Abstract
Aim:
To study the effects of testosterone on streptozotocin (STZ)-induced memory impairment in male rats.
Methods:
Adult male Wistar rats were intracerebroventricularly (icv) infused with STZ (750 μg) on d 1 and d 3, and a passive avoidance task was assessed 2 weeks after the first injection of STZ. Castration surgery was performed in another group of rats, and the passive avoidance task was assessed 4 weeks after the operation. Testosterone (1 mg·kg−1·d−1, sc), the androgen receptor antagonist flutamide (10 mg·kg−1·d−1, ip), the estrogen receptor antagonist tamoxifen (1 mg·kg−1·d−1, ip) or the aromatase inhibitor letrozole (4 mg·kg−1·d−1, ip) were administered for 6 d after the first injection of STZ.
Results:
STZ administration and castration markedly decreased both STL1 (the short memory) and STL2 (the long memory) in passive avoidance tests. Testosterone replacement almost restored the STL1 and STL2 in castrated rats, and significantly prolonged the STL1 and STL2 in STZ-treated rats. Administration of flutamide, letrozole or tamoxifen significantly impaired the memory in intact rats, and significantly attenuated the testosterone replacement in improving STZ- and castration-induced memory impairment.
Conclusion:
Testosterone administration ameliorates STZ- and castration-induced memory impairment in male Wistar rats.
Keywords: learning and memory, streptozotocin, testosterone, flutamide, letrozole, tamoxifen, Alzheimer's disease
Introduction
Alzheimer's disease is a common form of dementia in the elderly. Androgens, present in most bodily tissues, especially in the brain, perform specialized functions1,2. In addition, accumulating evidence indicates that age-related androgen loss can contribute to neurodegenerative disorders, including Alzheimer's disease3,4.
Alzheimer's disease is caused by the deposition of Aβ protein as well as the dystrophy of nerve cells in the terminal areas of the prefrontal cortex, hippocampus and other cerebral areas. Interestingly, there is a unique relationship between steroidal hormones and neurodegenerative diseases in that replacement with estrogen causes a decline in Alzheimer's disease progression5,6. The protective effects of estrogen due to the reduction in Aβ protein production5,6.
Some studies have shown that testosterone also affects the production and regulation of Aβ protein levels both in vitro7,8 and in vivo9. It has also been shown that, castrated rats are associated with a remarkable increase in Aβ levels in plasma10. More recently, it has been reported that low testosterone levels are associated with increased plasma Aβ -40 levels in elderly men with memory loss9. This protective effect has been shown to occur in the presence of an estrogen receptor blocker in experimental animals. Alzheimer's disease is also characterized by hyperphosphorylation of the intra-neural tau protein, which causes the development of neural tangles. Testosterone is capable of preventing the hyperphosphorylation of tau protein10,11. A study on age-matched controls and patients with Alzheimer's disease demonstrated that the reduction in neurosteroids in most areas of the body, especially in cerebral neurons, was the cause of the disease12. In addition, researchers have found synergistic effects among steroid sex hormones, such as testosterone and estrogen in relation to the level of Aβ in the rat brain13.
There are two types of internal androgens in the body: testosterone and its metabolite, dihydrotestosterone14, each of which performs a variety of functions in the central nervous system15.
Aromatase is an enzyme that converts androgen into estrogen and regulates the cerebral estrogen level in the brain. The activity of aromatase was first detected in the limbic system of the brain of the human fetus as well as in the rat hippocampus. Recent findings have revealed new unexpected roles for brain aromatase, including the regulation of synaptic activity, synaptic plasticity, neurogenesis and the response of neural tissue to injury, mood and cognition15. The brain aromatase enzyme was detected in the fetal human limbic system and in the rat hypothalamus16. A number of subsequent studies have shown the activity and distribution in the aromatase enzyme of the central system in some species of vertebrates. Thus, the activity of androgens in the brain's central system is mediated by both an indirect mechanism (ie, aromatization of testosterone and its conversion into estrogen) and a direct mechanism (ie, through androgen receptors)17. Anti-androgens were developed to competitively bind to androgen receptors to and interfere with androgen receptors association and action18. There are two general types of anti-androgen: steroidal and non steroidal19. The non steroidal anti-androgens such as flutamide are generally considered pure anti-androgens, as they do not exhibit androgen receptor agonist activity20.
