Abstract
Aim:
To evaluation the doxorubicin (DOX)-loaded pH-sensitive polymeric micelle release from tumor blood vessels into tumor interstitium using an animal vessel visibility model, the so-called dorsal skin-fold window chamber model.
Methods:
DOX-loaded pH-sensitive polyHis-b-PEG micelles and DOX-loaded pH-insensitive PLLA-b-PEG micelles were prepared. The uptake of the micelles by MDA-MB-231 breast cancer cells in vitro and in vivo was examined using flow cytometry. The pharmacokinetic parameters of the micelles were determined in SD rats after intravenous injection of a DOX dose (6 mg/kg). The release of the micelles from tumor vasculature and the antitumor efficacy were evaluated in MDA-MB-231 breast cancer xenografted in nude mice using a dorsal skin-fold window chamber.
Results:
The effective elimination half-life t1/2 of the pH-sensitive, pH-insensitive polymeric micelles and DOX-PBS in rats were 11.3 h, 9.4 h, and 2.1 h, respectively. Intravital microscopy in MDA-MB-231 breast cancer xenografted in nude mice showed that the pH-sensitive polymeric micelles rapidly extravasated from the tumor blood vessels, and DOX carried by the pH-sensitive micelles was preferentially released at the tumor site as compared to the pH-insensitive polymeric micelles. Furthermore, the pH-sensitive polymeric micelles exhibited significant greater efficacy in inhibition of tumor growth in the nude mice.
Conclusion:
When DOX is loaded into pH-sensitive polymeric micelles, the acidity in tumor interstitium causes the destabilization of the micelles and triggers drug release, resulting in high local concentrations within the tumor, thus more effectively inhibiting the tumor growth in vivo.
Keywords: anticancer drug, doxorubicin, nanoparticle, polymeric micelle, drug release, pharmacokinetics, pH, dorsal skin-fold window chamber model
Introduction
Triggered drug release in the tumor interstitium after drug-loaded nanovesicle release from tumor blood vessels may result in a high local concentration and lesser drug distribution to normal tissues. This has been one of the major goals in drug carrier design in chemotherapy1,2,3. The difference in pH between the solid tumors and normal tissues has been long-recognized among oncologists4,5,6,7. For example, the average extracellular pH of human solid tumors xenografted in mice falls below a pH of 7.08, while the pH value of normal tissue is approximately 7.4. The difference is small but apparent and could be used as a pathophysiological stimulus for triggering drug release. Some approaches have demonstrated that carriers with pH-sensitive chemical bonds could induce accelerated drug release at a lower endosomal pH9. This approach, however, is not currently applicable to effectively target the tumor extracellular pH effectively. Core-destabilizing polymeric micelles have been produced that might take advantage of tumor acidity10,11,12,13,14,15,16. Micelles 94 nm in size were constructed with poly(L-histidine) (∼5000 Da)-b-poly(ethylene glycol) (∼5000 Da) (polyHis-b-PEG). ℒ-histidine is a major basic amino acid responsible for the buffering capacity of biological systems, and its base form has a pKb of 6.52. PolyHis block forms the micellar core when the pH is above 7.4, but the core is destabilized by the ionization of His residues when the pH is below 7.0. The detailed synthesis of polyHis-b-PEG was described in our previous paper3. The characteristics of micelles including the pH-dependent stability of such micelles at approximately the tumor extracellular pH were also reported2. The result of anticancer drug delivery by such pH-sensitive micelles to solid tumors has demonstrated greatly improved antitumor efficacy and significantly reduced toxicity in an A2780 ovarian cancer animal model15,17. However, the mechanisms of the release of pH-sensitive micelles from tumor blood vessels and the release of the drug in weakly acidic tumor interstitia have not yet been clarified in vivo. In this study, we established a visible extravasation animal model, a so-called dorsal skin-fold window chamber model, for the evaluation of pH-sensitive micelle release from tumor blood vessels into interstitia.
Materials and methods
Materials
L-Histidine, carbobenzoxy (CBZ), nystatin, insulin, penicillin-streptomycin, triethylamine (TEA) and dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich Chemical Corp. Doxorubicin·HCl was purchased from Beijing Huafeng United Technology Co, Ltd. Trypsin, fetal bovine serum and RPMI-1640 media were provided by Hyclone. Other chemicals were of an analytical grade.
Cell lines and animals
MDA-MB-231 breast cancer cells were provided by the Department of Pathology, Institute of Medicinal Biotechnology in Peking Union Medical College. The cells were cultured in 75-cm2 cell culture flasks using RPMI-1640 media supplemented with 10% fetal bovine serum, 0.4% nystatin, 1.2% insulin and 1.2% penicillin-streptomycin. The media were changed every other day. The incubators were maintained at 5.0% CO2 and 36.5 °C. Cells in the logarithmic growth phase were used to conduct all the cell experiments in this study. Male Sprague-Dawley rats (Beijing Vital River Laboratories) were used for pharmacokinetics tests; female 6-week-old nude mice (Beijing Vital River Laboratories) were used for in vivo flow cytometry, dorsal skin-fold window chamber models and tumor growth inhibition tests. The animals were housed and maintained in the animal facility of the Animal Center of the Chinese Academy of Medical Sciences. The animal protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the Chinese Academy of Medical Sciences and the Animal Ethics Committee of the institute.
