOI] [PubMed] [Google Scholar]
- 21.Takahashi T, Murata T, Hamada T, et al. Changes in EEG and autonomic nervous activity during meditation and their association with personality traits. Int J Psychophysiol 2005;55:199–207 [DOI] [PubMed] [Google Scholar]
- 22.Brown BB. Recognition of aspects of consciousness through association with EEG alpha activity represented by a light signal. Psychophysiology 1970;6:442–452 [DOI] [PubMed] [Google Scholar]
- 23.Hardt J, Kamiya J. Anxiety change through electroencephalographic alpha feedback seen only in high anxiety subjects. Science 1978;201:79–81 [DOI] [PubMed] [Google Scholar]
- 24.Travis F. Autonomic and EEG patterns distinguish transcending from other experiences during Transcendental Meditation practice. Int J Psychophysiol 2001;42:1–9 [DOI] [PubMed] [Google Scholar]
- 25.Amodio P, Orsato R, Marchetti P, et al. Electroencephalographic analysis for the assessment of hepatic encephalopathy: comparison of non-parametric and parametric spectral estimation techniques. Clin Neurophysiol 2009;39:107–15 [DOI] [PubMed] [Google Scholar]
- 26.Lee SH, Ahn SC, Lee YJ, et al. Effectiveness of a meditation-based stress management program as an adjunct to pharmacotherapy in patients with anxiety disorder. J Psychosom Res 2007;62:189–195 [DOI] [PubMed] [Google Scholar]
- 27.Doufesh H, Faisal T, Lim K-S, et al. EEG spectral analysis on Muslim prayers. Appl Psychophysiol Biofeedback 2012;37:11–18 [DOI] [PubMed] [Google Scholar]
- 28.Malliani A, Pagani M, Lombardi F, et al. Cardiovascular neural regulation explored in the frequency domain. Circulation 1991;84:482–92 [DOI] [PubMed] [Google Scholar]