In this study, the effects of neuron protection were investigated using an androgen receptor antagonist (ie, Flutamide), estrogen receptor antagonist (ie, Tamoxifen) and aromatase inhibitor (ie, Letrozole) with the synergistic function of the androgen and estrogen receptors in neurodegenerative diseases, such as Alzheimer's disease.
Materials and methods
Chemicals
All chemicals were obtained from Sigma Chemical Co (USA) except for testosterone enanthate, which was purchased from Daroo Pakhsh (Tehran, Iran). Solutions were prepared freshly on the day of experimentation.
Tamoxifen and STZ were dissolved in sterile 5% dimethyl sulphoxide (DMSO), and artificial cerebrospinal fluid (ACSF: 120 mmol/L NaCl, 3 mmol/L KCl, 1.15 mmol/L CaCl2, 0.8 mmol/L MgCl2, 27 mmol/L NaHCO3, and 0.33 mmol/L NaH2PO4 adjusted to pH 7.2). Testosterone, flutamide and letrozole were dissolved in sterile Castor oil.
Animals
The study was conducted in male Wistar rats weighting 220–250 g. The animals were housed in standard polypropylene cages, four animals per cage, and under a 12 h light/12 h dark program at an ambient temperature of 25±2 °C with free access to food and water. The experiments were carried out under the ethical guidelines of the Tabriz University of Medical Sciences for the care and use of laboratory animals.
Castration
Animals were anesthetized by intraperitoneal (ip) injection of ketamine (60 mg/kg) and xylazine (6 mg/kg). The ventral scrotum was shaved and scrubbed with betadine (Behvazan Co, Rasht, Iran). Then, a 1.5-cm transverse incision was made at the midline scrotum, the testes were exteriorized through the incision, the tubules were tied with 0.4 silk sutures, and finally, the testes and testicular fat were removed. The sham surgery consisted of exposing the gonads without removing them. The behavioral studies were performed 4 weeks after castration.
Intracerebroventricular (icv) injection of STZ
The animals were anesthetized with ketamine (60 mg/kg, ip) and xylazine (6 mg/kg, ip), and then, the animals were mounted in a stereotaxic frame in the flat skull position. The scalp was shaved and swabbed with iodine and a small central incision was made to expose the skull. Then, two bilateral burr holes were drilled through the skull using coordinates according to the stereotaxic atlas19: anteroposterior from bregma (AP)=-0.8 mm, mediolateral from the midline (ML)=±1.6 mm and dorsoventral from the skull (DV)=3.4 mm. STZ (750 μg/10 μL ACSF/Rat, at d 1 and 3) was infused bilaterally into the cerebral ventricles using a Hamilton syringe and an infusion pump at a flow rate of 0.2 μL/min19. Behavioral investigations were carried out 2 weeks after the first injection of STZ.
Passive avoidance test
The apparatus (Azma Co, Tabriz, Iran) consisted of an illuminated chamber connected to a dark chamber by a guillotine door. Electric shocks were delivered to the grid floor by a stimulator. On the first and second days of testing, each rat was placed on the apparatus and left for 5 min to habituate to the apparatus. On the third day, an acquisition trial was carried out. The rats were individually placed in the illuminated chamber. After the habituation period (2 min), the guillotine door was opened and after the rat entered the dark chamber, the door was closed and an inescapable scrambled electric shock (1 mA, 50 Hz, 3 s once) was delivered. In this trial, the initial latency (IL) of entrance into the dark chamber was recorded and the rats with IL greater than 60 s were excluded from the study. Twenty-four hours later, each rat was placed of the illuminated chamber for a retention trial. The interval between the placement in the illuminated chamber and the entry into the dark chamber was measured as step-through latency (STL1, cut of time 900 s) for short memory. This test was conducted 3 weeks post-surgery, and each rat was tested only once for measuring STL2 for long memory.
Statistical analysis
Instate software was used to prepare the descriptive statistics and to compare the differences between the means of the data sets. All results were expressed as the mean±SEM and were analyzed by performing one-way ANOVA. The level of statistical significance was set at P<0.05. If statistical significance was observed at P<0.05, a post-hoc Tukey test was employed to identify where the differences occurred.
