An individualized, progressive, high-repetition, task-specific training protocol for inpatients with UE paresis after stroke yielded improvement on impairment and activity-level outcome measures.
MeSH TERMS: paresis, stroke, task performance and analysis, upper extremity
Abstract
OBJECTIVE. We investigated the feasibility of delivering an individualized, progressive, high-repetition upper-extremity (UE) task-specific training protocol for people with stroke in the inpatient rehabilitation setting.
METHOD. Fifteen patients with UE paresis participated in this study. Task-specific UE training was scheduled for 60 min/day, 4 days/wk, during occupational therapy for the duration of a participant’s inpatient stay. During each session, participants were challenged to complete ≥300 repetitions of various tasks.
RESULTS. Participants averaged 289 repetitions/session, spending 47 of 60 min in active training. Participants improved on impairment and activity level outcome measures.
CONCLUSION. People with stroke in an inpatient setting can achieve hundreds of repetitions of task-specific training in 1-hr sessions. As expected, all participants improved on functional outcome measures. Future studies are needed to determine whether this high-repetition training program results in better outcomes than current UE interventions.
One of the most debilitating deficits after stroke is upper-extremity (UE) paresis (Dobkin, 2005; Duncan et al., 1994; Foulkes, Wolf, Price, Mohr, & Hier, 1988; Harris, Eng, Miller, & Dawson, 2009; Legg et al., 2007; Wilkinson et al., 1997; Winstein et al., 2004). Surprisingly, interventions to address UE paresis are often inconsistently applied in routine clinical settings and even neglected at various stages of stroke recovery (Barker & Brauer, 2005). When provided, UE interventions often consist of various therapeutic approaches provided in low doses (Lang et al., 2009; Oujamaa, Relave, Froger, Mottet, & Pelissier, 2009), as observed during delivery of clinical services.
Upper-extremity task-specific training occurs in only about half of therapy sessions focused on the UE, and it involves an average of only 32 repetitions (Lang et al., 2009). Similarly, patients spend approximately 47 min/day in occupational therapy in the early phases of stroke rehabilitation, but only 4–11 min are spent on UE rehabilitation (Bernhardt, Chan, Nicola, & Collier, 2007; Harris et al., 2009). In the inpatient setting, therapy minutes can be allocated to locating equipment and setup, multiple competing clinical priorities (e.g., activities of daily living [ADLs], cognition, balance), and patients arriving late to sessions. Thus, the clinical reality early after stroke is in sharp contrast to research data illustrating the importance of individually tailored, progressive, high-repetition, task-specific UE training (Birkenmeier, Prager, & Lang, 2010; Boyd, Vidoni, & Wessel, 2010; Han, Wang, Meng, & Qi, 2013; Lang et al., 2009; Plautz, Milliken, & Nudo, 2000).
This type of individually tailored, progressive UE training is feasible in an outpatient setting, indicated by UE training that occurs for extended hours as part of constraint-induced movement therapy (CIMT; Barzel et al., 2009; Smania et al., 2012; Treger, Aidinof, Lehrer, & Kalichman, 2012; Wolf et al., 2006). Feasibility has also been demonstrated for 1-hr therapy sessions, typical of routine outpatient services (Birkenmeier et al., 2010). The next critical step is to determine the feasibility of delivering this type of training early after stroke, when people are in the inpatient rehabilitation facility (IRF) setting. The IRF setting is where most rehabilitation services are provided and money is spent and where recovery opportunity is greatest (Biernaskie, Chernenko, & Corbett, 2004; Horn et al., 2005; Krakauer, 2006). The potential to improve patient outcomes and reduce burden of care increases if such an intervention is feasible early in the IRF setting. The challenges to feasibility in the IRF environment include competing clinical priorities, rigid rules for therapy time, heavy emphasis on ADL retraining, and relatively high medical complexity.
Our primary aim was to examine the feasibility and tolerability of delivering high-repetition, individually tailored, progressive, task-specific training during occupational therapy in an IRF setting. The training was delivered in 1-hr sessions, thus requiring fewer therapy hours than the tested models of CIMT in the IRF setting (Dromerick et al., 2009). Our second aim was to gather preliminary efficacy data. We hypothesized that patients would be able to achieve hundreds of task-specific activities during 1-hr therapy sessions without inducing substantial fatigue or pain.
Method
This study was a single cohort, repeated-measures design (Figure 1). The institutional review board of Northwestern University approved the study, and informed consent was obtained from all participants.
Figure 1.
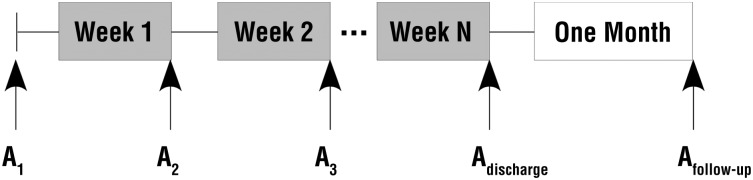
General time line of study. Assessments (A) were completed on a weekly basis for the duration of each participant’s admission to the inpatient rehabilitation facility, at discharge, and at 1-mo postdischarge.
Participants
We recruited a convenience sample of inpatients with poststroke unilateral paresis at an IRF. We selected the inclusion criteria on the basis of Birkenmeier et al.’s (2010) study examining the feasibility of this intervention in the outpatient setting. Inclusion criteria were as follows: Patient (1) meets ICD–9 criteria of unilateral stroke with residual UE paresis; (2) has a Motricity Index (Collin & Wade, 1990) score between 42 and 93 points; (3) has cognitive ability to follow commands, as indicated by a score of 0 to 1 on the Commands item of the National Institutes of Health Stroke Scale (NIHSS; Brott, et al., 1989); and (4) is at least age 18 yr. Participants were excluded if they (1) had severe neglect, as indicated by a score of 2 on the NIHSS Extinction and Inattention subtest, and (2) had a history of stroke more than 1 wk before the current index stroke located on or affecting the same side of the body.
