Abstract
Significance: Transforming growth factor β (TGFβ) has a crucial role in maintaining skin homeostasis. TGFβ signaling is important for re-epithelialization, inflammation, angiogenesis, and granulation tissue formation during wound healing. This review will discuss the most important findings regarding the role of TGFβ in epidermal maintenance and its restoration after injury.
Recent Advances: Latest findings on the role of TGFβ signaling in normal and impaired wound healing, including the role of TGFβ pathway in tissue regeneration observed in super-healer animal models, will be reviewed.
Critical Issues: The TGFβ pathway is attenuated in nonhealing wounds. Observed suppression of TGFβ signaling in chronic ulcers may contribute to the loss of tissue homeostasis and the inability of keratinocytes to migrate and close a wound.
Future Directions: A better understanding of TGFβ signaling may provide new insights not only in the normal epithelialization process, but also in tissue regeneration. Future studies focused on TGFβ-mediated crosstalk between multiple cell types involved in wound healing may lead to development of novel therapeutic advances for chronic wounds.

Irena Pastar, PhD
Scope and Significance
Keratinocyte migration and proliferation during epithelialization is regulated by multiple growth factors, including transforming growth factor β (TGFβ). In unwounded skin, TGFβ signaling contributes to tissue homeostasis through regulation of the keratinocyte cell cycle and inhibition of proliferation. During wound healing TGFβ regulates not only re-epithelialization, but also inflammation, angiogenesis, and granulation tissue formation. This article will review the role of TGFβ signaling in normal and impaired wound healing with particular focus on its effect on re-epithelialization. The most recent advances in this field, including the role of TGFβ pathway in tissue regeneration observed in super-healer animal models, will also be discussed.
Translational Relevance
TGFβ signaling is not only important for skin homeostasis and repair, but it is also often deregulated in cutaneous diseases. The role of TGFβ signaling in wound healing has been studied at length ever since it was shown that exogenous application of TGFβ enhanced wound healing in a murine model. Multiple studies have demonstrated the suppression of TGFβ signaling in the epidermis of chronic wounds. Attenuation of TGFβ pathway in non-healing wounds may contribute to the loss of tissue homeostasis, epidermal hyperproliferation, and the inability of keratinocytes to migrate and epithelialize a wound.
Clinical Relevance
Nonhealing wounds represent a tremendous clinical challenge and a significant burden to patients and healthcare professionals. Wound epithelialization is an essential component of wound repair. A wound cannot be acknowledged as closed, regardless of the totality of underlying dermal structures, if it lacks complete epithelialization. Keratinocyte migration is crucial for successful re-epithelialization, and TGFβ plays an important role in this process.
Discussion
TGFβ signaling pathway
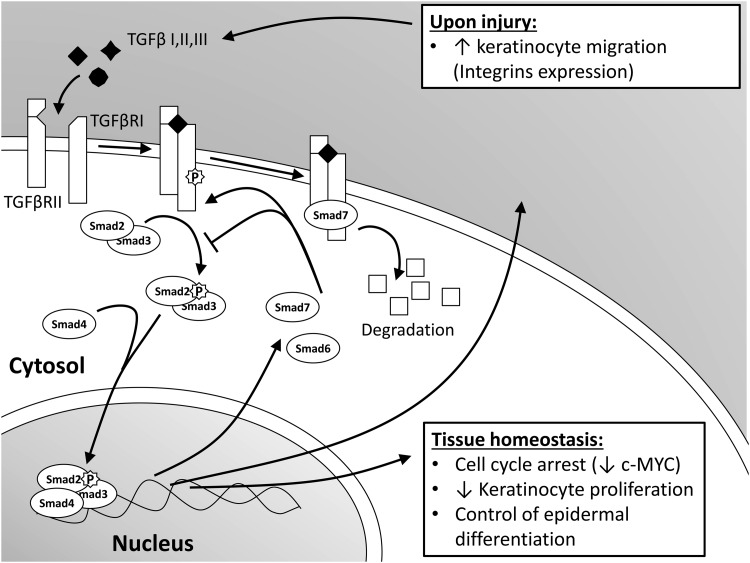
TGFβ is a family of pluripotent cytokines consisting of three isoforms: TGFβ1, 2, and 3 with a dominant role of TGFβ1 in cutaneous wound healing.1 These isoforms were found to be differentially expressed across epidermal layers. TGFβ1 localizes to the stratum granulosum and corneum, whereas TGFβ2 and, to a less extent TGFβ3, are present in supra-basal layers, thus indicating their different functions.2 All TGFβ isoforms are secreted as large pro-peptide molecules in an inactive latent form, in which the N-terminal latency-associated peptide remains noncovalently bound to the C-terminal mature TGFβ.3 These latent forms can be activated by proteases, integrins, thrombospodin-1, reactive oxygen species, low pH, heat, and shear forces to release the mature, biologically active growth factor.4 Once activated, TGFβ mediates its signaling by binding to transmembrane TGFβ receptor II (TGFβRII), followed by its heterodimerization and phosphorylation via serine/threonine kinases of transmembrane TGFβ receptor I (TGFβRI).4,5 The major intracellular mediators of TGFβ signaling are Smad proteins (Fig. 1). Activated TGFβRII binds and phosphorylates receptor-activated Smad2 or Smad3, which, upon heterodimerization with the Smad4, translocates into the nucleus. Within the nucleus, activated Smad complexes become transcriptional factors. They regulate expression of TGFβ target genes. Inhibitory Smad6 and Smad7 are also induced by TGFβ. These can prevent phosphorylation and nuclear translocation of receptor-associated Smads, and they can also cause degradation of TGFβ receptors, thereby acting as negative feedback.5 Smads are critical for TGFβ signaling; however, compelling evidence has suggested that Smad-independent pathways can also mediate TGFβ signal transduction. These independent pathways are activated by direct interaction or phosphorylation by the TGFβ receptors, and involve Mitogen-activated Protein kinase, Rho-like GTPase, and phosphatydilinositol-3-kinase signaling pathways.6 Thus, the effects of TGFβ signaling during wound healing can be achieved through both Smad-dependent and independent signaling.6,7
Figure 1.