Results
The effect of STZ and castration on memory impairments
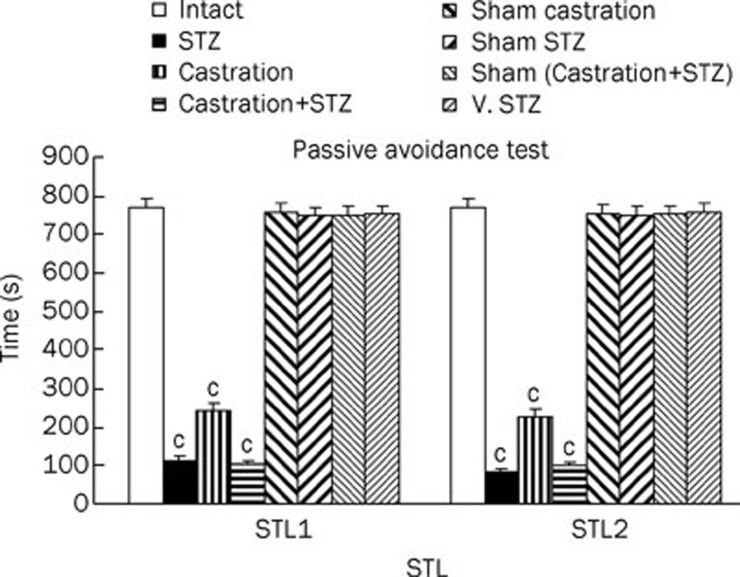
The step through latency (STL) was assessed in intact, STZ (750 μg/10 μL ACSF/Rat, at d 1 and 3), Castrated, Cas+STZ, sham (STZ+Cas) and vehicle-treated STZ-treated rats. As shown in Figure 1, STL1 and STL2 were significantly decreased (P<0.01) compared with the intact, STZ vehicle-treated and sham (STZ+Cas) animals.
Figure 1.
The passive avoidance test STL1 (short memory) and STL2 (long memory) results of intact, STZ (750 μg/rat in 10 μL ACSF at d 1 and 3), castration, castrated of STZ icv injection, sham operated of Cast+STZ and vehicle of STZ-lesion rats. Each bar represent the mean±SEM of STL time (s). n=8 rat for each group. cP<0.01 when compared with intact, sham operated and vehicle of STZ group (STZ=stroptozotocin, Cas=castration, V=vehicle).
The effect of testosterone replacement therapy on memory impairments
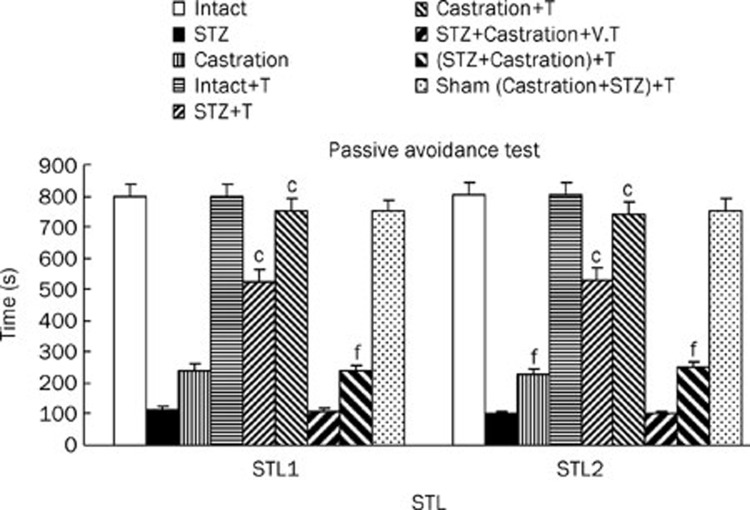
Nine groups of rats were included in this study: 1) Intact, 2) STZ, 3) Castrated, 4) Intact with testosterone (1 mg/kg, sc, for 6 d after the first STZ injection, 5) STZ with testosterone, 6) Castrated with testosterone, 7) Cas+STZ with vehicle, 8) Cas+STZ with testosterone and 9) Sham (Cas+STZ) with testosterone. STL1 and STL2 of the intact with testosterone and Sham (Cas+STZ) with testosterone groups were the same as the non-treated control animals. In the STZ with testosterone-treated and castrated with testosterone-treated rats, STL1 and STL2 were significantly (P<0.01) higher than the STZ-treated and castrated animals without testosterone. Furthermore, STL1 and STL2 were increased significantly (P<0.01) by testosterone replacement therapy in the STZ+Cas rats compared with the STZ+Cas group (Figure 2).