Synthesis of PolyHis-b-PEG
Poly(L-His)-b-PEG was synthesized as described before2. Briefly, after L-histidine (His) was derivatized by introducing a carbobenzoxy (CBZ) group onto the amino group, the amino group in the imidazole ring of N-CBZ-L-histidine was protected with a dinitrophenyl (DNP) group. N-CBZ-N-DNP-L-histidine was then transformed to the N-carboxyanhydride (NCA) form by thionyl chloride. The ring-opening polymerization of NCA was initiated with isopropylamine. The purified poly(N-DNP-His) was coupled with carboxylated PEG to yield diblock copolymers. The polymer was then deprotected by thiolysis with 2-mercaptoethanol by our routine method6.
DOX-loaded pH-sensitive micelles
The DOX-loaded polyHis/PEG micellar formulation was prepared as described previously2,3. Briefly, Doxorubicin·HCl (DOX·HCl) was stirred with excess triethylamine (TEA) (2 times DOX·HCl) in DMSO overnight to obtain the DOX base. The block polymer (50 mg) was dissolved in 10 mL of DMSO, mixed with a DOX base solution (10 mg DOX base in 10 mL DMSO) and stirred for 3 h. The solution was transferred to a pre-swollen dialysis membrane (Spectra/Por with molecular weight cutoff 15 000) and dialyzed against NaOH-Na2B4O7 buffer solution (pH 9.0) for 24 h at 4 °C. The medium was exchanged several times, and the contents of the dialysis tube were subsequently lyophilized.
Determination of encapsulation efficiency and DOX-loading capacity
The concentration of entrapped DOX was determined by HPLC after being dissolved in 10% DMSO in methanol17. The process was assayed on an Agilent 1200LC (Agilent Tech, USA) HPLC system. A Cosmosil ODS C18 column (5 μm, 4.6 mm×150 mm) was used. The mobile phase consisted of a buffer (1.44 g of sodium dodecylsulfate and 0.68 mL of phosphoric acid dissolved in 500 mL of water)-acetonitrile-methanol (500:500:60, v:v:v) delivered at a flow rate of 1.0 mL/min. The injection volume was 20 μL, and the wavelength was set at 254 nm. The column temperature was 25 °C. The concentration of DOX was determined based on the peak area.
The pH-sensitive micelles were destroyed by adding a 3× volume of methanol, and the total content of DOX was determined by using HPLC. Then, the solution was centrifuged through a 30 000 kDa MWCO filter (Millipore) at 4 °C. The content of DOX in the lower chamber of the filter device was also determined. The loading capacity of DOX was determined by HPLC after dissolving with acetonitrile (1:10, w/v). DOX Loading Capacity (w/w%)=amount of physically loaded DOX/amount of DOX-micelles×100%; Encapsulation Efficiency=amount of physically loaded DOX/amount of DOX initially added×100%.
CMC measurement
For measurement of critical micelle concentration (CMC) of the diblock copolymer, 6.0×10−7 mol/L of final pyrene concentration was added in each sample. The fluorescent intensity of each sample emission at 339 nm was measured. The CMC of each sample was calculated by plotting the emission spectrum profile against the logarithm of the diblock copolymer's concentration.
In vitro and in vivo flow cytometry
MDA-MB-231 breast cancer cells (1×106 cells/well) were seeded in a 6-well plate and incubated overnight and harvested by 0.2% (w/v) trypsin–0.1% (w/v) EDTA solution. Two milliliters of free DOX and DOX-loaded micelles, with 25 μg/mL DOX in the medium, was introduced to each well and incubated for 25 min. The cells were trypsinized and washed three times with PBS solution and then fixed with 2.5% glutaraldehyde. After filtering through a nylon mesh, cell fluorescence was measured by flow cytometry (FACSCAN, Becton Dickinson)18. For the in vivo tumor cell uptake of DOX-loaded micelles, DOX in various micelles was injected through the tail vein of mice. At 12 h after intravenous injection, the animals were sacrificed, and the tumors were excised and then dried on filter paper. After digestion by trypsin, the tumors were fixed with 2.5% glutaraldehyde, filtered through a nylon mesh, and measured by flow cytometry17.
