Abstract
A patient with a history of haemolytic anaemia and membranoproliferative glomerulonephritis type 1 since childhood developed relapsing atypical haemolytic uraemic syndrome (aHUS) at the age of 18. Despite several episodes of relapsing aHUS, she was successfully treated with plasmapheresis. aHUS is strongly associated with disorders of the complement pathway. Diagnostic work-up of the patient revealed normal serum values of complement factor H, I, B and membrane cofactor protein (MCP). Genetic analysis showed a homozygous mutation in the factor H gene. Extraordinarily, the homozygous mutation in this patient causes a normal amount but hypothetically functionally defective factor H in the plasma.
Keywords: atypical haemolytic uraemic syndrome, complement factor H, MPGN type 1
Background
Haemolytic uraemic syndrome (HUS) is characterized by the triad micro-angiopathic haemolytic anaemia, thrombocytopoenia and acute renal failure, mostly presenting in childhood following an episode of diarrhoea, caused by Shiga-like toxin-producing organisms. The atypical, non-diarrhoeal form (aHUS) can be familial or sporadic, and is associated with several underlying predisposing factors, such as autoimmune systemic diseases, neoplasm, pregnancy, HIV and certain medications [1, 2]. aHUS represents 5–10% of HUS in children, but the majority in adults. Half of the patients have relapses [2].
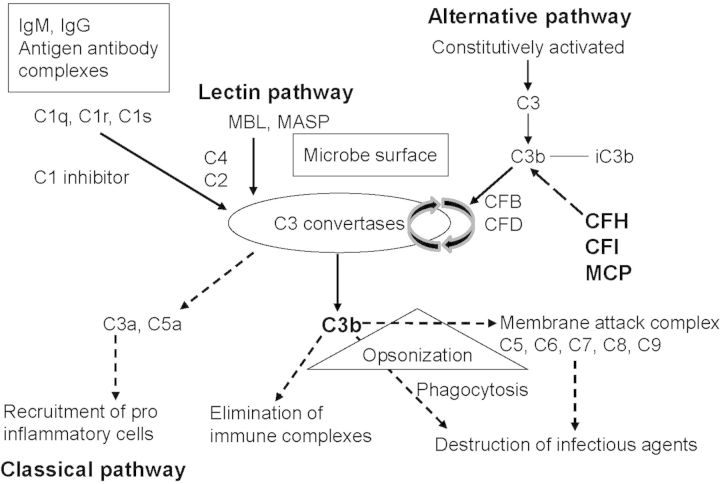
Advances in the past 15 years have shown aHUS to be a disorder of the alternative pathway of complement. Complement consists of multiple plasma and membrane-bound proteins, which trigger several pathways of enzymatic reactions designed for opsonization of pathologic targets, promotion of inflammatory and immune responses and promotion of anaphylatoxins release. It is activated by three pathways: the classical, lectin and alternative pathway. All three converge at the point of cleavage of C3. C3 is cleaved by the C3 convertase, into C3b and C3a. C3b binds indiscriminately to pathogens and host cells and its deposition on an antigen mark it for adherence and consequent elimination by phagocytic cells. The alternative pathway is continuously activated at a low level. Key factors of this pathway are factor B (CFB), factor H (CFH), factor I (CFI) and membrane cofactor protein (MCP). The activation of the alternative pathway occurs in a tightly regulated, sequential manner. All factors operate at different levels of the pathway and control the spontaneous activity of C3 convertase (Figure 1) [2, 3]. In some patients, the presence of an autoantibody, the C3 nephritic factor (C3NeF), interferes with these normal regulatory mechanisms [2]. Mutations in the complement regulatory proteins CFH, CFI, and MCP and complement CFB and C3 all result in complement over activation [4–6].
Fig. 1.
The three pathways of complement activation. Classical, lectin and alternative pathways converge at the point of C3 activation. The lytic pathway then leads to the assembly of the membrane attack complex that destroys infectious agents. Regulators of the alternative pathway CFH, CFI and MCP cooperate to inactivate endothelial cell surface-bound C3b, thus protecting endothelial cells from complement attack. CFH, factor H; CFI, factor I; CFB, factor B; CFD, factor D; MCP, membrane cofactor protein. Adapted with permission from C Loirat and V Frémeaux-Bacchi [2].