Schematic overview of transforming growth factor β (TGFβ) signaling pathway. Upon binding of ligands, TGFβ receptor (TGFβR)I and II heterodimerize and phosphorylate Smad2 or 3. These activated Smads form a complex with Smad4 and translocate to the nucleus to serve as transcriptional factors. Under normal conditions, TGFβ pathway maintains epidermal tissue homeostasis acting as a growth suppression cytokine. After a skin injury, TGFβ signaling regulates re-epithelialization by promoting keratinocyte migration.
TGFβ signaling in epidermal homeostasis
In unwounded epidermis, TGFβ1 participates in maintenance of tissue homeostasis by acting as a growth-inhibitory cytokine.8 The TGFβ pathway directly affects and arrests the cell cycle at the early G1 phase via Smad-mediated transcriptional regulation of multiple cell-cycle regulators, including the oncogene c-myc (Fig. 1).9 Therefore, TGFβ is known to act as a tumor suppressor in early stages of tumorigenesis. However, it can also promote advanced tumor cell invasiveness and metastasis.10 The in vitro findings on growth suppression roles of TGFβ and maintenance of epidermal homeostasis have also been confirmed in vivo. Epidermal targeted ablation of TGFβRII in mice led to loss-of-tissue homeostasis and induction of keratinocyte hyperproliferation.11,12 Furthermore, mice expressing constitutive TGFβ1 in epidermis, under keratin (K) 1 promoter, died shortly after birth due to decreased epidermal proliferation.13,14 In contrast with these findings, overexpression of TGFβ1 under a K10 promoter induced proliferation in suprabasal layers.15 Nevertheless, TGFβ1 inhibited cell growth in these animals when hyperplasia was induced by 12-tetradecanoyl-phorbol-13-acetate (TPA) treatment.15 Similar findings were observed in another transgenic mouse model over-expressing TGFβ1 under a TPA inducible K6 promoter,14 therefore confirming the role of TGFβ in keratinocyte cell-cycle control and suppression of epidermal hyperplasia induced by external factors.
To further elucidate the mechanisms of action of TGFβ1 in epidermis, Ito et al. developed a transgenic mice over-expressing Smad2 under the K14 promoter.16 These mice had defects in their skin, as the epidermis showed hyperproliferation of basal keratinocytes and hyperkeratosis. In addition, mice over-expressing Smad2 had deregulation of differentiation markers such as K10, K14, loricrin, and filaggrin, suggesting a regulatory role of TGFβ1 during epidermal differentiation.16 Although the role of TGFβ signaling in keratinocyte differentiation remains to be fully elucidated, one of the suggested mechanisms involves the regulation of Inhibitor of DNA-binding proteins and kruppel-like transcription factor 4.17,18 The epithelial overexpression of a negative regulator Smad7 in transgenic mice also resulted in hyperproliferative epidermis and hair follicle defects.19 Moreover, both TGFβRI and TGFβRII were almost completely absent in these animals, confirming the function of Smad7 in degradation of the TGFβ receptors.
In order to investigate the role of TGFβ signaling in human skin, Buschke et al.17 used organotypic cultures constructed of human keratinocyte cell lines and dermal fibroblasts. Abrogation of TGFβ signaling in this system by either over-expression of Smad7 or simultaneous knockdown of Smads 2, 3, and 4 resulted in an unrestricted response to mitogens such as keratinocyte growth factor and epidermal growth factor, consequent hyperplasia, and deregulated terminal differentiation. These aforementioned findings confirm the crucial role of TGFβ pathways in epidermal tissue homeostasis, not only in murine but also in human skin.17
In summary, TGFβ1 contributes to skin homeostasis through inhibition of keratinocyte proliferation and regulation of differentiation. When the wound occurs and the epidermal barrier is compromised, TGFβ signaling continues to have important effects on keratinocyte functions and the regulation of wound re-epithelialization.
TGFβ pathway in acute wound healing and epithelialization
TGFβ is not only crucial for epidermal homeostasis, but it has also been shown to be an important player in all phases of wound healing by regulating the functions of keratinocytes, fibroblasts, endothelial cells, monocytes, and other cell types. Although multiple growth factors modulate keratinocyte migration during wound healing,1 TGFβ1 has been the most extensively studied due to its importance and pleiotropic effects.
All three isoforms of TGFβ participate in wound healing and re-epithelialization. After acute injury, TGFβ1 is rapidly up-regulated and secreted by keratinocytes, platelets, monocytes, macrophages, and fibroblasts.1 TGFβ1 is essential for initiating inflammation and granulation tissue formation. It also stimulates wound contraction through induction of smooth muscle alpha actin expression in fibroblasts, and induction of myofibroblast differentiation. Further, TGFβ1 is involved in angiogenesis by up-regulating vascular endothelial growth factor.1 Lastly, TGFβ1 promotes keratinocyte migration during wound closure.