Dorsal skin-fold window chamber
To implant titanium window chamber frames in the mice5,19,20,21, the animals were anesthetized ip with ketamine at a dose of 100 mg/kg of body weight. One side of the skin was peeled, making a flap on the left side that was approximately 1 cm in diameter; the skin and underlying fascia tissue on the right side remained intact. A pair of titanium windows was mounted in alignment with the circular wound, which was then covered with a glass cover slip and a retaining ring after an MDA-MB-231 tumor fragment (approximately 0.1 mm3) was implanted onto the fascia. Following recovery from anesthesia and surgery, the animals were housed in an environmental chamber at 35 °C with 50% humidity and a 12-h light/12-h dark cycle, with access to rodent chow and water ad libitum. These conditions were necessary to maintain the viability of the chamber and to provide a high enough temperature to facilitate tumor growth. The fluorescent light intensities of the entire selected region and representative vascular regions were measured at each serial time point. Microcirculatory parameters were analyzed by intravital fluorescence microscopy22,23 using an Olympus microscope (Olympus BX51WI Microscope, Leeds Precision Instruments, Inc, MN, USA) specially designed for animal experiments.
Pharmacokinetics
To examine the pharmacokinetics of DOX micelles in the mouse body, DOX in PBS solution, DOX pH-insensitive micelles and DOX in pH-sensitive micelles were injected iv through the sublingual vein in rats at DOX base dose of 10 mg/kg. At given time intervals (5, 30 min, 1, 2, 4, 12, and 24 h) post-injection, the rats were anesthetized by methoxyflurane. Blood was collected via cardiac puncture and placed in microtubes with 10 μL 50 U/mL heparin. The whole blood (0.8 mL) was transferred to a tube, which contained 200 mg of daunomycin as the internal standard and 0.01 mol silver nitrate to prevent DOX from binding to DNA8. Triple extractions were performed after adding chloroform/isopropyl alcohol (3:1, v/v). The solutions were frozen overnight, thawed, and centrifuged at 16 000×g for 10 min. The organic phase was removed, and the resulting solutions were evaporated to dryness and re-dissolved in a mobile phase solution (methanol/isopropyl alcohol/Sorensen's buffer, 2:2:6, v/v/v). These samples were analyzed (at excitation wavelength 480 nm and emission wavelength 560 nm) using an HPLC system. HPLC of DOX was performed using a Supelco LC-18 column (250 mm×4.6 mm id, 5 mm particle size) and a Hitachi HPLC instrument (D-6000 interface, F-1080 Fluorescence Detector; L-6200A intelligent pump, and AS-2000 Autosampler). The non-compartmental pharmacokinetic parameters, which included the area under the drug concentration time curve (AUC), t1/2, clearance (CL), volume of distribution of drug (Vd) and mean retention time (MRT), were calculated using the trapezoidal rule. The data between the different formulations were compared for statistical significance by a one-way analysis of variance (ANOVA).
Tumor growth inhibition
The xenografts of human breast MDA-MB-231 carcinoma cells were developed after implanting 2 mm×2 mm tumor pieces into a window chamber. When the tumor volume reached approximately 50 mm3, all of the animals were treated thrice at 3-d intervals with either DOX-loaded pH-sensitive micelles or pH-insensitive micelles. The mice were safely treated without losing much weight during the treatment. Both the micelle formulations and free drugs were injected intravenously via tail vein at the DOX base dose of 10 mg/kg through 25G5/8 needles. The tumor inhibition activity was assessed with the tumor volume size, which was calculated by the following equation: V=(w)2×(l)/2, where (w) and (l) are width and length of the tumor measured by a caliper with a 3-d interval.
Results
The characterization of DTX-PM
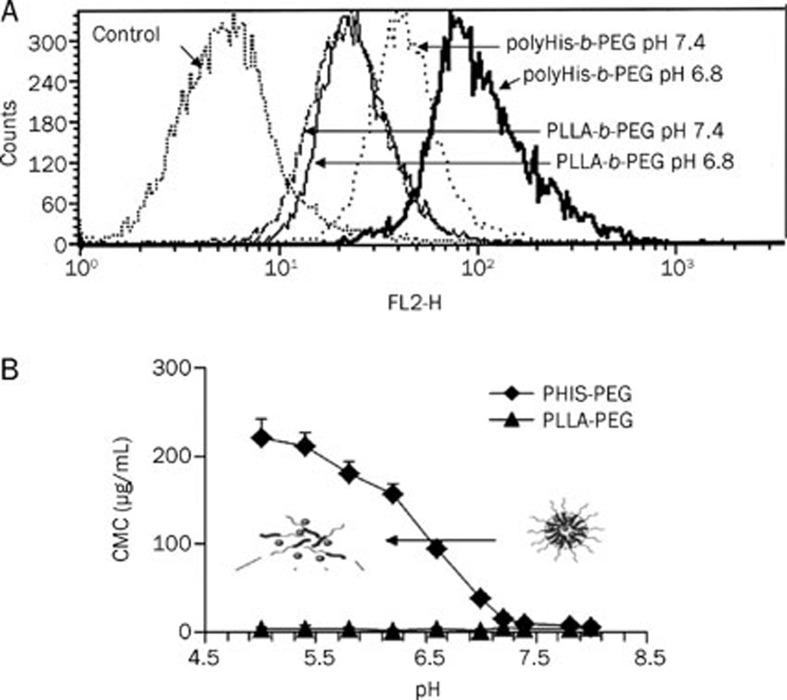
The encapsulation efficiency and loading capacity were 98% and 13%, respectively, for the pH-sensitive micelles and 99% and 23%, respectively, for the pH-insensitive micelles. The CMC values of the pH-sensitive micelles were 201 (at pH 5), 145 (pH 6), 72 (pH 6.8), 31 (pH 7.0), 5.6 (pH 7.4), and 1.2 (pH 8.0)×10−3mg/mL, respectively. These results indicated that increasing the pH level resulted in decreased CMC values.