The planned number of participants to recruit was 6–15; this number was considered sufficient to examine feasibility in the IRF environment and was similar to prior feasibility studies (Birkenmeier et al., 2010; Combs, Kelly, Barton, Ivaska, & Nowak, 2010; Page, Levine, & Leonard, 2005; Page, Levine, Sisto, & Johnston, 2001). Recruitment occurred through informing inpatient staff occupational therapists about the study and the criteria for participation. During the recruitment period (November 2012 to April 2013), 123 people with stroke were admitted. Staff occupational therapists referred 22 patients to the study during that time, and 15 were enrolled. Of the 15 patients enrolled, 3 failed their initial screen and were rescreened within 7 days for later enrollment. We closely monitored individuals who failed their initial screen because changes in the motor system can occur at a fast rate during the early phase of stroke rehabilitation. As increased UE movement emerged, participants were then rescreened and enrolled as appropriate.
Measures
Feasibility measures were taken during all sessions by the treating therapist. Outcome measures were administered by trained assessors at the time points shown in Figure 1. Because all participants received the intervention, the assessors could not be blinded. The primary assessor did not deliver treatment. All assessment sessions were video recorded and periodically reviewed by the research team to ensure accurate scoring.
Feasibility Measures.
A key measure of feasibility was the number of repetitions achieved during single sessions. A single repetition was characterized by a combination of most or all of the UE movement components: reaching, grasping or manipulating, transporting, and releasing (Birkenmeier et al., 2010). In calculating the average number of repetitions per session, we excluded the counts from the first two sessions because participants were acclimating to performing large amounts of practice. A second measure, time in active practice, captured the amount of training without the confound of defining repetitions and was defined as the number of minutes per session during which the person was actively practicing tasks. Rest breaks and setup time were not included. Consistent with calculating average repetitions, total time in task practice excluded the first two sessions. To document participant effort, fatigue ratings were recorded on completion of each session using the 0–10-point Stanford Fatigue Visual Numeric Scale (Stanford Patient Education Research Center, n.d.). Pain scores were measured pretreatment and posttreatment using a standard 0–10 numeric rating scale or the Wong-Baker Faces Pain Rating Scale (Keck, Gerkensmeyer, Joyce, & Schade, 1996). Feasibility was also assessed by the number of sessions attempted and attended for each participant.
Outcome Measures.
The primary outcome measure used was the Action Research Arm Test (ARAT; Lang, Edwards, Birkenmeier, & Dromerick, 2008), a criterion-rated, 19-item, activity-based assessment of the UE. Using ordinal scoring, the patient is scored on a scale of 0 (unable to complete) to 3 (completes with normal movement) with a maximum score of 57 indicating normal performance. The interrater reliability (.95–.98), test–retest reliability (.99), and construct validity (.92–.95) of the ARAT are well-established (Lin et al., 2009; Platz et al., 2005; Van der Lee et al., 2001), and this measure has been shown to be responsive to change early after stroke (Beebe & Lang, 2009; Lang, Wagner, Dromerick, & Edwards, 2006).
Impairment-level outcomes included grip and pinch strength measured with a Jamar hydraulic handheld dynamometer and pinch gauge (Patterson Medical, Warrenville, IL). Measurement of grip strength with a dynamometer has demonstrated excellent test–retest reliability, averaging >.80 (Mathiowetz, Weber, Volland, & Kashman, 1984; Roberts et al., 2011) and excellent interrater reliability, >.97 (Mathiowetz et al., 1984; Peolsson, Hedlund, & Oberg, 2001). Pinch was measured during a three-jaw-chuck pinch and demonstrated interrater reliability (.97–.99) and test–retest reliability (.74–.83; Mathiowetz et al., 1984).
The FIM (Ottenbacher, Hsu, Granger, & Fiedler, 1996) was an additional outcome measure to quantify independence in daily activities. The FIM has excellent consistency between raters (median interrater reliability = .95), demonstrates test–retest reliability (.92–.95), and is used as part of the Uniform Data System for Medical Rehabilitation. Scores for the five self-care domains (eating, grooming, upper body dressing, lower body dressing, and bathing) were recorded and summed as an upper-extremity FIM (UE–FIM) score (Dromerick et al., 2006; Lang et al., 2006; Lang, Wagner, Edwards, & Dromerick, 2007).
In combination with standard clinical assessments of function, emerging evidence supports the use of accelerometry as an objective measure for monitoring and recording UE use (Lang, Bland, Bailey, Schaefer, & Birkenmeier, 2013). Accelerometers (ActiGraph GT3×+; Actigraph LLC, Pensacola, FL) resembling the size of wristwatches measure movement in terms of acceleration (Lang et al., 2013). The test–retest reliability (.82–.94), construct validity (.93), and convergent validity (.53–.94) of accelerometers has been established (Lang et al., 2013; Uswatte et al., 2005; Uswatte, Giuliani, et al., 2006; Welk, Schaben, & Morrow, 2004). Additionally, intrarater reliability has been established when the same accelerometer unit is used (Welk et al., 2004). Accelerometers were placed on both wrists of each participant and worn for 24 hr at the time of study enrollment and for 24 hr before discharge from the IRF. Using the recorded activity counts, a use ratio was calculated to express the amount of use of the affected side compared with the nonaffected side (Uswatte et al., 2005). Use ratio values in community-dwelling adults are 0.95 ± 0.06 (mean ± standard deviation), indicating that the upper extremities are most frequently used together during daily life (Bailey & Lang, 2014).
Intervention
The intervention was an individually tailored UE training protocol (Birkenmeier et al., 2010) provided 60 min/day, 4 days/wk during occupational therapy for the duration of the IRF admission. All treating therapists were trained in the protocol and monitored to ensure fidelity. In this IRF, patients receive 6 days/wk of occupational therapy services. On the remaining 2 days, participants received interventions related to ADLs to ensure that basic self-care deficits were being addressed. ADL tasks, however, were regularly incorporated into task-specific training sessions. Participants were encouraged to incorporate the paretic UE into functional activity outside of the intervention hour but not in a repetitive manner. For example, participants were encouraged to use the paretic hand to assist with prepping a toothbrush with toothpaste or to answer a cell phone.