This section will focus on the role of TGFβ1 in re-epithelialization; however, TGFβ2 also participates in all the stages of wound healing and has been shown to accelerate re-epithelialization.20 TGFβ3 restricts scarring and improves collagen organization in vivo in contrast to the two other isoforms, which advance scar formation.1 It has been suggested that TGFβ3 supports scarless wound healing through modulation of the inflammatory response and the recruitment of fibroblasts to the wound site while simultaneously enhancing keratinocyte migration.21 Even though all three TGFβ isoforms bind to the same receptors, their effects may be different depending on genetic milieu and environment of the target cell.5
In vitro, TGFβ1 clearly promotes keratinocyte migration by increasing the expression of different integrins, which, in turn, promotes the adhesion and migration of keratinocytes.22 Interestingly, integrins can also regulate the TGFβ1 pathway during epithelialization, and the interaction between TGFβ and integrin-signaling pathways was confirmed in vivo.20 Namely, alpha 3 beta 1 integrin (α3β1)-null mice exhibited decreased epithelialization and overall suppression of the TGFβ pathway. Ablation of α3β1 integrin resulted in increased levels of inhibitory Smad7, decreased phosphorylation of Smad2 and 3, and reduction of Smad4 and TGFβRI.23 Moreover, increased Smad7 expression in α3β1 genetically depleted animals was identified as the main cause of inhibition of keratinocyte migration during wound healing, confirming that α3β1 down-regulates expression of Smad7 during the normal wound-healing process.23 In contrast to these results, Han et al. demonstrated that temporal epidermal Smad7 over-expression improved wound healing through both direct effects on keratinocyte proliferation and migration, and through indirect effects on wound stroma.24 Another example of TGFβ–integrin crosstalk is the interaction with integrin αvβ6, which has been shown to activate latent TGFβ1 by binding to the latency-associated protein and release of the TGFβ mature form.25 αvβ6 is also temporarily induced in the migrating epithelial edge during wound healing, and its expression is constitutively high in chronic wounds.26 Moreover, mice over-expressing this integrin develop spontaneous chronic ulcers with high levels of TGFβ1.26 Interestingly, these mice, as well as mutant mice lacking αvβ6, do not show any acute healing abnormalities,26 thereby strongly suggesting compensatory effects of alternative TGFβ activation mechanisms during the normal wound-healing process. However, Jacobsen et al.,27 demonstrated reduction of re-epithelialization due to impaired keratinocyte in β6−/− mice on induction of diabetes. Together, these data identified integrins as regulators of the TGFβ pathway during epithelialization,4 in addition to their well-described role as adhesion molecules.
Multiple studies using various animal wound models have demonstrated increased expression of TGFβ ligands and receptors in the epidermis adjacent to a wound after injury28 and in the leading edge of the migrating epithelial tongue.29 The levels of phosphorylated Smad2 (pSmad2) were also elevated in the nuclei of cells comprising migrating epithelial tongue in an ex vivo human acute wound model, thus confirming the activation of the pathway in human epidermis.30 Numerous studies have shown that exogenously applied recombinant TGFβ1 accelerates healing in animal models,31,32 while exogenous inhibition of TGFβ signaling using antibodies against all TGFβ isoforms resulted in impaired epithelialization and granulation tissue formation.33 However, in vivo studies using transgenic animal models have shown both beneficial and negative effects of TGFβ1 on wound closure depending on the wounding technique and the genetic approach used. In contrast to the beneficial effects of exogenous TGFβ1 on wound healing, abrogation of the pathway in TGFβ1-null mice resulted in smaller animals with thinner epidermis and dermis that showed improved wound closure of excisional wounds.34 However, excisional wounds are known to mostly heal by contraction; therefore, the effects on re-epithelialization could not be completely evaluated using this wounding technique. It is important to emphasize that these mice had to be treated by an immunosuppressive agent in order to reduce complications resulting from the inflammatory syndrome, as TGFβ1-null animals would otherwise die at about 3–4 weeks of age from an autoimmune-like inflammatory response.35 Therefore, results from the TGFβ1-knockout animals suggest that enhancement of wound healing could be attributed to the role of TGFβ1 signaling in the inflammatory response rather than epithelialization. Furthermore, when TGFβ1 knockout mice were crossed with immuno-deficient Scid−/− mice lacking B and T lymphocytes, double knockout animals demonstrated delayed healing when compared with Scid−/− or wild-type controls.36
Further studies using transgenic animals have demonstrated the complexity of TGFβ signaling in wound healing by showing that variability of the results depends on multiple factors, including the various wounding techniques used. The most illustrative example is epidermal over-expression of TGFβ1 driven by the K14 promoter, which not only resulted in delayed healing in full-thickness wounds37 but also caused opposite, beneficial effects in partial-thickness wounds, in which case increased re-epithelialization was observed.37 Such opposite results could be explained by the presence or absence of a substrate dermis, required for keratinocytes to migrate over in partial- or full-thickness wounds, respectively. Before keratinocyte migration, the substrate should be replaced by granulation tissue that provides a proper surface over which the keratinocytes can migrate, suggesting that the impairment of healing caused by TGFβ in full-thickness wounds is due to a different factor other than the effect of TGFβ on re-epithelialization.37 Interestingly, inhibition of TGFβ signaling in Smad3-null mice exhibited accelerated wound healing in excisional wounds mainly through reduction of inflammation, therefore supporting the role of TGFβ signaling in regulation of immune response during wound healing.38,39 Primary keratinocytes derived from these mice were less sensitive to TGFβ1 growth inhibition in vitro and exhibited increased proliferation at the wound edges in vivo.38 Conversely, epidermal deletion of Smad4 resulted in delayed wound closure and remodeling.40 Another transgenic mouse model expressing a dominant negative TGFβRII (under a K5 promoter) in the basal layer of the epidermis showed increased re-epithelialization of full-thickness wounds, increased proliferation, and reduced apoptosis of keratinocytes at the wound edges.41
TGFβ signaling is clearly important for successful wound closure. However, the complexity of the system (due to differential but still interacting effects of TGFβ isoforms and other ligands, cross-talk between multiple cell types, and the contribution of Smad-independent TGFβ signaling) makes experimental design and interpretations of the in vivo results challenging. Keratinocytes' response to TGFβ1 also depends on their contact with dermal fibroblasts as shown in co-culture experiments.42 Epidermal keratinocytes in these co-cultures showed increased proliferation and migration on TGFβ1 treatment; but higher concentrations of TGFβ1 were required to induce the same effect when keratinocytes were cultured alone.42
Biological functions of TGFβ in wound healing are complex, cell-type specific, and, most importantly, stringently regulated. Therefore, ideal expression and/or suppression systems to elucidate the full complexity of TGFβ effects on wound closure in vivo should be cell-type specific and temporally controlled. Although the role of TGFβ in wound healing has been extensively studied, we still need to learn more about the finer details of the TGFβ pathway in order to eventually convert latest advances in basic science into potential therapies for wound-healing disorders.