Cell uptake at different pH values
Here, we report on the visualization of pH-sensitive micelles from the blood vessels developed in MDA-MB-231 breast tumors and Doxorubicin accumulation in tumors of the mouse dorsal skin-fold window chamber model. Prior to the in vivo study, MDA-MB-231 breast cancer cells were incubated in pH 7.4 and pH 6.8 media at a base micelle concentration of 25 μg/mL DOX for an initial time period of 25 min. The resulting fluorescence histograms of the cellular uptake of DOX are presented in Figure 1A. All of the cell population profiles were unimodal, and the profiles were shifted to higher fluorescence intensity regions when the cells were treated with DOX-loaded polyHis-b-PEG micelles at two different pHs (normal blood pH and the tumor extracellular pH). The DOX fluorescence intensity of the cells treated at pH 6.8 appeared approximately 5 times higher than at pH 7.4, confirming a higher DOX availability to the tumor cells incubated at pH 6.8 from the beginning of the treatment with pH-sensitive micelles. This may be one of a variety of reasons why the inclusion of a sparingly water-soluble drug may overcome cancer cell multi-drug resistance and promote drug absorption by poly-histidine.
Figure 1.
Fluorescence histograms of breast cancer MDA-231 cells incubated with DOX loaded into polyHis-b-PEG pH-sensitive micelles. DOX (25 μg) was incorporated into 125 μg polyHis-b-PEG micelles per mL solution. Cells were incubated in pH 6.8 medium, pH 7.4 medium and control plain medium without DOX. All cells were incubated for 25 min after adding the formulations (A). The CMC of polyHis-b-PEG micelles as a function of pH value was present (B). pH sensitive micelles (particle size approximately 95 nm, measured by dynamic light scattering) were dissociated at a pH lower than 7.0 in the media (▪), whereas the pH-insensitive micelles remained intact as the pH was reduced to 4.5 (▴).
However, the DOX fluorescence intensity of the cells at pH 7.4 and 6.8 was similar when a control sample of pH-insensitive micelles prepared from poly (L-lactic acid)-b-PEG (PLLA-b-PEG) was used. The pH-sensitive micelles were destabilized at a lower pH, resulting in an accelerated DOX release4. This was consistent with the pH-dependent critical micelle concentration (CMC), which increased as the pH value decreased (Figure 1B).
Pharmacokinetic parameters
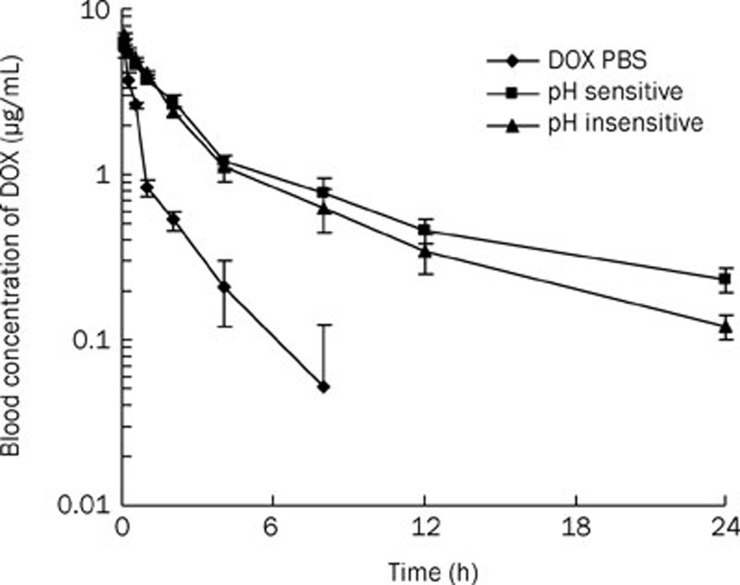
The blood concentration-time profiles of DOX after iv administration of free DOX dissolved in PBS, DOX in pH-insensitive micelles and DOX loaded into pH-sensitive micelles are shown in Figure 2. Each formulation was administered to rats (female, n=5) at a DOX dose of 6 mg/kg. The DOX concentration of DOX was approximately 1.5 μg/mL, 1.1 μg/mL and 0.21 μg/mL, respectively, after the administration of pH-sensitive, insensitive and DOX PBS at 4 h, and above 0.1 μg/mL of the blood DOX level, even at 24 h after the administration of DOX in micelles. The free DOX concentration was not detectable in our measurement condition (detection limit: 50 ng/mL) after 8 h. The non-compartmental pharmacokinetic parameters were calculated using the trapezoidal rule. The effective elimination half-life t1/2 of the pH-sensitive micelle formulation was 5 times longer than the DOX PBS solution (11.3 vs 2.1 h) and 1.2 times longer than the pH-insensitive micelles (11.3 vs 9.4 h), while the total CL decreased (1923 vs 367 mL·min−1·kg−1). The PEG graft on the surface of the micelles seemed to be responsible for the increased circulation time in the blood. In addition, the mean residence time (MRT) (2.6 vs 13.5 h) and VD at steady state (Vdss) (4024 vs 3987 I/kg) were calculated for the DOX-loaded pH-sensitive micelles and free DOX dissolved in PBS solution.
