Abstract
The β secretase, widely known as β-site amyloid precursor protein cleaving enzyme 1 (BACE1), initiates the production of the toxic amyloid β (Aβ) that plays a crucial early part in Alzheimer’s disease pathogenesis. BACE1 is a prime therapeutic target for lowering cerebral Aβ concentrations in Alzheimer’s disease, and clinical development of BACE1 inhibitors is being intensely pursued. Although BACE1 inhibitor drug development has proven challenging, several promising BACE1 inhibitors have recently entered human clinical trials. The safety and efficacy of these drugs are being tested at present in healthy individuals and patients with Alzheimer’s disease, and will soon be tested in individuals with presymptomatic Alzheimer’s disease. Although hopes are high that BACE1 inhibitors might be efficacious for the prevention or treatment of Alzheimer’s disease, concerns have been raised about potential mechanism-based side-effects of these drugs. The potential of therapeutic BACE1 inhibition might prove to be a watershed in the treatment of Alzheimer’s disease.
Introduction
Alzheimer’s disease is characterised by the cerebral accumulation of extracellular deposits called amyloid plaques that are composed of amyloid β peptides (Aβ) of 38–43 aminoacids. Amyloid β plaques are cardinal histopathological hallmarks of Alzheimer’s disease, fundamental to the amyloid cascade hypothesis of the disease, which posits cerebral Aβ accumulation as a crucial early player in disease pathogenesis, ultimately leading to neurodegeneration and dementia.1 If the amyloid hypothesis is correct, then inhibition of cerebral Aβ accumulation could benefit patients with Alzheimer’s disease.
The β secretase, referred to as β-site amyloid precursor protein (APP) cleaving enzyme 1 (BACE1), is the enzyme that initiates Aβ production by cleaving the extracellular domain of APP. Inhibitors of BACE1 are being considered at present for their potential to lower cerebral Aβ concentrations and to treat and prevent Alzheimer’s disease. Although several promising BACE1 inhibitors are being tested in human clinical trials, many questions remain about the safety of these drugs, the optimum level of BACE1 inhibition to achieve efficacy without unacceptable side-effects, and the stage of disease at which to treat for greatest therapeutic gain. Here, we review the potential of therapeutic BACE1 inhibition for Alzheimer’s disease at a crucial time in the search for effective approaches to treatment and prevention.
Amyloid β and Alzheimer’s disease
In the brain, Aβ is predominantly produced by neurons, although other cell types, including astrocytes and other glia, also generate Aβ especially under stress conditions that induce glial activation, as occurs in Alzheimer’s disease. Aβ is formed by the sequential proteolysis of the type 1 membrane protein APP (figure 1A). APP is first cleaved by the β-secretase enzyme to yield a membrane-bound C-terminal fragment called C99.2 A second enzyme named γ secretase, composed of four transmembrane proteins (presenilin, nicastrin, Pen2, and Aph1), then cuts C99 to liberate Aβ.3,4 A third protease, α secretase, can cleave APP at a site within Aβ, thus precluding its formation. Because both the β and γ secretases are required for production of Aβ, inhibition or modulation of these enzymes is considered a prime therapeutic goal for reducing cerebral Aβ concentrations in patients with Alzheimer’s disease. Conversely, activation of α secretase might also enable therapeutic Aβ reduction.
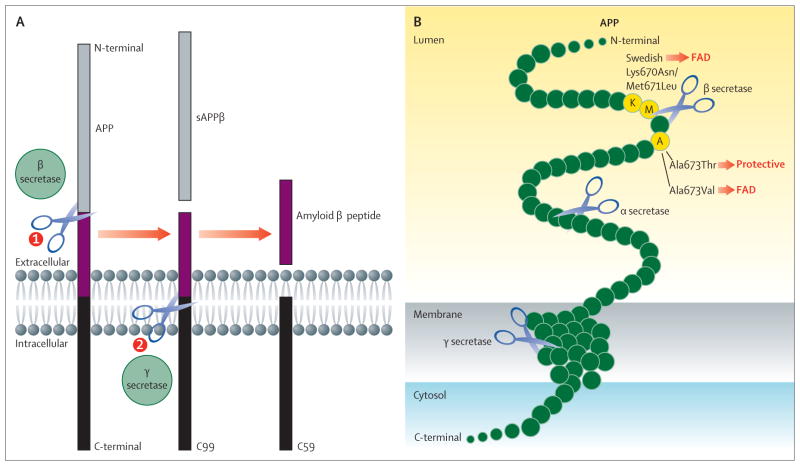
Figure 1. APP processing and mutations affecting β-secretase cleavage.