Regulation of TGFβ signaling by hormones during epithelialization
The effects of TGFβ can be manipulated by hormones, directly or indirectly, particularly via androgens.43,44 Importantly, androgen receptor (AR) as well as estrogen receptor β are expressed in fibroblasts, keratinocytes, and macrophages in the skin43 and are important for skin turnover and wound healing. AR can interact directly with pSmad3, which results in inhibition of binding to Smad-binding elements in epithelial cells.43 AR can also modulate TGFβ signaling indirectly through the Wnt/β-catenin pathway.44 Namely, testosterone induced nuclear translocation of AR-β-catenin complex, thereby increasing transcription of their target genes, including the antagonists of TGFβ pathway Smad7 and follistatin (Fst). Consistently, orchidectomy reduced Fst and Smad7 levels in skin, whereas topical testosterone treatment increased them in vivo.44 Furthermore, testosterone has recently been shown to diminish keratinocyte migration by interacting with and mobilizing a known inhibitor of wound healing, β-catenin,45 to the nucleus and, in turn, repressing the TGFβ/Smad pathway in vitro.43 This effect was successfully reversed by AR-antagonist, flutamide. Topical flutamide promoted keratinocyte migration and accelerated re-epithelialization without the harmful systemic corollary of androgen deprivation in vivo.43 On the other hand, the TGFβ pathway has also been found to modulate AR signaling via interaction with Smad3.10 It has been shown that transcriptional activity of AR can be either co-activated or co-repressed by direct interaction with Smad3, depending on the system and cell lines used.10,46 Beneficial effects of TGFβ1 on wound healing were also demonstrated in models for impaired epithelialization caused by glucocorticoids.45,47 TGFβ1 was able to reverse the glucocorticoid-induced wound-healing impairment in rats, although an interaction of TGFβ1 and glucocorticoid pathway in the contexts of epithelialization has not been studied in detail. In contrast to negative effects of androgens on wound healing, estrogens can increase epithelialization rates independently of Smad3 and TGFβ signaling.10 The crosstalk between TGFβ and sex hormones signaling does not imply differences in healing rates between genders, but rather points to the complexity of interaction between TGFβ and other pathways during the wound-healing process. However, declining levels of estrogen and sex steroid precursor-dehydroepiandrosterone with age in both genders may contribute to aging-related impaired wound healing.
Suppression of TGFβ signaling in chronic wounds
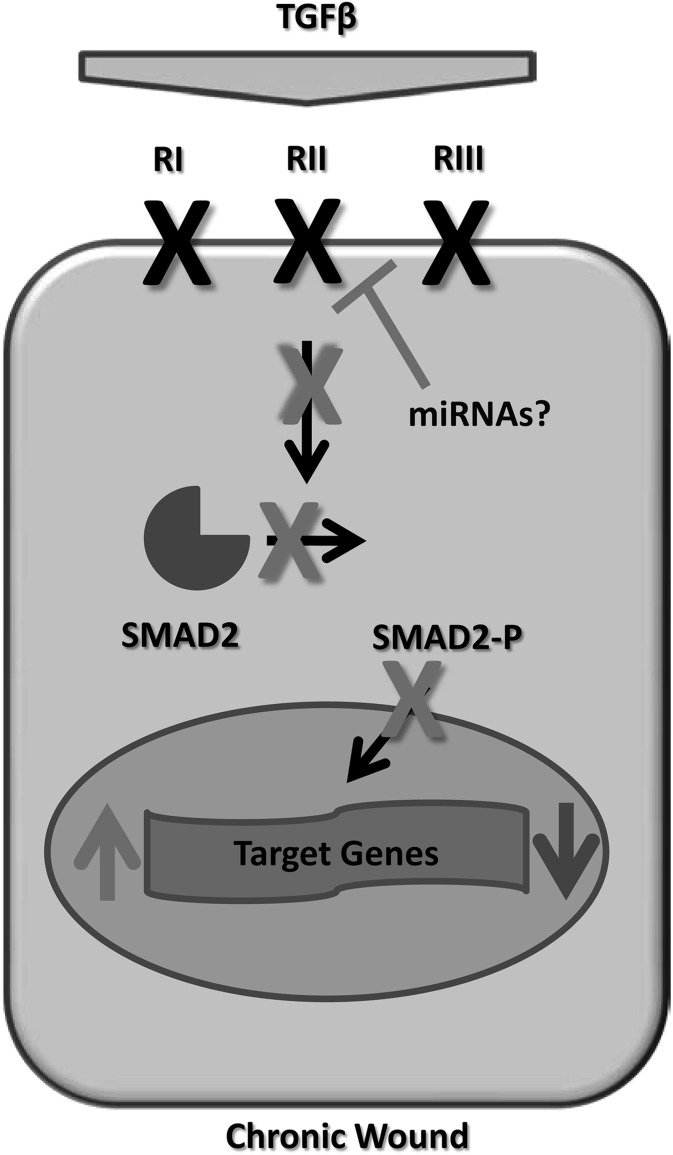
In contrast to normal wound-healing processes that are characterized by activation of the TGFβ pathway,48 multiple studies have demonstrated attenuation of TGFβ signaling in the epidermis of non-healing chronic wounds.30,48–50 We have recently shown that inhibition of TGFβ signaling in the keratinocytes of nonhealing venous ulcers (VUs) occurs at many levels (Fig. 2), including the following: decreased expression of all three TGFβ receptors, deregulation of TGFβ target genes, and loss of pSmad2.30 In addition, we also observed a decrease in the intrinsic inhibitor of TGFβ signaling, Smad7.30 Another important study analyzed the expression of TGFβ receptors on biopsies of nonhealing and healing VUs, and concluded that the absence of TGFβRII contributes to the chronicity of nonhealing ulcers, while the presence of TGFβRII correlated with positive healing outcomes.50 In rats and humans with impaired wound healing due to diabetes, TGFβ levels in wound fluid were diminished, and the normal elevation of TGFβ1 found during acute wound healing was absent.51,52 In addition, in vitro studies have revealed that VU-derived fibroblasts have reduced levels of the TGFβRII.49 Although the mechanism of TGFβ pathway suppression in chronic wounds remains to be fully elucidated, recent studies identified the potential role of post-transcriptional regulators, microRNAs (miRNAs), in the suppression of growth factor signaling in chronic wounds.53,54 Among other miRNA molecules, miRNA-20a was found to be over-expressed in nonhealing VUs,55 and TGFβRII has been recently confirmed as a target for this miRNA in human keratinocytes (Fig. 2).56 These data suggest that the observed induction of miRNA-20a can be responsible for suppression of TGFβ signaling in chronic wounds; however, further studies are needed to confirm the role of this miRNA in wound healing. Although growth factors including TGFβ used to be considered therapeutic modality for wound-healing disorders, small RNA therapeutics for modulation of miRNAs aberrantly expressed in chronic wounds may represent a novel promising therapeutic avenue.