Treatment activities were individualized, graded, and progressed for each participant. At enrollment, the treating occupational therapist and the participants discussed their meaningful activities to generate an occupational profile (American Occupational Therapy Association, 2008). They selected three activities to be used during the treatment sessions. If communication was a challenge (e.g., aphasia), the occupational therapist obtained an occupational profile from family members or friends. The goal for each treatment session was to achieve ≥300 repetitions in 1 hr through supervised, massed practice of the three selected activities (≥100 repetitions per activity).
Following the principle of shaping (Taub et al., 1994, 2013; Uswatte, Taub, Morris, Barman, & Crago, 2006), we graded and progressed activities to challenge each person without over- or underwhelming their motor capabilities, using established grading criteria (Birkenmeier et al., 2010). Examples of increasing task difficulty include increasing the height or distance to reach or place an item, increasing the weight or size of items, and changing the physical position of the participant (e.g., standing vs. sitting). Given the acuity of illness in the IRF, participants were provided rest breaks on request.
Statistical Analysis
Statistical analyses were completed with SPSS Version 21 software (IBM, Armonk, NY). Descriptive statistics were generated on feasibility measures. Data on outcome measures were normally distributed as indicated by Shapiro-Wilks tests. We used paired t tests to examine change in outcome measures between admission and discharge for all participants. For participants with 1-mo follow-up data, we ran an additional paired t test to look for changes between discharge and follow-up. We chose this method over the typical repeated-measures analysis of variance across three time points to capitalize on available data. The criterion for significance was set at p < .05. Pearson correlation coefficients were used to examine relationships between feasibility and outcome measures. Values in the text are means ± standard deviations unless otherwise indicated.
Results
Fifteen participants completed the study, and 11 were available for 1-mo follow-up assessments. General characteristics of all participants are provided in Table 1. Participants’ average age was 59 yr, and 80% of our sample size was male. Most participants were not enrolled immediately on admittance to the IRF: The average study enrollment length was 19 days, and the average stay length in the IRF was 29 days. Most participants had two or more comorbidities that could have limited their ability to participate in the intervention.
Table 1.
Participant Characteristics
| Participant Number | Age | Gender | Dominant/Affected UE | Duration Poststroke (days) | LOE in Study (days) | LOS in IRF (days) | Baseline ARAT Score | Comorbidities |
| 1 | 78 | M | R/L | 42 | 6 | 45 | 16 | CAD, A-fib, GERD |
| 2 | 34 | F | R/L | 11 | 21 | 28 | 14 | — |
| 3 | 89 | F | R/R | 12 | 12 | 20 | 39 | HTN, arthritis, unspecified cardiac |
| 4 | 68 | F | L/L | 10 | 29 | 45 | 48 | HTN, GERD, OB, unspecified cardiac |
| 5 | 48 | M | R/R | 23 | 10 | 26 | 26 | HTN, CA |
| 6 | 28 | M | R/L | 19 | 14 | 28 | 43 | HTN, diabetes, unspecified cardiac |
| 7 | 66 | M | R/R | 18 | 15 | 17 | 25 | HTN, GERD, TD, unspecified cardiac |
| 8 | 58 | M | R/L | 22 | 22 | 29 | 16 | HTN, diabetes, gout, arthritis |
| 9 | 53 | M | R/R | 7 | 20 | 21 | 35 | A-fib, HTN, CHF, HIV |
| 10 | 54 | M | R/R | 14 | 21 | 29 | 35 | CA, CHF, TM, renal failure, Sz, APLS |
| 11 | 54 | M | L/L | 10 | 23 | 28 | 31 | HTN, diabetes |
| 12 | 58 | M | R/L | 24 | 11 | 12 | 12 | HTN |
| 13 | 57 | M | L/R | 10 | 44 | 47 | 8 | HTN, RA, polio |
| 14 | 71 | M | R/L | Unknown | 20 | 37 | 29 | HTN, diabetes, alcohol dependence |
| 15 | 73 | M | R/R | 69 | 30 | 34 | 5 | HTN, unspecified cardiac |
| Mean or % | 59 | 80% M | 46% dominant | 20 | 19 | 29 | 25.47 |
Note. Duration Poststroke = No. of days poststroke to enrollment in the study. A-fib = atrial fibrillation; APLS = antiphospholipid antibody syndrome; ARAT = Action Research Arm Test; CA = cancer; CAD = coronary artery disease; CHF = congestive heart failure; F = female; GERD = gastroesophageal reflux disease; HIV = human immunodeficiency virus; HTN = hypertension; IRF = inpatient rehabilitation facility; L = left; LOE = length of enrollment; LOS = length of stay; M = male; OB = obesity; R = right; RA = rheumatoid arthritis; Sz = seizures; TD = thyroid disorder; TM = transverse myelitis.