Membranoproliferative glomerulonephritis (MPGN) is diagnosed on the basis of a glomerular-injury pattern that is common to a heterogeneous group of diseases. Dysregulation of the alternative pathway can occur because of mutations in or autoantibodies to C3 convertase (C3 nephritic factor). Certain genetic polymorphisms in factors H and B, MCP and C3 are also associated with MPGN [3]. Modern classification subdivided MPGN due to alternative pathway dysregulation into dense deposit disease (DDD) and C3 glomerulonephritis (C3GN). On the basis of the morphologic characteristics of C3GN on electron microscopy, C3GN is most likely to be termed MPGN I or MPGN III according to the older classification. The hypothesis that DDD and C3GN are part of a continuum is further supported by cases showing features that are intermediate between DDD and C3GN [3, 7]. In the underlying case, we prefer to preserve the term MPGN I.
Case
A healthy girl was born in 1989 to consanguineous parents, sharing the same great-grandparents. At the age of 1, she developed haemolytic anaemia and was treated with repeated blood transfusions. At the age of 8, she developed haematuria and proteinuria. At that time, C3 levels measured in plasma were low. A kidney biopsy showed MPGN type I. In seven glomeruli, there was high immunoactivity for C3, less for IgM and no activity for IgA, IgG, fibrinogen or C1q. No specific therapy was started and she grew up without major health problems.
At the age of 18, she consulted her general practitioner with complaints of overwhelming fatigue. Clinical evaluation showed peripheral oedema and a mild arterial hypertension. She used a contraceptive hormonal patch and was recently vaccinated against hepatitis B. Laboratory results showed decreased renal function (MDRD GFR 18 mL/min/1.73 m²), anaemia (Hb 9.9 g/dL) and thrombocytopoenia (27.109/L). C3 and C3b levels were low, but C4 levels were normal, as were levels of CFB, CFH, CFI and MCP. Haptoglobine was undetectably low. Antibodies against CFH could not be detected. C3 nephritic factor was found in one plasma sample during the first episode (Table 1). Diagnosis of aHUS was made and plasmapheresis (daily exchange of 1.5 × plasma volume/session during 2 weeks, tapered to three times a week) was applied until renal function and blood count normalized. As anti-hypertensive treatment, an ACE inhibitor was initiated. During the following 6 months, she had three relapses and each time plasmapheresis was re-instituted. After the last episode, she remained on a weekly plasmapheresis schedule during 11 months. She developed CT-confirmed bilateral lung emboli and pneumonia. There were no signs of nephrotic syndrome (total protein/total creatinine ratio < 1 mg/mg). Treatment existed of tinzaparine s.c., warfarin and antibiotics. Plasmapheresis was discontinued because of pulmonary infection.
Table 1.
Investigation of complement components
| C3 Ag | C4 Ag | Factor B Ag | Factor H Ag | Factor I Ag | CD46 | |||
|---|---|---|---|---|---|---|---|---|
| Normal values | 660–1250 mg/L² | 93–380 mg/L² | 90–320 mg/L² | 65–140%³ | 70–130%³ | Anti-FH Ab | C3 NeF | MFI5 |
| Sample 1 | 220 | 377 | 178 | 117 | 114 | N | Positive | na |
| Sample 2 | 735 | 297 | 155 | 117 | 121 | Negative | 938 | |
| Sample 3 | 582 | 381 | 208 | N | Negative | NA | ||
| Sample 4 | 462 | 609 | 389 | N | Negative | NA | ||
| Sample 5 | 436 | 637 | 305 | N | Negative | NA | ||
| Sample 6 | 225 | 318 | 144 | 119 | 135 | N | NA | NA |
Genetic analysis showed normal alleles for CFI, CFB, MCP and thrombomodulin [6, 8]. A homozygous mutation in the factor H gene was detected, located in SCR1 and showed a p.Arg53Cys CFH mutation.
During the last 2 years, there were no signs of aHUS anymore. Her renal function remained stable with a GFR of 69 mL/min/1.73 m² (MDRD formula), a total protein/creatinine ratio of 1.2 mg/mg and a slight elevation of C3d of 2.44 mg/dL.
Genetic work-up of her younger brother showed that he also carries the homozygous mutation. In his serum, C3NeF could not be detected. Clinically, he never suffered from haematuria, proteinuria, hypertension or HUS.
Discussion
This patient was diagnosed with MPGN type I at the age of 8 and aged 18 had several episodes of relapsing aHUS. She had none of the known risk factors for autoimmune diseases or malignancies. Although HUS is a well-known complication of oral contraceptive use [9], it is not described as an adverse effect of the contraceptive hormonal patch she used. She had recently been vaccinated, but prior vaccinations never caused any problem. Although rare, vaccination has been described as a trigger for HUS [10].
MPGN is classified as being mediated by immune complexes, by complement dysregulation that leads to persistent activation of the alternative complement pathway or rarely by mechanisms not involving immunoglobulin or complement deposition such as endothelial injury [11]. Complement-mediated MPGN is less common than immune complex-mediated MPGN and results from deregulation and persistent activation of the alternative complement pathway [11, 12]. Some of the genetic mutations that have been associated with these disorders are also associated with aHUS [13].