Figure 2.
The blood concentration-time profiles of DOX after iv administration of DOX PBS, DOX loaded into pH-sensitive micelles and DOX loaded into pH-insensitive micelles. Each formulation was administered to mice (female, n=5) at a DOX dose of 6 mg/kg.
Drug release from tumor blood vessel
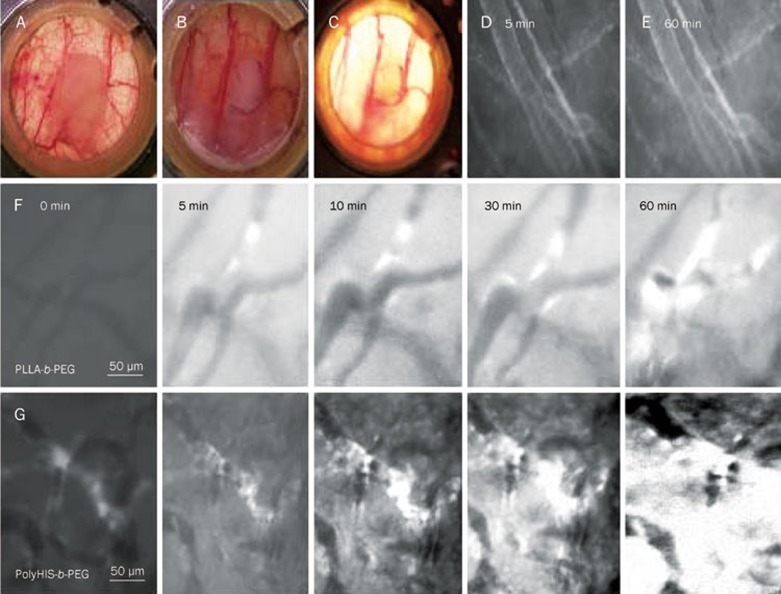
A 2 mm×2 mm tumor implanted into a BALB/c mouse window chamber is shown in Figure 3A. Blood vessel developments in the window chamber on d 1 and 15 after tumor implantation are shown in Figure 3B and Figure 3C, respectively. The fluorescence was confined to the vessels in physiology granulation tissue, as observed after intravenous injection of DOX-loaded pH-sensitive micelles through the mouse tail vein. The release of micelles from normal blood vessels was very limited at two initial time points (5 min to 60 min) as shown in Figure 3D and 3E.
Figure 3.
Mouse dorsal skin-fold window chamber made of two symmetrical titanium frames. A tumor xenograft was inoculated into a nu/nu mouse window chamber (A). After implanting the MDA-231 breast cancer tumor xenograft, tumor blood vessels were observed to be growing in the window chamber on d 1 (B) and d 15 (C). Normal blood vessel images after iv injection of DOX-loaded pH-sensitive micelles 5 min and 60 min are shown in (D) and (E), respectively. The tumor blood vessels after iv injection of DOX-loaded pH-insensitive and pH-sensitive micelles at the time course for 60 min are presented in (F) and (G) rows. The tight junctions between cells in the endothelial lining of blood vessels in normal tissues do not allow the drug-loaded micelles (indicated by cross) to enter into the tissues. In contrast, tumors are characterized by defective vasculature with large gaps between the endothelial cells, which allowed the drug-loaded micelles enter into the gaps, causing their accumulation in the tumor interstitium.
The endothelial cells of the blood vessels in normal tissue were joined by tight junctions that prevented penetration of nanoparticles, as shown in Figures 3D and 3E. In contrast, the tumors were characterized by a defective vasculature with large gaps between the endothelial cells that allowed the nanoparticles to enter into gaps up to 750 nm in size5,24. This permeability allows for accumulation of drug-loaded micelles in the tumor interstitium via the enhanced permeability and retention (EPR) effect25,26,27. The diffusion rate of DOX from tumor blood vessels for the pH-sensitive micelles was greater than the pH-insensitive micelles (Figure 3), suggesting that the rapid dissociation of pH-sensitive micelles in low-pH tumor tissue may reduce the barrier in tumor blood vessels.