Figure 2.
The passive avoidance test STL1 (short memory) and STL2 (long memory) results of intact, STZ, Cas, STZ+Cas, sham operated with replacement of the testosterone-treated in lesion rats. Each bar represent the mean±SEM of STL time(s). n=8 rat for each group. cP<0.01 when compared with intact rats. Also fP<0.01 when compared with STZ or (STZ+Cas) lesion rats (STZ=stroptozotocin group, Cas=castration group, V=vehicle, T=testosterone).
The effect of letrozole, flutamide and tamoxifen on memory impairments
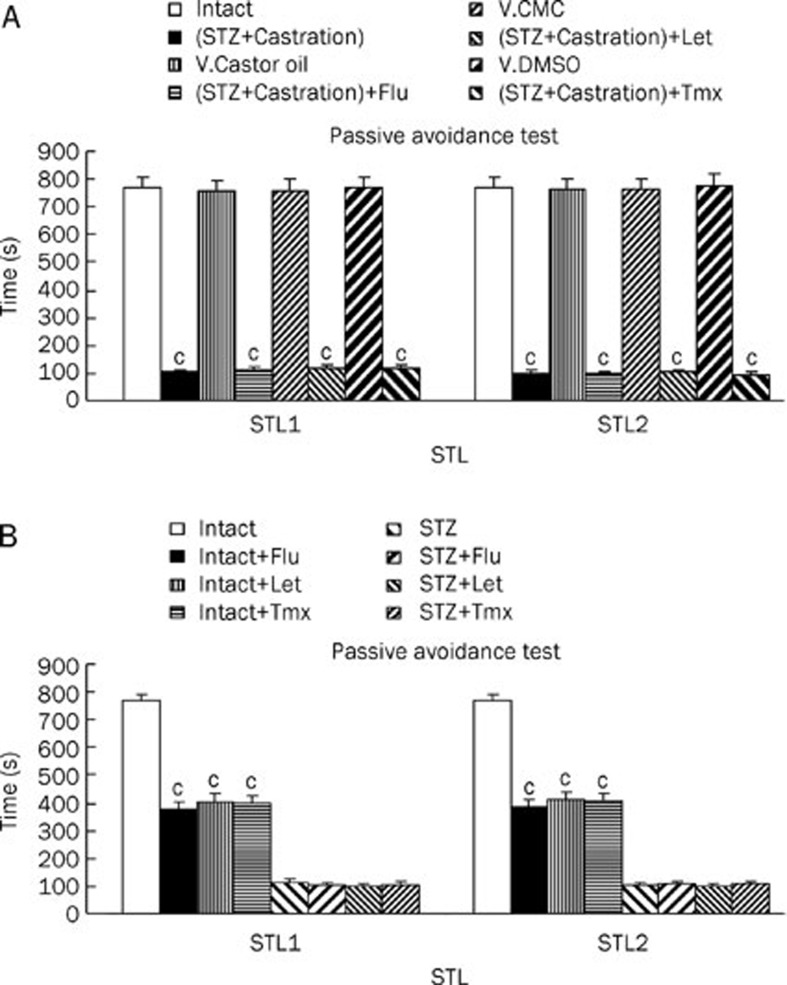
The effect of letrozole, flutamide and tamoxifen on short (STL1) and long (STL2) term memory was investigated using vehicle-treated (castor oil, carboxymethyl cellulose and 5% dimethyl sulfoxide) control rats. In these groups, step through latency was assessed using a passive avoidance test. The STL1 and STL2 of the vehicle-treated control rats (castor oil, carboxymethyl cellulose rats and 5% dimethyl sulfoxide), were the same as the intact animals. Additionally, the STL1 and STL2 of the castrated with STZ rats were significantly decreased (P<0.01) by flutamide, letrozole and tamoxifen compared with the intact rats treated with a vehicle control. In addition STLs were assessed in the intact and STZ rats treated with flutamide, letrozole and tamoxifen (Figure 3B). The STL1 and STL2 of the intact rats treated with flutamide, letrozole and tamoxifen were significantly decreased (P<0.01) compared with the intact animals. Lastly, the STL1 and STL2 of the STZ group treated with flutamide, letrozole and tamoxifen were similar to the STZ rats.