(A) APP is a type 1 membrane protein that is sequentially cleaved by two aspartic proteases to generate Aβ. First, the β-secretase enzyme cuts APP (1) to create the N-terminus of Aβ. Two APP fragments are produced: membrane-bound C99 and secreted sAPPβ ectodomain (grey). Second, C99 is cleaved by the γ-secretase enzyme (2) to generate the C-terminus of Aβ. Aβ (purple) is then released into the lumen of the endosome and secreted into the extracellular medium. An intracellular domain, C59 (black), is also produced. (B) The aminoacids in and around the Aβ domain of APP are represented as green circles. Aminoacids that affect β-secretase processing of APP in humans are shown in yellow circles, within which the wildtype residue is identified by the single-letter aminoacid code. The Lys670Asn/Met671Leu (Swedish) and Ala673Val mutations cause FAD by increasing the rate of β-secretase cleavage and Aβ production, whereas the Ala673Thr mutation protects against Alzheimer’s disease by doing the opposite. All three mutations occur at or within one aminoacid of the β-secretase cleavage site. Scissors show cleavage sites of the various secretases. APP=amyloid precursor protein. Aβ=amyloid β peptides. sAPPβ=soluble peptide APPβ. FAD=familial Alzheimer’s disease.
The genetics of human disorders provide insights into the pathogenic mechanisms of disease. For example, the discovery of mutations in the LDL receptor elucidated the pathogenic role of high serum cholesterol concentrations in familial hypercholesterolaemia and cardiovascular disease, ultimately leading to the development of the widely prescribed statins that inhibit HMG-CoA reductase and reduce serum cholesterol for the treatment of heart disease.5 Similarly, human genetics show that cerebral Aβ accumulation is crucially involved in the pathogenesis of Alzheimer’s disease.6 More than 200 autosomal dominant mutations have been identified in APP and presenilin (the catalytic subunit of γ secretase) that cause familial Alzheimer’s disease. Without exception, these mutations increase either the production of all isoforms of Aβ (total Aβ) or the proportion of the toxic 42-aminoacid isoform (Aβ42). Importantly, familial Alzheimer’s disease mutations in APP cluster near the β-secretase and γ-secretase cleavage sites and increase proteolysis of APP to generate elevated total Aβ or Aβ42. For example, the Swedish (Lys670Asn, Met671Leu)7 and Ala673Val8 mutations located near the β-secretase cleavage site increase the efficiency of β-secretase processing, and as a result lead to increased C99 and total Aβ production (figure 1B). APP duplication also causes familial Alzheimer’s disease via APP and Aβ overexpression. The ApoE ε4 allele, the major genetic risk factor for late-onset Alzheimer’s disease, is associated with increased Aβ accumulation. Furthermore, mutations in ADAM10, the physiologically relevant α secretase in neurons,9 cause late-onset Alzheimer’s disease by attenuating enzyme activity, resulting in increased β-secretase processing of APP and Aβ production.10 Thus, diverse genetic changes in at least five different genes all lead to increased cerebral Aβ accumulation associated with inherited forms of Alzheimer’s disease, strongly suggesting Aβ as a cause of Alzheimer’s disease pathogenesis.
Finally, a recently identified Ala673Thr APP variant confers protection against Alzheimer’s disease and cognitive decline in elderly individuals.11 This mutation occurs two aminoacids C-terminal to the β-secretase site (figure 1B), at the same position as the Ala673Val mutation that causes familial Alzheimer’s disease, but is less efficiently cleaved by β secretase and as a result reduces Aβ production by roughly 40%. Importantly, individuals that have one copy of the Ala673Thr mutation and are protected against Alzheimer’s disease might have a life-long reduction in Aβ production of about 20%, proof of principle that slight β-secretase inhibition might prevent Alzheimer’s disease.