Tumor growth inhibition
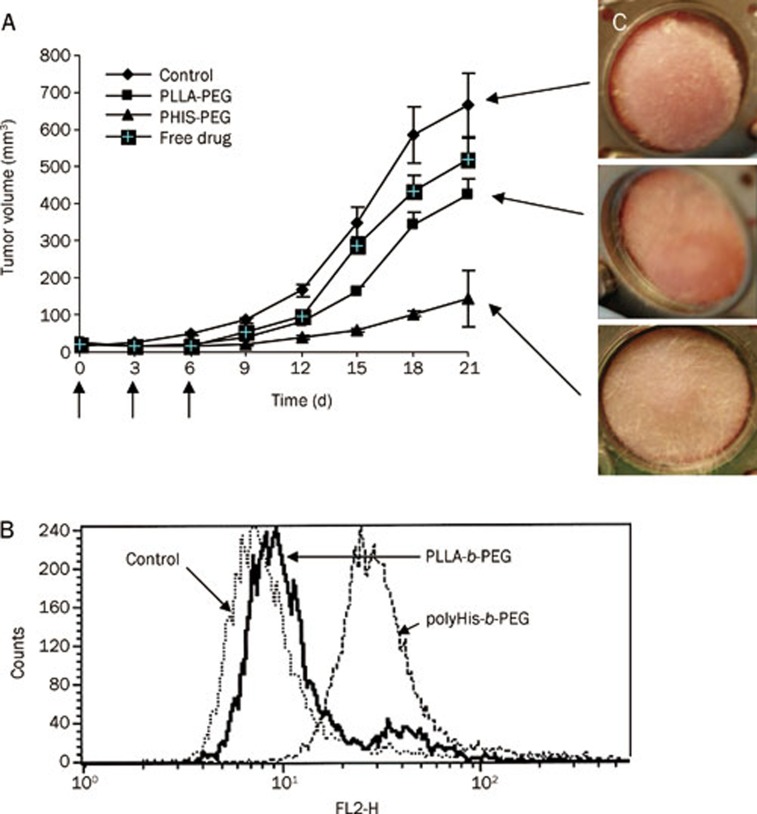
The serial images of DOX-loaded pH-sensitive and pH-insensitive micelles from tumor blood vessels are shown in two rows of Figures 3F and 3G. MDA-MB-231 cells xenografted in the nude mouse dorsal skin-fold window chamber model were used to study tumor growth inhibition in vivo. The antitumor activity of the DOX-loaded pH-sensitive micelles injected intravenously on d 0, 3, and 6 is shown in Figure 4A. After 21 d, the DOX-loaded pH-sensitive micelles significantly inhibited the growth of MDA-MB-231 xenografts in nude mice (5 mice per group) when compared with the DOX-loaded pH-insensitive micelles and control group (P<0.01, P<0.01 compared with control and pH-insensitive micelles; ANOVA test). After 21 d of treatment, the tumor volumes of the control and pH-insensitive micelle-treated groups were approximately 6.8- and 4.3-fold larger than the group treated with the pH-sensitive micelles. To further clarify drug concentrations in cancer cells in vivo, the cells were extracted from tumor tissues 12 h post-administration of 10 mg/kg of DOX and examined by flow cytometry. A high fluorescence intensity with a unimodal distribution of the cell population was observed when treated with pH-sensitive micelles. However, there was a bimodal distribution of cells with varying fluorescence intensity in the group treated with the pH-insensitive micelles. Approximately 14% of the total tumor cells appeared to show high DOX uptake. The intensity of cellular uptake of DOX supplied from the pH-sensitive micelles was approximately 3.3-fold higher than the pH-insensitive micelles, as shown in Figure 4B. Tumor cells with a unimodal distribution of high DOX fluorescence intensity in the cell population correlated with slowly growing tumors.
Figure 4.
Effects of DOX-loaded pH-sensitive micelles and pH-insensitive micelles on the growth of MDA 231 breast carcinoma in the window chamber model are presented in (A) and DOX uptake by MDA-231 breast tumor cells in vivo was evaluated by flow cytometry (B). Each formulation was administered three times at 3-day intervals (arrows) at a dose of 10 mg/kg for tumor growth inhibition study. For the in vivo tumor cell uptake test, tumor cells were extracted and evaluated by flow cytometry after a 12-h administration of DOX-loaded pH-sensitive micelles at a dose of 10 mg/kg.
The pH-sensitive micelles prepared with polyHis-b-PEG showed preferential drug accumulation in the tumor site, most likely by the combined effects of a longer circulation time in the bloodstream17, micellar disintegration and triggered drug release in the relatively acidic tumor interstitium. This enhanced the bioavailability of DOX in the tumor cells, resulting in improved antitumor efficacy. The drug accumulation processes were successfully observed using the dorsal window chamber model.