Figure 3.
The passive avoidance test STL1 (short memory) and STL2 (long memory) results of flutamide (10 mg/kg, ip, for 6 d), letrozole (4 mg/kg, ip, for 6 d) and tamoxifen (1 mg/kg, ip, for 6 d) with V. of castor oil, V. of CMC and V. of DMSO (A), intact and STZ-treated of lesion rats (B). Each bar represent the mean±SEM of STL time (s). n=8 rat for each group. cP<0.01 compared with intact, V. of castor oil, V. of CMC or V. of DMSO rats. Also compared with intact or STZ lesion rats (STZ=stroptozotocin, Cas=castration, V=vehicle, T=testosterone, Flu=flutamide, Let=letrozole, Tmx=tamoxifen, ip=intraperitoneal, CMC=carboximethilcellulose, DMSO=dimethyl solphoxide).
The effect of testosterone replacement and letrozole, flutamide and tamoxifen injection on memory impairment
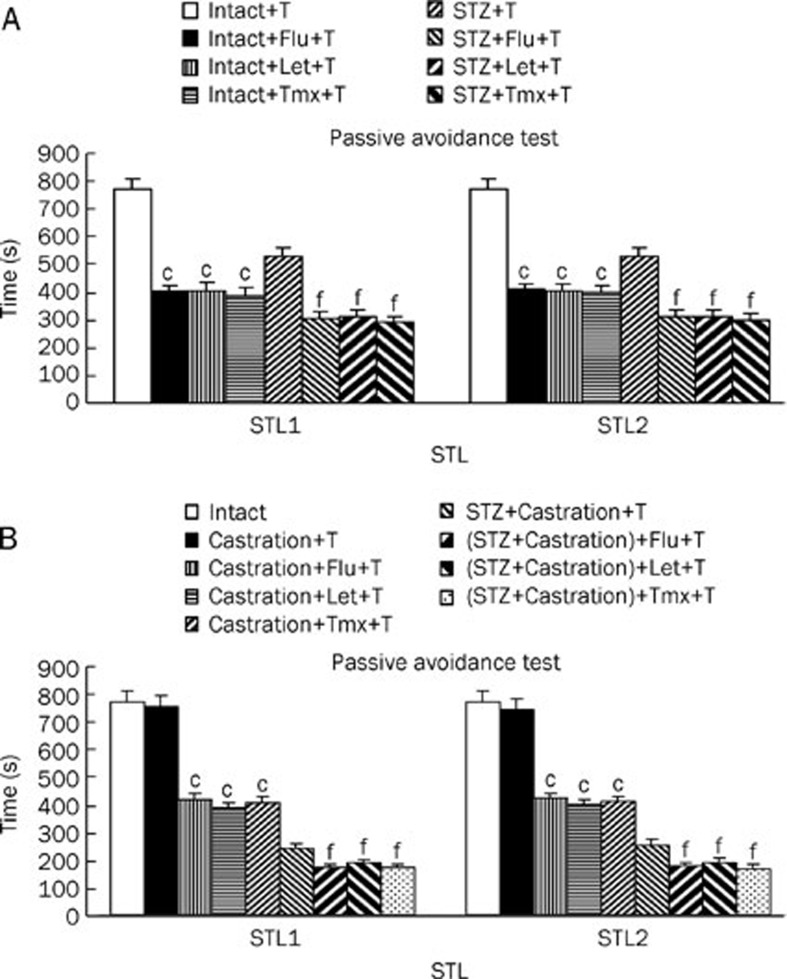
The effect of testosterone replacement in intact rats treated with letrozole, flutamide and tamoxifen on short term (STL1) and long term (STL2) memory were assessed (Figure 4A). The results demonstrated that the STL1 and STL2 of the intact rats treated with Let, Tmx, and Flu were significantly decreased (P<0.01) compared with the intact rats and the intact rats provided with testosterone replacement. Additionally, the STL1 and STL2 of the STZ-treated rats provided with testosterone replacement and injected letrozole, flutamide and tamoxifen were significantly decreased (P<0.01) compared with the same group without letrozole, flutamide and tamoxifen. In addition, STLs were assessed in the castrated and Cas+STZ groups provided with testosterone replacement and after Flu, Let, and Tmx treatment (Figure 4B). The STL1 and STL2 of the castrated rats provided with testosterone replacement (Flu, Let, and Tmx) were significantly decreased (P<0.01) compared with the same group without Flu, Let, and Tmx treatment. In addition, Figure 4B shows that the STL1 and STL2 of the STZ+Cas group provided with testosterone replacement and with Flu, Let, and Tmx treatment were significantly decreased (P<0.01) compared with the same group without Flu, Let, and Tmx treatment.