Feasibility Data
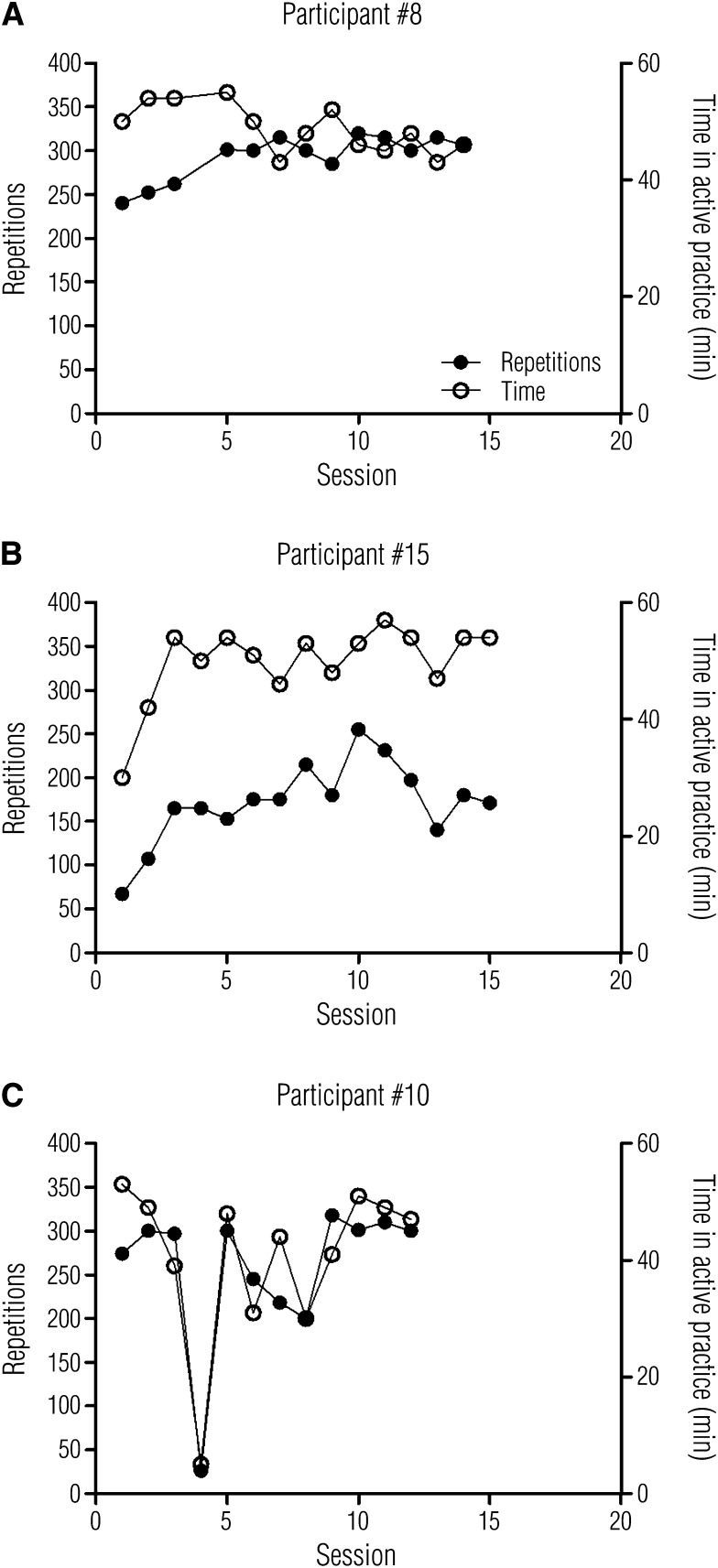
No participants were withdrawn or dropped out during the intervention. Individual examples of the number of repetitions and time in active practice across sessions are shown in Figure 2. These individual examples were selected to highlight participants who performed at and below the study means and also to illustrate the medical complexities and barriers to service delivery often found in an IRF. One participant (#8) increased the number of repetitions over the first few sessions, achieving an average of 302 after the first two sessions (Figure 2A). The number of minutes of active practice started higher and was reduced slightly as the intervention progressed. Another participant (#15) achieved fewer repetitions per session (average of 183 after the first two sessions) but spent nearly the whole hour in active practice (Figure 2B). Another participant’s (#10) treatment was disrupted by a hospital acquired infection (C. difficile; Figure 2C). In many sessions, this participant (#10) achieved a high number of repetitions and spent a substantial amount of the hour in active practice. In other sessions, his treatment was limited by toileting needs, fatigue, and frustration.
Figure 2.
Individual examples of number of repetitions achieved and time spent in active practice per session over the course of the intervention.
The average number of repetitions achieved per session was 289 ± 35, 95% confidence interval (CI) [280, 299]. The total repetitions achieved across the intervention ranged from 1,207 to 5,963, with an average of 2,956 ± 1,197. Time spent in active task practice per session was 47 ± 3.7 min, 95% CI [46.1, 48.0]. Effort to achieve the desired number of repetitions was high, as indicated by an average fatigue score of 6.5 ± 1.2, 95% CI [6.2, 6.8] on the 0–10 point scale. Pain scores at the beginning of each session were 1.6 ± 1.6, 95% CI [1.2, 2.1] and after each session were 2.7 ± 2.7, 95% CI [2.0, 3.4]. The average change in pain per session was 1.1 points (different pre- and postsession, p = .02).
The number of sessions attempted was 11.5 ± 5.3, 95% CI [10.2, 12.9], and the number of sessions attended was 10.9 ± 4.9, 95% CI [9.7, 12.2], indicating that participants did not often miss scheduled sessions. The highest number of sessions attempted and attended was 24 (Participant 13), whereas the lowest number of both attempted and attended sessions was four (Participant 5).
Preliminary Outcomes
Table 2 presents baseline, discharge, and 1-mo data scores for all outcome measures and their statistical significance. As expected at this early period poststroke, improvement in ARAT, grip, pinch, UE–FIM items, and the use ratio from baseline to discharge was found. Eleven of the 15 participants were available for 1-mo follow-up assessments. Participants who were unavailable (1 lived out of state, 2 were unreachable, 1 died) were those who improved the least during the IRF stay. Thus, the major improvements seen from discharge to 1 mo (last column of Table 2) occurred in those who would be most likely to experience such changes.
Table 2.
Preliminary Outcome Data
| Score |
p |
||||
| Measurea | Baseline (n = 15) | Discharge (n = 15) | 1 mo (n = 11) | Δ Dischargeb | Δ 1 moc |
| ARAT | 25 ± 13 | 35 ± 11 | 40 ± 8.2 | .000 | .018 |
| Grip strength (in kg) | 7.5 ± 5.9 | 14 ± 11 | 19 ± 14 | .007 | .052 |
| Pinch strength (in kg) | 0.87 ± 1.3 | 2.4 ± 1.9 | 3.7 ± 2.2 | .001 | .002 |
| UE–FIM | 20 ± 4.5 | 26 ± 0.98 | 30 ± 2.9 | .000 | .009 |
| Use ratiod | 0.47 ± 0.14 | 0.68 ± 0.21 | — | .005 | — |
Note. ARAT = Action Research Arm Test; FIM = Functional Independence Measure; UE = upper extremity; — = not measured.
ARAT, 0–57-point scale; UE–FIM, 0–35-point scale; higher numbers indicate better results on all scales. Values reported for scores are mean ± standard deviation. bp reported on change from baseline to discharge for all measures, n = 15. cp reported on change from discharge to 1-mo postdischarge, n = 11. dFrom accelerometers, normal values in persons without stroke are 0.95 ± 0.06.