Figure 2.
Attenuation of TGFβ signaling in chronic wounds. Cartoon summarizes attenuation of signaling cascade in chronic wounds. Decreased levels of TGFβ, down-regulation of receptors, and subsequent loss of pSmad2 resulted in deregulation of TGFβ target genes in nonhealing ulcers. Induction of specific miRNAs may be responsible for suppression of TGFβ receptors in VUs.
The reported suppression of TGFβ signaling in the nonhealing edges of chronic wound epidermis can result in multiple consequences. It is well established that keratinocytes at the nonhealing edges of chronic wounds do not properly execute either activation or differentiation pathways, resulting in a thick, hyperproliferative, hyper- and para-keratotic epidermis,1 partly due to c-myc overexpression.45 Since TGFβ activity involves suppression of growth-promoting transcription factors, especially c-myc,9 the diminished TGFβ signaling in VUs and diabetic foot ulcers may play a role in loss of cytostatic control and observed c-myc induction in hyperproliferative epidermis of the non-healing wound. The lack of TGFβ signaling in chronic wounds could also have multiple consequences and even lead to the increased inducible nitric oxide synthase (iNOS) activity and greater NO synthesis,30 as TGFβ1 has been demonstrated to down-regulate iNOS activity in epithelial cells and macrophages.57 Although NO can stimulate keratinocyte migration and angiogenesis, excessive amounts may have an inhibitory effect on these important, wound-healing processes.58 Functional loss of the TGFβ/Smad signaling cascade in the epidermis of chronic wounds clearly contributes to a nonhealing phenotype and also implies an explanation for the limited ability of the exogenous applications of TGFβ to accelerate wound healing in patients with recurrent ulcers.59
TGFβ pathway in tissue regeneration
Activation of TGFβ signaling can be observed not only in acute wound healing but also in tissue regeneration. Some of the first studies that documented mammalian regeneration involved work with Murphy Roths Large (MRL) mice, which have the unique capacity for complete wound closure after through-and-through ear hole punches.60,61 The healed tissue maintained normal tissue architecture, thus mimicking amphibian regeneration as opposed to the expected human scar tissue formation.60,61 Expansion of work with MRL mice showed alterations in the Smad signaling pathway, which might contribute to the observed regenerative phenotype by reduction of the pro-inflammatory responses and overall enhanced TGFβ signaling.62 A more recent study by Liu J et al.63 further explored the underlying molecular mechanisms of regeneration in mammals by inducing mutations in mice and screening for regenerative wound healing phenotypes in the same ear hole punch model. Prospective genetic screens of obtained regenerative phenotypes (Fig. 3) identified a single point mutation in a TGFβR1.63 This mutation resulted in enhanced TGFβ signaling and a super-healer phenotype that epithelialized and healed faster with newly generated tissue rather than fibrosis.63 In addition, embryonic fibroblasts from these mice had increased expression of a subset of TGFβ target genes, confirming that the mutation, indeed, caused activation of the receptor and the TGFβ signaling pathway.63 In contrast to these results, abrogation of TGFβ1 signaling in TGFβ1/Rag2 double knockout mice resulted in improved closure of ear holes in comparison to wild-type or Rag2−/− animals.64 These double mutants can overcome the lethal inflammatory response in TGFβ1-null mice due to complete lack of mature B and T lymphocytes caused by Rag2 abrogation. Interestingly, even though improved wound closure was observed in TGFβ1−/− Rag2−/− mice, these ear hole wounds still remained inflamed and not fully closed.64 These findings again highlight the variability of the in vivo data resulting from the broad spectrum of TGFβ1 functions in wound healing, regeneration, and inflammation.39
Figure 3.
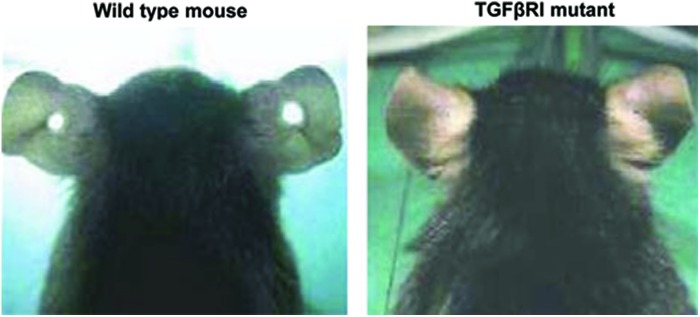
Accelerated and regenerative healing in super-healer TGFβRI mutant mouse. A 2-mm through-and-through hole was punched in the ears of normal mice and super healer mutant mice to screen for acceleration of wound healing. Incomplete ear hole closing was observed in normal mice (left), and complete ear hole closing occurred in the super healer TGFβRI mutant (right) after 5 weeks. Reprinted by permission from Liu et al. 63 To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Another recently described wild-type model of mammalian regeneration is the African spiny mice, Acomys, with the extraordinary ability to regenerate skin along with complete wound closure of the through-and-through ear hole punch.65 Although the mechanism of regeneration in Acomys remains to be elucidated, the possibility of a TGFβ role cannot be excluded.