Figure 4.
The passive avoidance test STL1 (short memory) and STL2 (long memory) results of testosterone replacement-treated with flutamide (10 mg/kg, ip, for 6 d), letrozole (4 mg/kg, ip, for 6 d) and tamoxifen (1 mg/kg, ip, for 6 d) in intact and STZ (A), castrated and (STZ+Cas)- lesion rats (B). Each bar represent the mean±SEM of STL time (s). n=8 rat for each group. cP<0.01 compared with intact or testosterone replacement of castrated rats. Also fP<0.01 compared with testosterone replacement of STZ or STZ+Cas groups. (STZ=stroptozotocin, Cas=castration, T=testosterone, Flu=flutamide, Let=letrozole, Tmx=tamoxifen, ip=intraperitoneal).
Discussion
Alzheimer's disease is a neurodegenerative disease that is caused by the destruction of the neurons in different areas of the cerebral cortex, particularly the cholinergic neurons of the hippocampus and the frontal cortex. Drastic abnormalities have been found to occur in cerebral glucose and energy metabolism in sporadic Alzheimer's disease, pointing to a primary disturbance in neuronal insulin and insulin receptor signal transduction, which contributes to the cause of dementia. Streptozotocin (STZ) is known to inhibit insulin receptor function and oxidative stress. Herein, the acute administration of streptozotocin at a dose of 750 μg/rat/10 μL ACSF on the first and third days induced a model of sporadic Alzheimer's disease. The characterization and validation of the STZ-icv treated rat as a model for sporadic AD has been ongoing for more than 20 years. Important findings regarding the pathophysiology of sporadic Alzheimer's disease have already been provided by means of STZ-icv treated rats, which revealed that the development of an insulin resistant brain state induced by STZ-icv treatment precedes and eventually leads to tau and Aβ pathology21. This study observed significant reduction in STL1 and STL2 in the group receiving STZ compared with the control group (P<0.01). Thus, STZ may contribute to memory and learning disorders in male Wistar rats. The dose of STZ used in this study is safe and has been shown not to cause any change in peripheral blood glucose levels22. In previous studies, the effect of testosterone on rats treated with STZ in relation to androgen or estrogen receptors has not been thoroughly addressed23. Therefore, in the current study, an androgen receptors antagonist (ie, Flutamide with the dose of 10 mg/kg, ip, on d 1–6), estrogen receptors antagonist (ie, Tamoxifen with the dose of 1 mg/kg, ip, on d 1–6) and aromatase inhibitor (ie, Letrozole with the dose of 4 mg/kg, ip, on d 1–6) were employed. The results from the passive avoidance test revealed obvious memory disorders in the 4-week castrated rats. These results support the data from a previous report that tested the effect of testosterone on rat spatial memory24. The results from the present study demonstrated that STL1 (short memory) and STL2 (long memory) were significantly decreased in the control group and the STZ-treated group treated with flutamide, tamoxifen, and letrozole, indicating memory impairment and the subsequent development of AD in these groups of rats.
Testosterone can modulate brain function via aromatization to 17b-estradiol to influence estrogen receptor-mediated gene transcription. In some cases, the effect of testosterone supplementation on verbal memory appears to be due to the aromatization to 17b-estradiol, whereas the effect on spatial memory is dependent of aromatization25,26. In rodents, androgen manipulation modulates spatial working memory wherein animals must use new spatial information that is learned in each trial26.
Similarly, intra-hippocampus injections of estradiol improve rat performance in a Morris water maze test27. Thus, the metabolic role of androgens in memory improvement should not be underestimated.