Three important relationships were present in the data. First, there was a moderate negative relationship between time to enrollment in the study and baseline ARAT, r = −.54, p = .04, indicating that those who were enrolled later had worse UE motor capabilities at baseline. Second, no relationship was found between total repetitions achieved and baseline ARAT score, r = .19, p = .48, indicating that patients with different levels of UE capabilities could engage in the high-repetition intervention. Finally, a moderate-to-good relationship was revealed between total repetitions and change in ARAT, r = .58, p = .02, indicating that higher doses were associated with better outcomes.
Discussion
These data show that it is feasible to deliver a substantially higher number of repetitions of individually tailored, progressive, task-specific training in the inpatient setting than what is currently delivered in routine clinical practice (Lang et al., 2009). The available therapy time was maximized, indicated by hundreds of repetitions and long durations in active task practice per session. The average amount of time spent in task practice for this study was equal to the total number of minutes typically spent in occupational therapy in the IRF setting (Harris et al., 2009). The number of sessions attempted or attended varied as a result of differing lengths of stay. Sessions were rarely missed despite high fatigue scores. All participants improved, including on the UE–FIM items, indicating that this intervention did not detract from other critical inpatient priorities of ADL retraining.
Feasibility
The finding of feasibility without detracting from ADL retraining is important to clinicians trying to implement higher doses of therapy within the IRF setting and for clinical scientists wanting to study the potential benefits of higher doses. We do not know whether the higher doses achieved were optimal; the target of 300 repetitions was somewhat arbitrary. No data are available on the optimal dose of task-specific training needed to promote motor recovery for UE paresis, although a trial is under way (NCT01146379).
As anticipated, reports of fatigue were high. The substantial effort expended, however, did not limit overall participation, as indicated by a long duration of time spent in task practice and nearly identical values for the average number of sessions attempted and the average number of sessions attended for each participant.
Pain scores changed significantly from pre- to postintervention. Participants reported more pain at the end than at the beginning of sessions. The magnitude of the scores was, however, relatively low. Even by the end of sessions, the pain scores were mild (<3/10) and substantially lower than pain values reported in the hemiparetic shoulder pain literature (Castiglione, Bagnato, Boccagni, Romano, & Galardi, 2011; Ratmansky, Defrin, & Soroker, 2012). Pain did not limit participation, evidenced by the similar number of sessions attempted and attended. By visual inspection, individual participant pain scores showed that pain reports had returned to baseline before the next scheduled session.
Preliminary Outcomes
Participants improved on all outcome measures from baseline to discharge and in the subset followed up 1 mo later. Change scores on the ARAT are similar to published minimally clinically important difference values during this early time poststroke (Lang et al., 2008) of 12 to 17 points for the dominant and nondominant sides from baseline to 1 mo. Participants were discharged more independent in self-care than at admission (UE–FIM score). This finding is noteworthy because it shows that implementing a high-repetition, individually tailored, progressive, task-specific training intervention 4 days/wk did not detract from the importance of ADL retraining in the inpatient setting.
Participants also improved use of the affected UE outside of scheduled therapy time, indicated by the change in use ratio. At discharge, the use of the affected limb was still limited (0.68 ± 0.21) but closer to typical values (0.95 ± 0.06) in people without stroke (Bailey & Lang, 2014).
Finally, the moderate-to-good relationship between total repetitions and change in ARAT scores is consistent with similar published correlations (Birkenmeier et al., 2010; Moore, Roth, Killian, & Hornby, 2010), indicating that higher doses are associated with better outcomes. Finally, we were able to individualize and deliver this intervention to various UE abilities, indicated by the lack of a relationship between baseline ARAT scores and total repetitions achieved.
Limitations and Future Research
Several limitations need to be considered. First, our sample size was small, limiting not only the generalization of results but also the ability to use further statistical analysis to examine individual factors within the sample. Future studies with larger sample sizes are needed to explore relationships between other stroke-related impairments, individual comorbidities, and their influence on feasibility and outcome.
Second, a control group was absent. The functional gains are likely because of some combination of natural recovery and provided rehabilitation services. Future studies are needed to determine how much improvement might be because of the high-repetition intervention. A larger comparison study evaluating the overall efficacy of an individually tailored, progressive, high-repetition, task-specific UE training intervention compared with standard occupational therapy would be beneficial.
Third, we did not control the number of sessions experienced by each participant. The range of sessions attended was large (4–24). Although it is likely that four sessions are too few, it is not possible to determine whether 24 sessions are ideal. Thus, these data should be viewed as the beginning of an exploration of how the dose of movement practice early after stroke might be manipulated to optimize functional outcomes.
Implications for Occupational Therapy Practice
Patients with stroke in the IRF setting can achieve hundreds of repetitions of task-specific training in 1-hr therapy sessions. Using an established protocol, each task can be selected from the patient’s occupational profile and individualized, graded, and progressed to various UE capabilities. This intervention can be delivered within the confines of current service delivery (i.e., no additional sessions were added to the participant’s regular therapy schedule).
The results of this study have the following implications for occupational therapy practice:
Patients with UE paresis after a stroke, staying in an IRF, can achieve hundreds of repetitions of task-specific training.
As is expected at this level of rehabilitation, patients improved on all outcome measures after engaging in this intervention.
No additional occupational therapy sessions were added to the normal 3-hr/day therapy schedule for the IRF setting.
Engaging in a high-repetition, task-specific intervention does not detract from the critical inpatient priority of ADL retraining.
Conclusion
It is feasible to deliver hundreds of repetitions of task-specific training for the paretic UE within the confines of current IRF practice. The intervention was delivered as part of regularly scheduled therapy, no additional occupational therapy sessions were added, and no special equipment was required. Engaging in an individually tailored, progressive, high-repetition, task-specific UE training intervention does not detract from other critical inpatient priorities such as ADL retraining.
Acknowledgments
We acknowledge Marghuretta Bland, Jill Seelbach, and Brittany Hill for their ongoing contributions to data acquisition and processing. We also acknowledge Caitlin Doman and Kathleen Froehlich for their involvement in data acquisition and administering outcome measures for this study. Support for this project comes from a National Institutes of Health grant no. R01 HD068290 to Catherine Lang and from a grant from the Buchanan Family Fellowship to Kimberly Waddell.