Together, these findings unveil the possibility of mammals having a better regenerative capability than previously expected, thus motivating the research community to clarify the molecular pathways involved.65 Even though these super-healing phenotypes have been linked to TGFβ signaling,62,63,65 further studies are warranted to elucidate the specific roles of TGFβ and other molecules and pathways in these fascinating tissue-regeneration processes.
TAKE-HOME MESSAGES.
• TGFβ is a growth control cytokine that is involved in maintaining skin homeostasis by suppressing keratinocyte proliferation.
• TGFβ signaling regulates keratinocyte migration and proliferation during epithelialization.
• TGFβ pathway is attenuated in chronic wounds, leading to loss-of-tissue homeostasis and inability of keratinocytes to migrate and close a wound.
• Suppression of TGFβ signaling cascade in chronic wounds is an underlying factor for failure of topical TGFβ treatments in clinical trials.
• The super-healer mouse with a point mutation in TGFβRI exhibited enhanced TGFβ signaling cascade and healed through-and-through ear wounds with complete tissue regeneration.
• A better understanding of TGFβ signaling may provide new insights not only into the normal epithelialization process, but also into tissue regeneration.
Abbreviations and Acronyms
- α3β1
alpha 3 beta 1 integrin
- AR
androgen receptor
- Fst
follistatin
- iNOS
inducible nitric oxide synthase
- K
keratin
- MAP
Mitogen-activated Protein
- miRNA
microRNA
- MRL
Murphy Roths large (mice)
- pSmad
phosphorylated Smad
- TGFβ
transforming growth factor β
- TGFβR
transforming growth factor β receptor
- TPA
12-tetradecanoyl-phorbol-13-acetate
- VU
venous ulcers
Acknowledgments
The authors would like to give special thanks to Drs. Marjana Tomic-Canic and Olivera Stojadinovic and the members of their labs for their continuous support.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the author(s) listed.
About the Authors
Horacio Ramirez, BS, is a Graduate student in the Human Genomics and Genetics program at the University of Miami Miller School of Medicine. He received his undergraduate education in Argentina and graduated with a major in Biotechnology from “Universidad Nacional del Litoral.” He is currently working on elucidating the roles of miRNAs in the pathogenesis of chronic wounds. Shailee Patel, BS, is currently a medical student research fellow in the Department of Dermatology and Cutaneous Surgery at the University of Miami Miller School of Medicine. Irena Pastar, PhD, holds faculty appointment as a Research Assistant Professor at the Department of Dermatology and Cutaneous Surgery, University of Miami Miller School of Medicine. Dr. Pastar's research focuses on molecular mechanisms of epithelialization in acute and chronic wounds. Her laboratory also investigates host response mechanisms to bacterial skin and wound infections.
References
- 1.Barrientos S, Stojadinovic O, Golinko MS, Brem H, and Tomic-Canic M: Growth factors and cytokines in wound healing. Wound Repair Regen 2008; 16:585. [DOI] [PubMed] [Google Scholar]
- 2.Cho HR, Hong SB, Kim YI, Lee JW, and Kim NI: Differential expression of TGF-beta isoforms during differentiation of HaCaT human keratinocyte cells: implication for the separate role in epidermal differentiation. J Korean Med Sci 2004; 19:853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annes JP, Munger JS, and Rifkin DB: Making sense of latent TGFbeta activation. J Cell Sci 2003; 116 (Pt 2):217. [DOI] [PubMed] [Google Scholar]
- 4.Worthington JJ, Klementowicz JE, and Travis MA: TGFbeta: a sleeping giant awoken by integrins. Trends Biochem Sci 2011; 36:47. [DOI] [PubMed] [Google Scholar]
- 5.Shi Y. and Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003; 113: 685. [DOI] [PubMed] [Google Scholar]
- 6.Zhang YE: Non-Smad pathways in TGF-beta signaling. Cell Res 2009; 19:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo F, Hutchenreuther J, Carter DE, and Leask A: TAK1 is required for dermal wound healing and homeostasis. J Invest Dermatol 2013; 133:1646. [DOI] [PubMed] [Google Scholar]
- 8.Siegel PM. and Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer 2003; 3: 807. [DOI] [PubMed] [Google Scholar]
- 9.Pietenpol JA, Holt JT, Stein RW, and Moses HL: Transforming growth factor beta 1 suppression of c-myc gene transcription: role in inhibition of keratinocyte proliferation. Proc Natl Acad Sci USA 1990; 87:3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashcroft GS, Mills SJ, Flanders KC, et al. : Role of Smad3 in the hormonal modulation of in vivo wound healing responses. Wound Repair Regen 2003; 11:468. [DOI] [PubMed] [Google Scholar]
- 11.Guasch G, Schober M, Pasolli HA, Conn EB, Polak L, and Fuchs E: Loss of TGFbeta signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell 2007; 12:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang XJ, Greenhalgh DA, Bickenbach JR, et al. : Expression of a dominant-negative type II transforming growth factor beta (TGF-beta) receptor in the epidermis of transgenic mice blocks TGF-beta-mediated growth inhibition. Proc Natl Acad Sci USA 1997; 94:2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sellheyer K, Bickenbach JR, Rothnagel JA, et al. : Inhibition of skin development by overexpression of transforming growth factor beta 1 in the epidermis of transgenic mice. Proc Natl Acad Sci USA 1993; 90:5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fowlis DJ, Cui W, Johnson SA, Balmain A, and Akhurst RJ: Altered epidermal cell growth control in vivo by inducible expression of transforming growth factor beta 1 in the skin of transgenic mice. Cell Growth Differ 1996; 7:679. [PubMed] [Google Scholar]