Discussion
As described in this study, the chemotherapy drug, DOX, when loaded into pH-sensitive micelles and released from tumor blood vessels, as shown in the dorsal window chamber model, effectively inhibited tumor volume growth in breast cancer cell tumor-bearing mice. The core of the micelle was composed of pH-sensitive poly-histidine dissociates under the lower pH caused by deprotonation. The hydrophobic drug contained in the micelle core was released upon micelle dissociation, leading to enhanced drug uptake by cancer cells, as shown in Figure 1. In contrast, the release of the drug in the pH-insensitive micelle composed of PLLA-PEG was much reduced compared to the pH-sensitive micelle because the strong hydrophobic core architecture still existed in the micelle, as we previously described3. The CMC was increased with a reduction of the pH in the medium because of the prompt deprotonation of the lone electronic pairs on the nitrogen of the imidazole ring in the poly-histidine structure, while the pH-insensitive micelle still maintained a low CMC under various pH conditions. It was demonstrated that the pH-sensitive poly-histidine micelle could be stable at a normal of pH 7.4 and could disintegrate at a low pH, which is very useful for acidic solid tumor targets.
DOX itself emits red fluorescence, and its excitation and emission wavelengths are 488 nm and 510 nm, respectively. The release of DOX from tumor blood vessels can be visualized in the window chamber by an intravital fluorescent microscope. A 2 mm×2 mm tumor implanted into a BALB/c mouse via the window chamber model is shown in Figure 3A. The endothelial cells of the blood vessels in normal tissue were joined by tight junctions that prevented penetration of nanoparticles, as shown in Figure 3D and 3E. In contrast, the tumors were characterized by a defective vasculature with large gaps between the endothelial cells, which allowed the nanoparticles to enter into gaps up to 750 nm in size5,24. This permeability allowed for the accumulation of drug-loaded micelles in the tumor interstitium via the enhanced permeability and retention (EPR) effect25,26,27. The diffusion rate of DOX from tumor blood vessels in pH-sensitive micelles was greater than in pH-insensitive micelles (Figure 3), suggesting that the rapid dissociation of pH-sensitive micelles in low-pH tumor tissues may reduce the barrier in tumor blood vessels.
The pH-sensitive micelles prepared with polyHis-b-PEG showed preferential drug accumulation in the tumor site, most likely by the combined effects of a longer circulation time in the bloodstream17, micellar disintegration and triggered drug release in the relatively acidic tumor interstitium. This enhanced the bioavailability of DOX into the tumor cells, resulting in improved antitumor efficacy. The drug accumulation processes were successfully observed by the dorsal window chamber model.
In conclusion, we successfully established a visible extravasation animal model, the so-called dorsal skin-fold window chamber model, for the evaluation and clarification of the mechanisms of drug delivery released from tumor blood vessels into interstitia. The cores of pH non-sensitive PEG-PLLA and pH-sensitive micelles were composed of poly(L-histidine)-b-poly(ethylene glycol) and were prepared for comparing drug release patterns in the acidic environment of solid tumors. Intravital microscope evaluation showed that the pH-sensitive micelle was preferentially released at the tumor site when compared to the pH-insensitive micelle system. The destabilization of the pH-sensitive micelles and the triggered drug release caused by the tumor acidity were the major mechanisms resulting in high local concentrations of DOX in the tumor. The pH-sensitive micelles significantly inhibited tumor growth in vivo when chemotherapy drugs were loaded into the hydrophobic core.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No 81373342), and the Natural Science Foundation of Beijing (No 2141004 and 7142114).
References
- Kataoka K, Matsumoto T, Yokoyama M, Okano T, Sakurai Y, Fukushima S, et al. Doxorubicin-loaded poly(ethylene glycol)-poly(beta-benzyl-ℒ-aspartate) copolymer micelles: Their pharmaceutical characteristics and biological significance. J Control Release. 2000;64:143–53. doi: 10.1016/s0168-3659(99)00133-9. [DOI] [PubMed] [Google Scholar]
- Lee ES, Na K, Bae YH. Polymeric micelle for tumor pH and folate-mediated targeting. J Control Release. 2003;91:103–13. doi: 10.1016/s0168-3659(03)00239-6. [DOI] [PubMed] [Google Scholar]
- Lee ES, Shin HJ, Na K, Bae YH. Poly(ℒ-histidine)-peg block copolymer micelles and pH-induced destabilization. J Control Release. 2003;90:363–74. doi: 10.1016/s0168-3659(03)00205-0. [DOI] [PubMed] [Google Scholar]
- Tannock IF, Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989;49:4373–84. [PubMed] [Google Scholar]
- Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, et al. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc Natl Acad Sci U S A. 1998;95:4607–12. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs M, McSheehy PM, Griffiths JR, Bashford CL. Causes and consequences of tumour acidity and implications for treatment. Mol Med Today. 2000;6:15–9. doi: 10.1016/s1357-4310(99)01615-9. [DOI] [PubMed] [Google Scholar]