References
- American Occupational Therapy Association. (2008). Occupational therapy practice framework: Domain and process (2nd ed.). American Journal of Occupational Therapy,, 62, 625–683 http://dx.doi.org/10.5014/ajot.62.6.625 [DOI] [PubMed] [Google Scholar]
- Bailey, R. R., & Lang, C. E. (2014). Upper-limb activity in adults: Referent values using accelerometry. Journal of Rehabilitation Research and Development, 50, 1213–1222 http://dx.doi.org/10.1682/JRRD.2012.12.0222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker, R. N., & Brauer, S. G. (2005). Upper limb recovery after stroke: The stroke survivors’ perspective. Disability and Rehabilitation, 27, 1213–1223 http://dx.doi.org/10.1080/09638280500075717 [DOI] [PubMed] [Google Scholar]
- Barzel, A., Liepert, J., Haevernick, K., Eisele, M., Ketels, G., Rijntjes, M., & van den Bussche, H. (2009). Comparison of two types of constraint-induced movement therapy in chronic stroke patients: A pilot study. Restorative Neurology and Neuroscience, 27, 673–680 http://dx.doi.org/10.3233/RNN-2009-0524 [DOI] [PubMed] [Google Scholar]
- Beebe, J. A., & Lang, C. E. (2009). Relationships and responsiveness of six upper extremity function tests during the first six months of recovery after stroke. Journal of Neurologic Physical Therapy, 33, 96–103 http://dx.doi.org/10.1097/NPT.0b013e3181a33638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt, J., Chan, J., Nicola, I., & Collier, J. M. (2007). Little therapy, little physical activity: Rehabilitation within the first 14 days of organized stroke unit care. Journal of Rehabilitation Medicine, 39, 43–48 http://dx.doi.org/10.2340/16501977-0013 [DOI] [PubMed] [Google Scholar]
- Biernaskie, J., Chernenko, G., & Corbett, D. (2004). Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. Journal of Neuroscience, 24, 1245–1254 http://dx.doi.org/10.1523/JNEUROSCI.3834-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeier, R. L., Prager, E. M., & Lang, C. E. (2010). Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: A proof-of-concept study. Neurorehabilitation and Neural Repair, 24, 620–635 http://dx.doi.org/10.1177/1545968310361957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd, L. A., Vidoni, E. D., & Wessel, B. D. (2010). Motor learning after stroke: Is skill acquisition a prerequisite for contralesional neuroplastic change? Neuroscience Letters, 482, 21–25 http://dx.doi.org/10.1016/j.neulet.2010.06.082 [DOI] [PubMed] [Google Scholar]
- Brott, T., Adams, H. P., Jr., Olinger, C. P., Marler, J. R., Barsan, W. G., Biller, J., …Hertzberg, V. (1989). Measurements of acute cerebral infarction: A clinical examination scale. Stroke, 20, 864–870 http://dx.doi.org/10.1161/01.STR.20.7.864 [DOI] [PubMed] [Google Scholar]
- Castiglione, A., Bagnato, S., Boccagni, C., Romano, M. C., & Galardi, G. (2011). Efficacy of intra-articular injection of botulinum toxin type A in refractory hemiplegic shoulder pain. Archives of Physical Medicine and Rehabilitation, 92, 1034–1037 http://dx.doi.org/10.1016/j.apmr.2011.01.015 [DOI] [PubMed] [Google Scholar]
- Collin, C., & Wade, D. (1990). Assessing motor impairment after stroke: A pilot reliability study. Journal of Neurology, Neurosurgery and Psychiatry, 53, 576–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs, S. A., Kelly, S. P., Barton, R., Ivaska, M., & Nowak, K. (2010). Effects of an intensive, task-specific rehabilitation program for individuals with chronic stroke: A case series. Disability and Rehabilitation, 32, 669–678 http://dx.doi.org/10.3109/09638280903242716 [DOI] [PubMed] [Google Scholar]
- Dobkin, B. H. (2005). Clinical practice: Rehabilitation after stroke. New England Journal of Medicine, 352, 1677–1684 http://dx.doi.org/10.1056/NEJMcp043511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromerick, A. W., Lang, C. E., Birkenmeier, R., Hahn, M. G., Sahrmann, S. A., & Edwards, D. F. (2006). Relationships between upper-limb functional limitation and self-reported disability 3 months after stroke. Journal of Rehabilitation Research and Development, 43, 401–408 http://dx.doi.org/10.1682/JRRD.2005.04.0075 [DOI] [PubMed] [Google Scholar]
- Dromerick, A. W., Lang, C. E., Birkenmeier, R. L., Wagner, J. M., Miller, J. P., Videen, T. O., …Edwards, D. F. (2009). Very Early Constraint-Induced Movement during Stroke Rehabilitation (VECTORS): A single-center RCT. Neurology, 73, 195–201 http://dx.doi.org/10.1212/WNL.0b013e3181ab2b27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, P. W., Goldstein, L. B., Horner, R. D., Landsman, P. B., Samsa, G. P., & Matchar, D. B. (1994). Similar motor recovery of upper and lower extremities after stroke. Stroke, 25, 1181–1188 http://dx.doi.org/10.1161/01.STR.25.6.1181 [DOI] [PubMed] [Google Scholar]
- Foulkes, M. A., Wolf, P. A., Price, T. R., Mohr, J. P., & Hier, D. B. (1988). The Stroke Data Bank: Design, methods, and baseline characteristics. Stroke, 19, 547–554 http://dx.doi.org/10.1161/01.STR.19.5.547 [DOI] [PubMed] [Google Scholar]
- Han, C., Wang, Q., Meng, P. P., & Qi, M. Z. (2013). Effects of intensity of arm training on hemiplegic upper extremity motor recovery in stroke patients: A randomized controlled trial. Clinical Rehabilitation, 27, 75–81 http://dx.doi.org/10.1177/0269215512447223 [DOI] [PubMed] [Google Scholar]