- 15.Cui W, Fowlis DJ, Cousins FM, et al. : Concerted action of TGF-beta 1 and its type II receptor in control of epidermal homeostasis in transgenic mice. Genes Dev 1995; 9:945. [DOI] [PubMed] [Google Scholar]
- 16.Ho Y, Sarkar P, Mi Q, et al. : overexpression of smad2 reveals its concerted action with smad4 in regulating TGF-beta-mediated epidermal homeostasis. Dev Biol 2001; 236:18. [DOI] [PubMed] [Google Scholar]
- 17.Buschke S, Stark HJ, Cerezo A, et al. : A decisive function of transforming growth factor-beta/Smad signaling in tissue morphogenesis and differentiation of human HaCaT keratinocytes. Mol Biol Cell 2011; 22:782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang B, Yoo N, Vu M, et al. : Transforming growth factor-beta can suppress tumorigenesis through effects on the putative cancer stem or early progenitor cell and committed progeny in a breast cancer xenograft model. Cancer Res 2007; 67:8643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owens P, Han G, Li AG, and Wang XJ: The role of Smads in skin development. J Invest Dermatol 2008; 128: 783. [DOI] [PubMed] [Google Scholar]
- 20.Cox DA, Kunz S, Cerletti N, McMaster GK, and Burk RR: Wound healing in aged animals—effects of locally applied transforming growth factor beta 2 in different model systems. EXS 1992; 61:287. [DOI] [PubMed] [Google Scholar]
- 21.Tyrone JW, Marcus JR, Bonomo SR, Mogford JE, Xia Y, and Mustoe TA. Transforming growth factor beta3 promotes fascial wound healing in a new animal model. Arch Surg 2000; 135: 1154. [DOI] [PubMed] [Google Scholar]
- 22.Jeong HW. and Kim IS. TGF-beta1 enhances betaig-h3-mediated keratinocyte cell migration through the alpha3beta1 integrin and PI3K. J Cell Biochem 2004; 92: 770. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds LE, Conti FJ, Silva R, et al. : alpha3beta1 integrin-controlled Smad7 regulates reepithelialization during wound healing in mice. J Clin Invest 2008; 118:965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han G, Li F, Ten Dijke P, and Wang XJ: Temporal smad7 transgene induction in mouse epidermis accelerates skin wound healing. Am J Pathol 2011; 179:1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Annes JP, Chen Y, Munger JS, and Rifkin DB. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol 2004; 165:723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie Y, Gao K, Hakkinen L, and Larjava HS. Mice lacking beta6 integrin in skin show accelerated wound repair in dexamethasone impaired wound healing model. Wound Repair Regen 2009; 17:326. [DOI] [PubMed] [Google Scholar]
- 27.Jacobsen JN, Steffensen B, Hakkinen L, Krogfelt KA, and Larjava HS. Skin wound healing in diabetic beta6 integrin-deficient mice. APMIS 2010; 118:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gold LI, Sung JJ, Siebert JW, and Longaker MT: Type I (RI) and type II (RII) receptors for transforming growth factor-beta isoforms are expressed subsequent to transforming growth factor-beta ligands during excisional wound repair. Am J Pathol 1997; 150:209. [PMC free article] [PubMed] [Google Scholar]
- 29.Kane CJ, Hebda PA, Mansbridge JN, and Hanawalt PC. Direct evidence for spatial and temporal regulation of transforming growth factor beta 1 expression during cutaneous wound healing. J Cell Physiol 1991; 148:157. [DOI] [PubMed] [Google Scholar]
- 30.Pastar I, Stojadinovic O, Krzyzanowska A, et al. : Attenuation of the transforming growth factor beta-signaling pathway in chronic venous ulcers. Mol Med 2010; 16:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puolakkainen PA, Reed MJ, Gombotz WR, Twardzik DR, Abrass IB, and Sage HE. Acceleration of wound healing in aged rats by topical application of transforming growth factor-beta(1). Wound Repair Regen 1995; 3:330. [DOI] [PubMed] [Google Scholar]
- 32.Mustoe TA, Pierce GF, Thomason A, Gramates P, Sporn MB, and Deuel TF: Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science 1987; 237:1333. [DOI] [PubMed] [Google Scholar]
- 33.Lu L, Saulis AS, Liu WR, et al. : The temporal effects of anti-TGF-beta1, 2, and 3 monoclonal antibody on wound healing and hypertrophic scar formation. J Am Coll Surg 2005; 201:391. [DOI] [PubMed] [Google Scholar]
- 34.Koch RM, Roche NS, Parks WT, Ashcroft GS, Letterio JJ, and Roberts AB. Incisional wound healing in transforming growth factor-beta1 null mice. Wound Repair Regen 2000; 8:179. [DOI] [PubMed] [Google Scholar]
- 35.Kulkarni AB, Huh CG, Becker D, et al. : Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A 1993; 90:770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crowe MJ, Doetschman T, and Greenhalgh DG: Delayed wound healing in immunodeficient TGF-beta 1 knockout mice. J Invest Dermatol 2000; 115:3. [DOI] [PubMed] [Google Scholar]
- 37.Tredget EB, Demare J, Chandran G, Tredget EE, Yang L, and Ghahary A: Transforming growth factor-beta and its effect on reepithelialization of partial-thickness ear wounds in transgenic mice. Wound Repair Regen 2005; 13:61. [DOI] [PubMed] [Google Scholar]
- 38.Ashcroft GS, Yang X, Glick AB, et al. : Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol 1999; 1:260. [DOI] [PubMed] [Google Scholar]
- 39.Wang XJ, Han G, Owens P, Siddiqui Y, and Li AG: Role of TGF beta-mediated inflammation in cutaneous wound healing. J Investig Dermatol Symp Proc 2006; 11:112. [DOI] [PubMed] [Google Scholar]