This study showed that testosterone did not produce any significant effect on the time of STL1 and STL2 in the control group. Consequently, it could be concluded that testosterone, beyond the physiological limit of the body, did not have any effect on improving memory and learning in the non-castrated and control groups. Testosterone did improve the memory and learning of the STZ-treated rats, but not to the same extent as the control group. Another finding of this study was that castor oil, carboxy methyl cellulous (CMC) and dimethyl sulphoxide (DMSO) did not significantly change the STL1 or STL2 of both the control and castrated groups. Therefore, significant changes were only associated with the replacement of letrozole, flutamide, and tamoxifen in rats. Androgen receptor antagonists, such as flutamide, affect Androgen receptors by blocking them. Tamoxifen and Letrozole treatment in the control group was the main reason for the significant changes in STLs (P<0.01), which induced memory and learning disorders. These drugs influence pathways in the CNS that are affected by testosterone, causing learning and memory disorders in rats. In addition, testosterone, through direct and indirect mechanisms, may decrease memory impairment in rats. According to the obtained results, castration impaired memory in the rats treated with STZ, while testosterone replacement reversed memory impairment. Therefore, testosterone may potentially be used to treat learning and memory disorders. Furthermore, based on our study, androgen and estrogen receptors may have synergistic effects; therefore, therapy with testosterone in combination with routine anti-AD drugs may useful in the prevention and treatment of AD disease.
Acknowledgments
We wish to thank the Director of the Drug Applied Research Center and the Research Vice-Chancellor, Tabriz University of Medical Sciences for supporting this study.
References
- Pike CJ, Nguyen TV, Ramsden M, Yao M, Murphy MP, Rosario ER. Androgen cell signaling pathways involved in neuroprotective actions. Horm Behav. 2008;53:693–705. doi: 10.1016/j.yhbeh.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CJ, Rosario ER, Nguyen TV. Androgens, aging, and Alzheimer's disease. Endocrine. 2006;29:233–41. doi: 10.1385/ENDO:29:2:233. [DOI] [PubMed] [Google Scholar]
- Rosario ER, Chang L, Stanczyk FZ, Pike CJ. Age-related testosterone depletion and the development of Alzheimer disease. JAMA. 2004;292:1431–2. doi: 10.1001/jama.292.12.1431-b. [DOI] [PubMed] [Google Scholar]
- Compton J, van Amelsvoort T, Murphy D. Mood, cognition and Alzheimer's disease. Best Pract Res Clin Obstet Gynaecol. 2002;16:357–70. doi: 10.1053/beog.2002.0285. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Toran-Allerand CD, Greengard P, Gandy SE. Estrogen regulates metabolism of Alzheimer amyloid beta precursor protein. J Biol Chem. 1994;269:13065–8. [PubMed] [Google Scholar]
- Levin-Allerhand JA, Lominska CE, Wang J, Smith JD. 17alpha-Estradiol and 17beta-estradiol treatments are effective in lowering cerebral amyloid-beta levels in Abeta PPSWE transgenic mice. J Alzheimers Dis. 2002;4:449–57. doi: 10.3233/jad-2002-4601. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Xu H, Gross RS, Greenfield JP, Hai B, Wang R, et al. Testosterone reduces neuronal secretion of Alzheimer's beta-amyloid peptides. Proc Natl Acad Sci U S A. 2000;97:1202–5. doi: 10.1073/pnas.97.3.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough S, Engert S, Behl C. Testosterone stimulates rapid secretory amyloid precursor protein release from rat hypothalamic cells via the activation of the mitogen activated protein kinase pathway. Neurosci Lett. 2000;296:49–52. doi: 10.1016/s0304-3940(00)01622-0. [DOI] [PubMed] [Google Scholar]
- Gillett MJ, Martins RN, Clarnette RM, Chubb SA, Bruce DG, Yeap BB. Relationship between testosterone, sex hormone binding globulin and plasma amyloid beta peptide 40 in older men with subjective memory loss or dementia. J Alzheimers Dis. 2003;5:267–9. doi: 10.3233/jad-2003-5401. [DOI] [PubMed] [Google Scholar]