- Harris, J. E., Eng, J. J., Miller, W. C., & Dawson, A. S. (2009). A self-administered Graded Repetitive Arm Supplementary Program (GRASP) improves arm function during inpatient stroke rehabilitation: A multi-site randomized controlled trial. Stroke, 40, 2123–2128 http://dx.doi.org/10.1161/STROKEAHA.108.544585 [DOI] [PubMed] [Google Scholar]
- Horn, S. D., DeJong, G., Smout, R. J., Gassaway, J., James, R., & Conroy, B. (2005). Stroke rehabilitation patients, practice, and outcomes: Is earlier and more aggressive therapy better? Archives of Physical Medicine and Rehabilitation, 86(Suppl. 2), S101–S114 http://dx.doi.org/10.1016/j.apmr.2005.09.016 [DOI] [PubMed] [Google Scholar]
- Keck, J. F., Gerkensmeyer, J. E., Joyce, B. A., & Schade, J. G. (1996). Reliability of the faces and word descriptor scales to measure procedural pain. Journal of Pediatric Nursing, 11, 368–374 http://dx.doi.org/10.1016/S0882-5963(96)80081-9 [DOI] [PubMed] [Google Scholar]
- Krakauer, J. W. (2006). Motor learning: Its relevance to stroke recovery and neurorehabilitation. Current Opinion in Neurology, 19, 84–90 http://dx.doi.org/10.1097/01.wco.0000200544.29915.cc [DOI] [PubMed] [Google Scholar]
- Lang, C. E., Bland, M. D., Bailey, R. R., Schaefer, S. Y., & Birkenmeier, R. L. (2013). Assessment of upper extremity impairment, function, and activity after stroke: Foundations for clinical decision making. Journal of Hand Therapy, 26, 104–114, quiz 115 http://dx.doi.org/10.1016/j.jht.2012.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, C. E., Edwards, D. F., Birkenmeier, R. L., & Dromerick, A. W. (2008). Estimating minimal clinically important differences of upper-extremity measures early after stroke. Archives of Physical Medicine and Rehabilitation, 89, 1693–1700 http://dx.doi.org/10.1016/j.apmr.2008.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, C. E., Macdonald, J. R., Reisman, D. S., Boyd, L., Jacobson Kimberley, T., Schindler-Ivens, S. M., …Scheets, P. L. (2009). Observation of amounts of movement practice provided during stroke rehabilitation. Archives of Physical Medicine and Rehabilitation, 90, 1692–1698 http://dx.doi.org/10.1016/j.apmr.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, C. E., Wagner, J. M., Dromerick, A. W., & Edwards, D. F. (2006). Measurement of upper-extremity function early after stroke: Properties of the Action Research Arm Test. Archives of Physical Medicine and Rehabilitation, 87, 1605–1610 http://dx.doi.org/10.1016/j.apmr.2006.09.003 [DOI] [PubMed] [Google Scholar]
- Lang, C. E., Wagner, J. M., Edwards, D. F., & Dromerick, A. W. (2007). Upper extremity use in people with hemiparesis in the first few weeks after stroke. Journal of Neurologic Physical Therapy, 31, 56–63 http://dx.doi.org/10.1097/NPT.0b013e31806748bd [DOI] [PubMed] [Google Scholar]
- Legg, L., Drummond, A., Leonardi-Bee, J., Gladman, J. R., Corr, S., Donkervoort, M., …Langhorne, P. (2007). Occupational therapy for patients with problems in personal activities of daily living after stroke: Systematic review of randomised trials. BMJ, 335, 922 http://dx.doi.org/10.1136/bmj.39343.466863.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, J. H., Hsu, M. J., Sheu, C. F., Wu, T. S., Lin, R. T., Chen, C. H., & Hsieh, C. L. (2009). Psychometric comparisons of 4 measures for assessing upper-extremity function in people with stroke. Physical Therapy, 89, 840–850 http://dx.doi.org/10.2522/ptj.20080285 [DOI] [PubMed] [Google Scholar]
- Mathiowetz, V., Weber, K., Volland, G., & Kashman, N. (1984). Reliability and validity of grip and pinch strength evaluations. Journal of Hand Surgery, 9, 222–226 http://dx.doi.org/10.1016/S0363-5023(84)80146-X [DOI] [PubMed] [Google Scholar]
- Moore, J. L., Roth, E. J., Killian, C., & Hornby, T. G. (2010). Locomotor training improves daily stepping activity and gait efficiency in individuals poststroke who have reached a “plateau” in recovery. Stroke, 41, 129–135 http://dx.doi.org/10.1161/STROKEAHA.109.563247 [DOI] [PubMed] [Google Scholar]
- Ottenbacher, K. J., Hsu, Y., Granger, C. V., & Fiedler, R. C. (1996). The reliability of the Functional Independence Measure: A quantitative review. Archives of Physical Medicine and Rehabilitation, 77, 1226–1232 http://dx.doi.org/10.1016/S0003-9993(96)90184-7 [DOI] [PubMed] [Google Scholar]
- Oujamaa, L., Relave, I., Froger, J., Mottet, D., & Pelissier, J. Y. (2009). Rehabilitation of arm function after stroke: Literature review. Annals of Physical and Rehabilitation Medicine, 52, 269–293 http://dx.doi.org/10.1016/j.rehab.2008.10.003 [DOI] [PubMed] [Google Scholar]
- Page, S. J., Levine, P., & Leonard, A. C. (2005). Modified constraint-induced therapy in acute stroke: A randomized controlled pilot study. Neurorehabilitation and Neural Repair, 19, 27–32 http://dx.doi.org/10.1177/1545968304272701 [DOI] [PubMed] [Google Scholar]
- Page, S. J., Levine, P., Sisto, S., & Johnston, M. V. (2001). A randomized efficacy and feasibility study of imagery in acute stroke. Clinical Rehabilitation, 15, 233–240 http://dx.doi.org/10.1191/026921501672063235 [DOI] [PubMed] [Google Scholar]
- Peolsson, A., Hedlund, R., & Oberg, B. (2001). Intra- and inter-tester reliability and reference values for hand strength. Journal of Rehabilitation Medicine, 33, 36–41 http://dx.doi.org/10.1080/165019701300006524 [DOI] [PubMed] [Google Scholar]