- 40.Owens P, Engelking E, Han G, Haeger SM, and Wang XJ: Epidermal Smad4 deletion results in aberrant wound healing. Am J Pathol 2010; 176:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amendt C, Mann A, Schirmacher P, and Blessing M: Resistance of keratinocytes to TGFbeta-mediated growth restriction and apoptosis induction accelerates re-epithelialization in skin wounds. J Cell Sci 2002; 115 (Pt 10):2189. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z, Wang Y, Farhangfar F, Zimmer M, and Zhang Y: Enhanced keratinocyte proliferation and migration in co-culture with fibroblasts. PloS One 2012; 7:e40951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toraldo G, Bhasin S, Bakhit M, et al. : Topical androgen antagonism promotes cutaneous wound healing without systemic androgen deprivation by blocking beta-catenin nuclear translocation and cross-talk with TGF-beta signaling in keratinocytes. Wound Repair Regen 2012; 20:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chipuk JE, Cornelius SC, Pultz NJ, et al. : The androgen receptor represses transforming growth factor-beta signaling through interaction with smad3. J Biol Chem 2002; 277:1240. [DOI] [PubMed] [Google Scholar]
- 45.Stojadinovic O, Brem H, Vouthounis C, et al. : Molecular pathogenesis of chronic wounds: the role of beta-catenin and c-myc in the inhibition of epithelialization and wound healing. Am J Pathol 2005; 167:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayes SA, Zarnegar M, Sharma M, et al. : SMAD3 represses androgen receptor-mediated transcription. Cancer Res 2001; 61:2112. [PubMed] [Google Scholar]
- 47.Pierce GF, Mustoe TA, Lingelbach J, Masakowski VR, Gramates P, and Deuel TF: Transforming growth factor beta reverses the glucocorticoid-induced wound-healing deficit in rats: possible regulation in macrophages by platelet-derived growth factor. Proc Natl Acad Sci USA 1989; 86:2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmid P, Cox D, Bilbe G, et al. : TGF-beta s and TGF-beta type II receptor in human epidermis: differential expression in acute and chronic skin wounds. J Pathol 1993; 171:191. [DOI] [PubMed] [Google Scholar]
- 49.Kim BC, Kim HT, Park SH, et al. : Fibroblasts from chronic wounds show altered TGF-beta-signaling and decreased TGF-beta Type II receptor expression. J Cell Physiol 2003; 195:331. [DOI] [PubMed] [Google Scholar]
- 50.Cowin AJ, Hatzirodos N, Holding CA, et al. : Effect of healing on the expression of transforming growth factor beta(s) and their receptors in chronic venous leg ulcers. J Invest Dermatol 2001; 117:1282. [DOI] [PubMed] [Google Scholar]
- 51.Bitar MS. and Labbad ZN. Transforming growth factor-beta and insulin-like growth factor-I in relation to diabetes-induced impairment of wound healing. J Surg Res 1996; 61:113. [DOI] [PubMed] [Google Scholar]
- 52.Jude EB, Blakytny R, Bulmer J, Boulton AJ, and Ferguson MW: Transforming growth factor-beta 1, 2, 3 and receptor type I and II in diabetic foot ulcers. Diabet Med 2002; 19:440. [DOI] [PubMed] [Google Scholar]
- 53.Pastar I, Ramirez H, Stojadinovic O, Brem H, Kirsner RS, and Tomic-Canic M: Micro-RNAs: new regulators of wound healing. Surg Tech Int 2011; XXI:51. [PubMed] [Google Scholar]
- 54.Banerjee J, Chan YC, and Sen CK: MicroRNAs in skin and wound healing. Physiol Genomics 2011; 43:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pastar I, Khan AA, Stojadinovic O, et al. : Induction of specific MicroRNAs inhibits cutaneous wound healing. J Biol Chem 2012; 287:29324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schultz N, Marenstein DR, De Angelis DA, et al. : Off-target effects dominate a large-scale RNAi screen for modulators of the TGF-beta pathway and reveal microRNA regulation of TGFBR2. Silence 2011; 2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vodovotz Y, Letterio JJ, Geiser AG, Chesler L, Roberts AB, and Sparrow J. Control of nitric oxide production by endogenous TGF-beta1 and systemic nitric oxide in retinal pigment epithelial cells and peritoneal macrophages. J Leukoc Biol 1996; 60:261. [DOI] [PubMed] [Google Scholar]
- 58.Jude EB, Tentolouris N, Appleton I, Anderson S, and Boulton AJ. Role of neuropathy and plasma nitric oxide in recurrent neuropathic and neuroischemic diabetic foot ulcers. Wound Repair Regen 2001; 9:353. [DOI] [PubMed] [Google Scholar]
- 59.Robson MC, Phillip LG, Cooper DM, et al. : Safety and effect of transforming growth factor-beta(2) for treatment of venous stasis ulcers. Wound Repair Regen 1995; 3:157. [DOI] [PubMed] [Google Scholar]
- 60.Heber-Katz E, Leferovich JM, Bedelbaeva K, and Gourevitch D: Spallanzani's mouse: a model of restoration and regeneration. Curr Top Microbiol Immunol 2004; 280:165. [DOI] [PubMed] [Google Scholar]
- 61.Clark LD, Clark RK, and Heber-Katz E: A new murine model for mammalian wound repair and regeneration. Clin Immunol Immunopathol 1998; 88:35. [DOI] [PubMed] [Google Scholar]
- 62.Tolba RH, Schildberg FA, Decker D, et al. : Mechanisms of improved wound healing in Murphy Roths Large (MRL) mice after skin transplantation. Wound Repair Regen 2010; 18:662. [DOI] [PubMed] [Google Scholar]
- 63.Liu J, Johnson K, Li J, et al. : Regenerative phenotype in mice with a point mutation in transforming growth factor beta type I receptor (TGFBR1). Proc Natl Acad Sci USA 2011; 108:14560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arthur LM, Demarest RM, Clark L, et al. : Epimorphic regeneration in mice is p53-independent. Cell Cycle 2010; 9:3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seifert AW, Kiama SG, Seifert MG, Goheen JR, Palmer TM, and Maden M: Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature 2012; 489:561. [DOI] [PMC free article] [PubMed] [Google Scholar]