Abstract
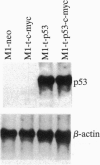
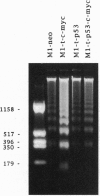
Myeloid leukemic M1 cells that do not express p53 and transfected M1 clones that constitutively express the [Val135]p53 mutant or deregulated c-myc or coexpressing both genes grew autonomously in culture with a similar growth rate and cloning efficiency. Expression of deregulated c-myc in M1 leukemic cells enhanced susceptibility to induction of apoptotic cell death and resulted in a reduced leukemogenicity when injected into isologous mice. Expression of the [Val135]p53 mutant did not change cell susceptibility to induction of apoptosis or leukemogenicity, but expression of this mutant p53 suppressed the effects of deregulated c-myc on these properties. The results indicate that the [Val135]p53 mutant can show a gain of function for susceptibility to apoptosis and leukemogenicity in leukemic cells with deregulated c-myc and, thus, enhance tumor development.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Cory S. Transgenic models of tumor development. Science. 1991 Nov 22;254(5035):1161–1167. doi: 10.1126/science.1957168. [DOI] [PubMed] [Google Scholar]
- Ashwell J. D., Berger N. A., Cidlowski J. A., Lane D. P., Korsmeyer S. J. Coming to terms with death: apoptosis in cancer and immune development. Immunol Today. 1994 Apr;15(4):147–151. doi: 10.1016/0167-5699(94)90309-3. [DOI] [PubMed] [Google Scholar]
- Askew D. S., Ashmun R. A., Simmons B. C., Cleveland J. L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991 Oct;6(10):1915–1922. [PubMed] [Google Scholar]
- Askew D. S., Ihle J. N., Cleveland J. L. Activation of apoptosis associated with enforced myc expression in myeloid progenitor cells is dominant to the suppression of apoptosis by interleukin-3 or erythropoietin. Blood. 1993 Oct 1;82(7):2079–2087. [PubMed] [Google Scholar]
- Bissonnette R. P., Echeverri F., Mahboubi A., Green D. R. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992 Oct 8;359(6395):552–554. doi: 10.1038/359552a0. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Dittmer D., Pati S., Zambetti G., Chu S., Teresky A. K., Moore M., Finlay C., Levine A. J. Gain of function mutations in p53. Nat Genet. 1993 May;4(1):42–46. doi: 10.1038/ng0593-42. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Harvey M., Slagle B. L., McArthur M. J., Montgomery C. A., Jr, Butel J. S., Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992 Mar 19;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Fanidi A., Harrington E. A., Evan G. I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992 Oct 8;359(6395):554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- Harvey M., Vogel H., Morris D., Bradley A., Bernstein A., Donehower L. A. A mutant p53 transgene accelerates tumour development in heterozygous but not nullizygous p53-deficient mice. Nat Genet. 1995 Mar;9(3):305–311. doi: 10.1038/ng0395-305. [DOI] [PubMed] [Google Scholar]
- Hermeking H., Eick D. Mediation of c-Myc-induced apoptosis by p53. Science. 1994 Sep 30;265(5181):2091–2093. doi: 10.1126/science.8091232. [DOI] [PubMed] [Google Scholar]
- Jacobsen K. A., Prasad V. S., Sidman C. L., Osmond D. G. Apoptosis and macrophage-mediated deletion of precursor B cells in the bone marrow of E mu-myc transgenic mice. Blood. 1994 Oct 15;84(8):2784–2794. [PubMed] [Google Scholar]
- Klein G., Klein E. Evolution of tumours and the impact of molecular oncology. Nature. 1985 May 16;315(6016):190–195. doi: 10.1038/315190a0. [DOI] [PubMed] [Google Scholar]
- Lavigueur A., Maltby V., Mock D., Rossant J., Pawson T., Bernstein A. High incidence of lung, bone, and lymphoid tumors in transgenic mice overexpressing mutant alleles of the p53 oncogene. Mol Cell Biol. 1989 Sep;9(9):3982–3991. doi: 10.1128/mcb.9.9.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Levy N., Yonish-Rouach E., Oren M., Kimchi A. Complementation by wild-type p53 of interleukin-6 effects on M1 cells: induction of cell cycle exit and cooperativity with c-myc suppression. Mol Cell Biol. 1993 Dec;13(12):7942–7952. doi: 10.1128/mcb.13.12.7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Hematopoietic cells from mice deficient in wild-type p53 are more resistant to induction of apoptosis by some agents. Blood. 1993 Aug 15;82(4):1092–1096. [PubMed] [Google Scholar]
- Lotem J., Sachs L. Regulation by bcl-2, c-myc, and p53 of susceptibility to induction of apoptosis by heat shock and cancer chemotherapy compounds in differentiation-competent and -defective myeloid leukemic cells. Cell Growth Differ. 1993 Jan;4(1):41–47. [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Michalovitz D., Halevy O., Oren M. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell. 1990 Aug 24;62(4):671–680. doi: 10.1016/0092-8674(90)90113-s. [DOI] [PubMed] [Google Scholar]
- Nudel U., Zakut R., Shani M., Neuman S., Levy Z., Yaffe D. The nucleotide sequence of the rat cytoplasmic beta-actin gene. Nucleic Acids Res. 1983 Mar 25;11(6):1759–1771. doi: 10.1093/nar/11.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltvai Z. N., Korsmeyer S. J. Checkpoints of dueling dimers foil death wishes. Cell. 1994 Oct 21;79(2):189–192. doi: 10.1016/0092-8674(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Oren M. p53: the ultimate tumor suppressor gene? FASEB J. 1992 Oct;6(13):3169–3176. doi: 10.1096/fasebj.6.13.1397838. [DOI] [PubMed] [Google Scholar]
- Reed J. C., Cuddy M., Haldar S., Croce C., Nowell P., Makover D., Bradley K. BCL2-mediated tumorigenicity of a human T-lymphoid cell line: synergy with MYC and inhibition by BCL2 antisense. Proc Natl Acad Sci U S A. 1990 May;87(10):3660–3664. doi: 10.1073/pnas.87.10.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnitzky D., Kimchi A. Deregulated c-myc expression abrogates the interferon- and interleukin 6-mediated G0/G1 cell cycle arrest but not other inhibitory responses in M1 myeloblastic cells. Cell Growth Differ. 1991 Jan;2(1):33–41. [PubMed] [Google Scholar]
- Sachs L., Lotem J. Control of programmed cell death in normal and leukemic cells: new implications for therapy. Blood. 1993 Jul 1;82(1):15–21. [PubMed] [Google Scholar]
- Sachs L. The adventures of a biologist: prenatal diagnosis, hematopoiesis, leukemia, carcinogenesis, and tumor suppression. Adv Cancer Res. 1995;66:1–40. [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Bath M. L., Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990 Nov 22;348(6299):331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Jacks T., Cory S. DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell. 1994 Oct 21;79(2):329–339. doi: 10.1016/0092-8674(94)90201-1. [DOI] [PubMed] [Google Scholar]
- Wattel E., Preudhomme C., Hecquet B., Vanrumbeke M., Quesnel B., Dervite I., Morel P., Fenaux P. p53 mutations are associated with resistance to chemotherapy and short survival in hematologic malignancies. Blood. 1994 Nov 1;84(9):3148–3157. [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]