BACE1
In view of the role of Aβ in Alzheimer’s disease pathogenesis, the molecular cloning of the secretase enzymes became a major goal for their value as drug targets. The characteristics of Aβ production and secretase activities in cultured cells allowed the development of cell-based assays for secretase identification. Five groups independently reported the molecular cloning of the β-secretase enzyme, variously named β-site APP cleaving enzyme (BACE), Asp2, and memapsin 2.12–16 Although the five groups used different approaches to identify the β secretase (henceforth referred to as BACE1) they all agreed on the same polypeptide sequence, strongly supporting the conclusion that the cloned protein was indeed β secretase.
BACE1 has all the characteristics predicted for the β secretase;2 it is a 501 aminoacid type 1 transmembrane aspartic protease related to the pepsin family (figure 2A). The BACE1 catalytic domain contains two signature aspartic protease motifs (Asp-Thr/Ser-Gly-Ser/Thr) that form the active site of the enzyme and are oriented in the lumen of acidic intracellular compartments for cleaving the β-secretase site of APP. BACE1 has highest concentrations in neurons, has the correct sequence specificity and acidic pH optimum for enzymatic activity, undertakes β-secretase processing of APP, and increases Aβ generation.
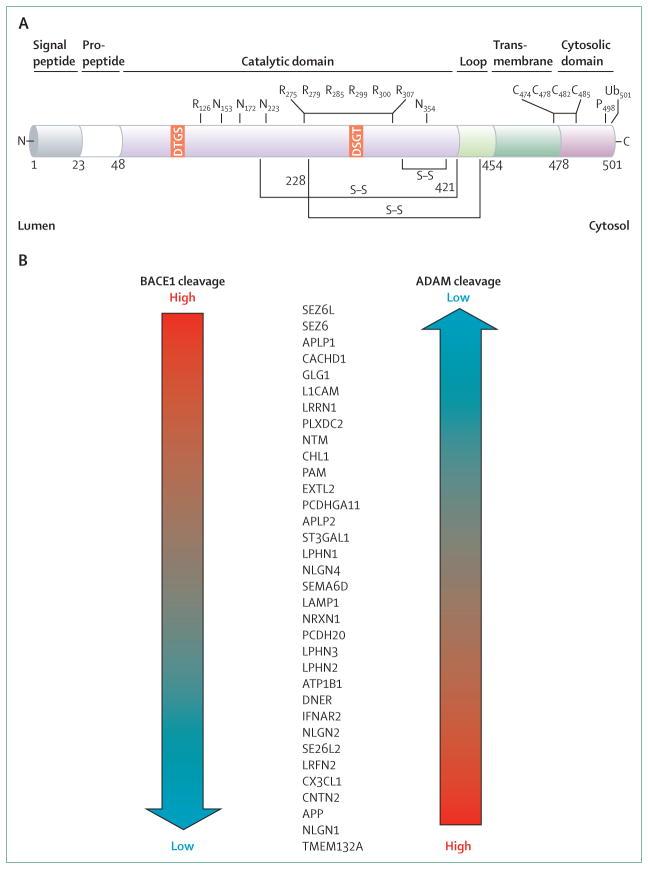
Figure 2. Primary structure and neuronal substrates of BACE1.
(A) BACE1 is a 501 aminoacid type 1 transmembrane aspartic protease. The various subdomains of BACE1 are shown above the structure. Numbers refer to aminoacid positions. The two signature aspartic protease active site motifs at positions 93 and 289 are shaded orange. S–S denotes positions of disulphide bridges within the catalytic domain. N represents positions of N-linked glycosylation sites. R shows the positions of acetylated arginine residues. C marks the positions of S-palmitoylated cysteine residues. P shows the phosphorylation of serine 498. Ub denotes ubiquitination of lysine 501. (B) BACE1 substrates identified in primary cultured neurons are listed from those that are predominantly cleaved by BACE1 (BACE1 cleavage high; top) to those that are processed by BACE1 at a low level (bottom). These substrates also are cleaved by other proteases in the ADAM family, but the ADAM cleavage preference is opposite to that of BACE1. Adapted from Kuhn and colleagues,17 by permission of the European Molecular Biology Organization. ADAM=a disintegrin and metalloproteinase domain family.