This patient has a genetic predisposition to complement activation, due to a homozygous mutation in the complement factor H gene. It remains uncertain if the first episode of anaemia during childhood was an early presentation of aHUS. A relationship between MPGN type I and aHUS has been described before in familial HUS [14]. Some authors even suggest that MPGN and HUS represent two forms of a disease spectrum with a common pathogenic principle [15]. The presence of C3NeF is also described in patients with MPGN, but whether in this case C3NeF was present at the time of diagnosis of MPGN is unknown.
Some mutations (type 1 mutations) are associated with quantitative deficiency in CFH (decreased plasma levels), but many are associated with normal plasma levels of CFH, the mutant CFH being functionally deficient (type 2 mutations) [2]. The homozygous mutation in this patient causes hypothetical a normal amount of functionally defective CFH (mutation type 2) to be present in the plasma. This functional deficiency has several consequences: firstly, degradation and regeneration of the renal glomerular basement membrane. The deposition of immunoglobulin, complement or both in the mesangium and sub-endothelial region of the capillary wall triggers an acute injury, which is often followed by an inflammatory phase, with an influx of inflammatory cells. A subsequent reparative phase occurs, during which new mesangial matrix results in mesangial expansion, along with the generation of a new glomerular basement membrane [3]. The second consequence is development to aHUS. It remains unclear what finally triggered the evolution to HUS. The formation of C3NeF might further compromise this regulation. There still might be a role for the contraceptive patch, or for the vaccination. This supports a multiple hit theory for the development of aHUS in this patient. Thirdly, the defective complement regulation in an infected environment (pneumonia) might have triggered the formation of thrombi locally and causing lung emboli at the age of 20. It is known that, for instance, C-terminal CFH mutants have a reduced ability to bind platelets, resulting in complement activation on the surface of platelets, aggregation and release of tissue-factor expressing micro-particles, all of which participate to the formation of thrombi within the microcirculation. Alteration of the endothelial cells by inflammation may participate actively in this process [2].
Recently, Pechtl et al. investigated the consequences of aHUS-linked R53H mutations. According to their research, they conclude that diminished cofactor activity of (R53H)FH1–4 cannot be explained by the lower affinity for C3b alone. A functionally deficient (R53H)FH mutant in heterozygous individuals would likely compete with wild-type FH for binding C3b and could thereby further diminish complement regulatory capacity [16]. The homozygous mutation in our patient causes a normal amount but hypothetically functionally defective CFH in the plasma and therefore cannot underline this research entirely. The R53H mutation disrupted cofactor activity while binding C3b with normal affinity, results in the conclusion that a mutation at this amino acid impairs the binding of CFH to CFI.
Servais et al. analysed the role of acquired and genetic complement abnormalities in a cohort of 134 patients, of whom 49 have MPGN type I. Two patients have the R53C abnormality, one homozygous, one heterozygous and have MPGN1/C3GN.
Our case was not mentioned in their results [17]. However, this case might be important because of the changes of MPGN transforming to aHUS.
Plasma infusions or plasmapheresis replete the body with normal functioning CFH, but competition with the defective CFH at the binding sites might prevent normalization of the complement cascade. Besides, existing C3NeF needs to be eliminated from the circulation [1, 2, 18]. In case of recurrence of HUS in this patient, re-institution of plasmapheresis is preferred to plasma infusion. Genetic screening of people with aHUS provides important prognostic information guiding clinical decision making. aHUS, CFH mutations are the most frequent ones and patients with such mutations have a poor prognosis; 60–70% die or reach end-stage renal failure within 3 years.
Renal function of survivors does not recover completely [2, 5, 6, 18]. Adequate and intensive plasmapheresis could obtain long-term preservation of renal function in aHUS. Patients with abnormalities in soluble regulators such as CFH respond better to plasma exchange than patients with abnormalities in the transmembrane regulator MCP [2, 6]. Treatment with eculizumab, an anti-C5 monoclonal humanized mouse antibody has been shown to be successful in several reported aHUS cases. However, eculizumab is very costly and has severe side effects. Although rare, meningitis in particular is a risk though vaccination and prophylaxis reduce the likelihood of this. The best option for treating aHUS related to CFH mutation might be a human-plasma-derived CFH concentrate, presently being developed in France [18]. Further research to understand the molecular mechanisms responsible for the pathogenesis of the disease and for the development of adequate therapy, such as recombinant CFH, which reduces the risk from CFH-pooled serum, is a challenge for the near future [19].
Conflict of interest statement
None declared.