- Platz, T., Pinkowski, C., van Wijck, F., Kim, I. H., di Bella, P., & Johnson, G. (2005). Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: A multicentre study. Clinical Rehabilitation, 19, 404–411 http://dx.doi.org/10.1191/0269215505cr832oa [DOI] [PubMed] [Google Scholar]
- Plautz, E. J., Milliken, G. W., & Nudo, R. J. (2000). Effects of repetitive motor training on movement representations in adult squirrel monkeys: Role of use versus learning. Neurobiology of Learning and Memory, 74, 27–55 http://dx.doi.org/10.1006/nlme.1999.3934 [DOI] [PubMed] [Google Scholar]
- Ratmansky, M., Defrin, R., & Soroker, N. (2012). A randomized controlled study of segmental neuromyotherapy for post-stroke hemiplegic shoulder pain. Journal of Rehabilitation Medicine, 44, 830–836 http://dx.doi.org/10.2340/16501977-1021 [DOI] [PubMed] [Google Scholar]
- Roberts, H. C., Denison, H. J., Martin, H. J., Patel, H. P., Syddall, H., Cooper, C., & Sayer, A. A. (2011). A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age and Ageing, 40, 423–429 http://dx.doi.org/10.1093/ageing/afr051 [DOI] [PubMed] [Google Scholar]
- Smania, N., Gandolfi, M., Paolucci, S., Iosa, M., Ianes, P., Recchia, S., …Farina, S. (2012). Reduced-intensity modified constraint-induced movement therapy versus conventional therapy for upper extremity rehabilitation after stroke: A multicenter trial. Neurorehabilitation and Neural Repair, 26, 1035–1045 http://dx.doi.org/10.1177/1545968312446003 [DOI] [PubMed] [Google Scholar]
- Stanford Patient Education Research Center. (n.d.). Stanford Fatigue Visual Numeric Scale. Retrieved from http://patienteducation.stanford.edu/research/vnsfatigue.html [Google Scholar]
- Taub, E., Crago, J. E., Burgio, L. D., Groomes, T. E., Cook, E. W., 3rd, DeLuca, S. C., & Miller, N. E. (1994). An operant approach to rehabilitation medicine: Overcoming learned nonuse by shaping. Journal of the Experimental Analysis of Behavior, 61, 281–293 http://dx.doi.org/10.1901/jeab.1994.61-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub, E., Uswatte, G., Mark, V. W., Morris, D. M., Barman, J., Bowman, M. H., …Bishop-McKay, S. (2013). Method for enhancing real-world use of a more affected arm in chronic stroke: Transfer package of constraint-induced movement therapy. Stroke, 44, 1383–1388 http://dx.doi.org/10.1161/STROKEAHA.111.000559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treger, I., Aidinof, L., Lehrer, H., & Kalichman, L. (2012). Modified constraint-induced movement therapy improved upper limb function in subacute poststroke patients: A small-scale clinical trial. Topics in Stroke Rehabilitation, 19, 287–293 http://dx.doi.org/10.1310/tsr1904-287 [DOI] [PubMed] [Google Scholar]
- Uswatte, G., Foo, W. L., Olmstead, H., Lopez, K., Holand, A., & Simms, L. B. (2005). Ambulatory monitoring of arm movement using accelerometry: An objective measure of upper-extremity rehabilitation in persons with chronic stroke. Archives of Physical Medicine and Rehabilitation, 86, 1498–1501 http://dx.doi.org/10.1016/j.apmr.2005.01.010 [DOI] [PubMed] [Google Scholar]
- Uswatte, G., Giuliani, C., Winstein, C., Zeringue, A., Hobbs, L., & Wolf, S. L. (2006). Validity of accelerometry for monitoring real-world arm activity in patients with subacute stroke: Evidence from the Extremity Constraint-Induced Therapy Evaluation Trial. Archives of Physical Medicine and Rehabilitation, 87, 1340–1345 http://dx.doi.org/10.1016/j.apmr.2006.06.006 [DOI] [PubMed] [Google Scholar]
- Uswatte, G., Taub, E., Morris, D., Barman, J., & Crago, J. (2006). Contribution of the shaping and restraint components of constraint-induced movement therapy to treatment outcome. NeuroRehabilitation, 21, 147–156 [PubMed] [Google Scholar]
- Van der Lee, J. H., De Groot, V., Beckerman, H., Wagenaar, R. C., Lankhorst, G. J., & Bouter, L. M. (2001). The intra- and interrater reliability of the Action Research Arm Test: A practical test of upper extremity function in patients with stroke. Archives of Physical Medicine and Rehabilitation, 82, 14–19 http://dx.doi.org/10.1053/apmr.2001.18668 [DOI] [PubMed] [Google Scholar]
- Welk, G. J., Schaben, J. A., & Morrow, J. R., Jr. (2004). Reliability of accelerometry-based activity monitors: A generalizability study. Medicine and Science in Sports and Exercise, 36, 1637–1645 [PubMed] [Google Scholar]
- Wilkinson, P. R., Wolfe, C. D. A., Warburton, F. G., Rudd, A. G., Howard, R. S., Ross-Russell, R. W., & Beech, R. R. (1997). A long-term follow-up of stroke patients. Stroke, 28, 507–512 http://dx.doi.org/10.1161/01.STR.28.3.507 [DOI] [PubMed] [Google Scholar]
- Winstein, C. J., Rose, D. K., Tan, S. M., Lewthwaite, R., Chui, H. C., & Azen, S. P. (2004). A randomized controlled comparison of upper-extremity rehabilitation strategies in acute stroke: A pilot study of immediate and long-term outcomes. Archives of Physical Medicine and Rehabilitation, 85, 620–628 http://dx.doi.org/10.1016/j.apmr.2003.06.027 [DOI] [PubMed] [Google Scholar]
- Wolf, S. L., Winstein, C. J., Miller, J. P., Taub, E., Uswatte, G., Morris, D., …, Nichols-Larsen, D.; EXCITE Investigators. (2006). Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: The EXCITE randomized clinical trial. JAMA, 296, 2095–2104 http://dx.doi.org/10.1001/jama.296.17.2095 [DOI] [PubMed] [Google Scholar]