- Papasozomenos SC. The heat shock-induced hyperphosphorylation of tau is estrogen-independent and prevented by androgens: implications for Alzheimer's disease. Proc Natl Acad Sci U S A. 1997;94:6612–7. doi: 10.1073/pnas.94.13.6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papasozomenos SCh, Shanavas A. Testosterone prevents the heat shock-induced overactivation of glycogen synthase kinase-3 beta but not of cyclin-dependent kinase 5 and c-Jun NH2-terminal kinase and concomitantly abolishes hyperphosphorylation of tau: implications for Alzheimer's disease. Proc Natl Acad Sci U S A. 2002;99:1140–5. doi: 10.1073/pnas.032646799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weill-Engerer S, David JP, Sazdovitch V, Liere P, Eychenne B, Pianos A, et al. Neurosteroid quantification in human brain regions: comparison between Alzheimer's and no demented patients. J Clin Endocrinol Metab. 2002;87:5138–43. doi: 10.1210/jc.2002-020878. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Davis SR. Minireview: aromatase and the regulation of estrogen biosynthesis-some new perspectives. Endocrinology. 2001;142:4589–94. doi: 10.1210/endo.142.11.8547. [DOI] [PubMed] [Google Scholar]
- Mooradian AD, Morley JE, Korenman SG. Biological actions of androgens. Endocr Rev. 1987;8:1–28. doi: 10.1210/edrv-8-1-1. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci. 2004;24:495–9. doi: 10.1523/JNEUROSCI.4516-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Petro Z. Aromatization of androstenedione by the anterior hypothalamus of adult male and female rats. Endocrinology. 1972;90:295–8. doi: 10.1210/endo-90-1-295. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Horvath TL, Leranth C, Harada N, Naftolin F. Aromataseimmunoreactivity in the rat brain gonadectomy-sensitive hypothalamicneurons and an unresponsive 'limbic ring' of the lateral septum-bednucleus-amygdala complex. J Steroid Biochem Mol Biol. 1993;44:481–98. doi: 10.1016/0960-0760(93)90253-s. [DOI] [PubMed] [Google Scholar]
- Kemppainen JA, Wilson EM. Agonist and antagonist activities of hydroxyflutamideand Casodex relate to androgen receptor stabilization. Urology. 1996;48:157–63. doi: 10.1016/s0090-4295(96)00117-3. [DOI] [PubMed] [Google Scholar]
- Singh SM, Gauthier S, Labrie F. Androgen receptor antagonists (anti-androgens): structure-activity relationships. Curr Med Chem. 2000;7:211–47. doi: 10.2174/0929867003375371. [DOI] [PubMed] [Google Scholar]
- Berrevoets CA, Umar A, Brinkmann AO. Anti-androgens: selective androgen receptor modulators. Mol Cell Endocrinol. 2002;198:97–103. doi: 10.1016/s0303-7207(02)00373-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C.The rat brain in stereotaxic coordinates2nd ed. San Diego: Academic Press; 1986.
- Shingo AS, Kanabayashi T, Murase T, Kito S. Cognitive decline in STZ-3V rats is largely due to dysfunctional insulin signalling through the dentate gyrus. Behav Brain Res. 2012;229:378–83. doi: 10.1016/j.bbr.2012.01.034. [DOI] [PubMed] [Google Scholar]
- Seyedreza P, Alireza MN, Seyedebrahim H. Role of testosterone in memory impairment of Alzheimer's disease induced by Streptozotocin in male rats. Daru. 2012;20:98. doi: 10.1186/2008-2231-20-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer MD, Daviau ED, Coneeny MK, Engelman SM, Prince WT, Rodriguez-Wisdom KN. Effects of testosterone on spatial learning and memory in adult male rats. Horm Behav. 2011;59:484–96. doi: 10.1016/j.yhbeh.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier MM, Matsumoto AM, Amory JK, Ahmed S, Bremner W, Peskind ER, et al. The role of aromatization in testosterone supplementation: effects on cognition in older men. Neurology. 2005;64:290–6. doi: 10.1212/01.WNL.0000149639.25136.CA. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm Behav. 2005;48:268–77. doi: 10.1016/j.yhbeh.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Kohlimaire JR, Alexander GM. Post training intrahippocampal estradiol injections enhance spatial memory in male rats: interaction with cholinergic system. Behav Neurosci. 1996;110:626–32. doi: 10.1037//0735-7044.110.3.626. [DOI] [PubMed] [Google Scholar]