- Engin K, Leeper DB, Cater JR, Thistlethwaite AJ, Tupchong L, McFarlane JD. Extracellular pH distribution in human tumours. Int J Hyperthermia. 1995;11:211–6. doi: 10.3109/02656739509022457. [DOI] [PubMed] [Google Scholar]
- Gao ZG, Fain HD, Rapoport N. Controlled and targeted tumor chemotherapy by micellar-encapsulated drug and ultrasound. J Control Release. 2005;102:203–22. doi: 10.1016/j.jconrel.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Park TG. Perfusion culture of hepatocytes within galactose-derivatized biodegradable poly(lactide-co-glycolide) scaffolds prepared by gas foaming of effervescent salts. J Biomed Mater Res. 2002;59:127–35. doi: 10.1002/jbm.1224. [DOI] [PubMed] [Google Scholar]
- Na K, Bae YH. Self-assembled hydrogel nanoparticles responsive to tumor extracellular pH from pullulan derivative/sulfonamide conjugate: Characterization, aggregation, and adriamycin release in vitro. Pharm Res. 2002;19:681–8. doi: 10.1023/a:1015370532543. [DOI] [PubMed] [Google Scholar]
- Na K, Lee ES, Bae YH. Adriamycin loaded pullulan acetate/sulfonamide conjugate nanoparticles responding to tumor pH: pH-dependent cell interaction, internalization and cytotoxicity in vitro. J Control Release. 2003;87:3–13. doi: 10.1016/s0168-3659(02)00345-0. [DOI] [PubMed] [Google Scholar]
- Sethuraman VA, Na K, Bae YH. Ph-responsive sulfonamide/pei system for tumor specific gene delivery: An in vitro study. Biomacromolecules. 2006;7:64–70. doi: 10.1021/bm0503571. [DOI] [PubMed] [Google Scholar]
- Kang SI, Bae YH. pH-induced solubility transition of sulfonamide-based polymers. J Control Release. 2002;80:145–55. doi: 10.1016/s0168-3659(02)00021-4. [DOI] [PubMed] [Google Scholar]
- Kang SI, Bae YH. pH-dependent elution profiles of selected proteins in hplC having a stationary phase modified with pH-sensitive sulfonamide polymers. J Biomater Sci Polym Ed. 2004;15:879–94. doi: 10.1163/1568562041271110. [DOI] [PubMed] [Google Scholar]
- Lee ES, Na K, Bae YH. Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor. J Control Release. 2005;103:405–18. doi: 10.1016/j.jconrel.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Lee ES, Na K, Bae YH. Super pH-sensitive multifunctional polymeric micelle. Nano Lett. 2005;5:325–9. doi: 10.1021/nl0479987. [DOI] [PubMed] [Google Scholar]
- Gao ZG, Lee DH, Kim DI, Bae YH. Doxorubicin loaded pH-sensitive micelle targeting acidic extracellular pH of human ovarian a2780 tumor in mice. J Drug Target. 2005;13:391–7. doi: 10.1080/10611860500376741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Fain HD, Rapoport N. Ultrasound-enhanced tumor targeting of polymeric micellar drug carriers. Mol Pharm. 2004;1:317–30. doi: 10.1021/mp049958h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsky WL, Fukumura D, Gohongi T, Ancukiewcz M, Weich HA, Torchilin VP, et al. Augmentation of transvascular transport of macromolecules and nanoparticles in tumors using vascular endothelial growth factor. Cancer Res. 1999;59:4129–35. [PubMed] [Google Scholar]
- Wu NZ, Braun RD, Gaber MH, Lin GM, Ong ET, Shan S, et al. Simultaneous measurement of liposome extravasation and content release in tumors. Microcirculation. 1997;4:83–101. doi: 10.3109/10739689709148320. [DOI] [PubMed] [Google Scholar]
- Lichtenbeld HC, Yuan F, Michel CC, Jain RK. Perfusion of single tumor microvessels: Application to vascular permeability measurement. Microcirculation. 1996;3:349–57. doi: 10.3109/10739689609148307. [DOI] [PubMed] [Google Scholar]
- Tozer GM, Prise VE, Wilson J, Cemazar M, Shan S, Dewhirst MW, et al. Mechanisms associated with tumor vascular shut-down induced by combretastatin a-4 phosphate: Intravital microscopy and measurement of vascular permeability. Cancer Res. 2001;61:6413–22. [PubMed] [Google Scholar]
- Dewhirst MW, Shan S, Cao Y, Moeller B, Yuan F, Li CY. Intravital fluorescence facilitates measurement of multiple physiologic functions and gene expression in tumors of live animals. Dis Markers. 2002;18:293–311. doi: 10.1155/2002/820102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RB. Tumor physiology and delivery of nanopharmaceuticals. Anticancer Agents Med Chem. 2006;6:503–12. doi: 10.2174/187152006778699077. [DOI] [PubMed] [Google Scholar]
- Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of sirna nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci U S A. 2007;104:15549–54. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan S, Flowers C, Peltz CD, Sweet H, Maurer N, Kwon EJ, et al. Preferential extravasation and accumulation of liposomal vincristine in tumor comparing to normal tissue enhances antitumor activity. Cancer Chemother Pharmacol. 2006;58:245–55. doi: 10.1007/s00280-005-0145-x. [DOI] [PubMed] [Google Scholar]
- Gao ZG, Lukyanov AN, Singhal A, Torchilin VP. Diacyllipid-polymer micelles as nanocarriers for poorly soluble anticancer drugs. Nano Lett. 2002;2:979–82. [Google Scholar]