Acknowledgements
We thank our patient for giving permission to publish this case.
References
- 1.Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;22(361):1676–1687. doi: 10.1056/NEJMra0902814. doi:10.1186/1757-1626-1-161. [DOI] [PubMed] [Google Scholar]
- 2.Loirat C, Frémeaux-Bacchi V. Atypical haemolytic uremic syndrome. Orphanet J Rare Dis. 2011;6:60. doi: 10.1186/1750-1172-6-60. doi:10.1148/rg.24si045503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis—a new look at an old entity. N Engl J Med. 2012;366:1119–1131. doi: 10.1056/NEJMra1108178. doi:10.1038/sj.ki.5002124. [DOI] [PubMed] [Google Scholar]
- 4.Loirat C, Noris M, Fremeaux-Bacchi V. Complement and the atypical haemolytic uremic syndrome in children. Pediatr Nephrol. 2008;23:1957–1972. doi: 10.1007/s00467-008-0872-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kavanagh D, Anderson HE. Interpretation of genetic variants of uncertain significance in atypical haemolytic uremic syndrome. Kidney Int. 2012;81:11–13. doi: 10.1038/ki.2011.330. [DOI] [PubMed] [Google Scholar]
- 6.Kavanagh D, Goodship THJ. Atypical haemolytic Uremic syndrome, genetic basis and clinical manifestations. Hematology Am Soc Hematol Educ Program. 2011;2011:15–20. doi: 10.1182/asheducation-2011.1.15. [DOI] [PubMed] [Google Scholar]
- 7.Malik TH, Lavin PJ, Goicoechea de Jorge E, et al. A hybrid CFHR3-1 gene causes familial C3 glomerulopathy. J Am Soc Nephrol. 2012;23:1155–1160. doi: 10.1681/ASN.2012020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delvaeye M, Noris M, De Vriese A, et al. Thrombomodulin mutations in atypical haemolytic-uremic syndrome. N Engl J Med. 2009;361:345–357. doi: 10.1056/NEJMoa0810739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauglustaine D, Vanrenterghem Y, Michielsen OP, et al. Oestrogen containing oral contraceptives, decreased prostacyclin production, and haemolytic uraemic syndrome. Lancet. 1981;317:328–329. doi: 10.1016/s0140-6736(81)91943-7. [DOI] [PubMed] [Google Scholar]
- 10.Karim Y, Masood A. Haemolytic uremic syndrome following measles, mumps and rubella vaccination. Nephrol Dial Transplant. 2002;17:941–942. doi: 10.1093/ndt/17.5.941-a. [DOI] [PubMed] [Google Scholar]
- 11.Sethi S, Fervenza FC, Zhang Y, et al. Proliferative glomerulonephritis secondary to dysfunction of the alternative pathway of complement. Clin J Am Soc Nephrol. 2011;6:1009–1017. doi: 10.2215/CJN.07110810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol. 2011;31:341–348. doi: 10.1016/j.semnephrol.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Servais A, Frémeaux-Bacci V, Lequintrec M, et al. Primary glomerulonephritis with isolated C3 deposits: a new entity which shares common genetic risk factors with haemolytic syndrome. J Med Genet. 2007;44:193–199. doi: 10.1136/jmg.2006.045328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper M, McGraw ME, Unsworth DJ, et al. Familial mesangio-capillary glomerulonephritis with initial presentation as haemolytic uremic syndrome. Nephrol Dial Transplant. 2004;19:230–233. doi: 10.1093/ndt/gfg470. [DOI] [PubMed] [Google Scholar]
- 15.Skerka C, Licht C, Mengel M, et al. Autoimmune forms of thrombotic microangiopathy and membranoproliferative glomerulonephritis: indications for a disease spectrum and common pathogenic principles. Mol Immunol. 2009;46:2801–2807. doi: 10.1016/j.molimm.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Pechtl IC, Kavanagh D, Mcintosh N, et al. Disease-associated N-terminal complement factor H mutations perturb cofactor and decay-accelerating activities. J Biol Chem. 2011;286:11082–11090. doi: 10.1074/jbc.M110.211839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Servais A, Noël LH, Roumenina LT, et al. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82:454–464. doi: 10.1038/ki.2012.63. [DOI] [PubMed] [Google Scholar]
- 18.Davin J-C, Groothoff J, Gracchi V, et al. Long-term renal function under plasma exchange in atypical uremic syndrome. Pediatr Nephrol. 2011;26:1915–1916. doi: 10.1007/s00467-011-1925-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt CQ, Slingsby FC, Richards A, et al. Production of biologically active complement factor H in therapeutically useful quantities. Protein Expr Purif. 2011;76:254–263. doi: 10.1016/j.pep.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]