A homologue, BACE2, was identified with roughly 64% aminoacid similarity to BACE1. The high homology between the two proteases initially suggested that BACE2 is also a β secretase. However, unlike β secretase, BACE2 has low neuronal expression.18,19 Additionally, although BACE2 can generate Aβ in vitro, the preferred BACE2 cleavage site in APP is within Aβ,20–23 thus precluding the formation of Aβ. These results show that BACE2 is unlikely to be a major β secretase in the brain, although concerns have been raised that BACE1 inhibitors might also inhibit BACE2 and cause BACE2-related mechanism-based side-effects.
BACE physiological functions
BACE1 knockout mice
To unequivocally show in vivo that BACE1 is the β secretase implicated in Alzheimer’s disease, several groups used gene targeting strategies to generate BACE1 knockout (−/−) mice.24–27 These mice were initially reported to be viable and fertile with no overt phenotype, and to have normal gross morphology and behaviour, tissue histology, and blood cell and clinical chemistry characteristics, implying that therapeutic inhibition of BACE1 might be free of mechanism-based side-effects. The BACE1−/− mice crossed with APP transgenic mice that develop amyloid plaques do not produce Aβ, and do not show Aβ deposits or Aβ-dependent memory deficits.19,28–31 These results show that BACE2 cannot compensate for BACE1 in terms of Aβ generation, and thus validate BACE1 as the major β secretase in the brain, making BACE1 inhibition a viable treatment strategy for Alzheimer’s disease.
Despite initial findings that BACE1−/− mice are normal, further investigations of BACE1 were necessary to understand its physiological functions and predict potential mechanism-based side-effects of BACE1 inhibition. BACE1 is concentrated in neuronal presynaptic terminals,32,33 suggesting an important role for BACE1 at the synapse. Consistent with BACE1 neuronal localisation, recent studies of BACE1−/− mice have shown complex neurological phenotypes, including axon guidance defects,34–36 hypomyelination,37–39 memory deficits,19,28,30,40,41 muscle spindle reduction,42 neurochemical deficits,43 neurogenesis and astrogenesis abnormalities,44 neurodegeneration with age,45 spine density reduction,46 retinal pathology,47 schizophrenia endophenotypes,46 and seizures,40,45,48 among other phenotypes (table 1). Any of the BACE1 knockout phenotypes might mimic potential mechanism-based side-effects of BACE1 inhibitor drugs in humans, thus raising a note of caution that BACE1 inhibition might not be wholly free of toxic effects.
Table 1.
Phenotypes of BACE1 and BACE2 knockout mice
| Putative substrate | References | |
|---|---|---|
| BACE1 knockout mouse phenotypes | ||
|
| ||
| Astrogenesis increase, neurogenesis decrease | JAG1 | Hu et al (2013)44 |
| Axon guidance defects | CHL1 | Rajapaksha et al (2011),34 Cao et al (2012),35 Hitt et al (2012)36 |
| Hyperactivity | NRG1 | Dominguez et al (2005),27 Savonenko et al (2008)46 |
| Hypomyelination | NRG1 | Willem et al (2006),37 Hu et al (2006),38 Hu et al (2008)39 |
| Memory deficits | Unknown | Laird et al (2005),19 Ohno et al (2004),28 Ohno et al (2007),30 Ohno et al (2006),41 Kobayashi et al (2008)40 |
| Insulin sensitivity enhanced | Unknown | Dominguez et al (2005),27 Meakin et al (2012),49 Hoffmeister et al (2013)50 |
| Muscle spindle reduction | NRG1 | Cheret et al (2013)42 |
| Neurochemical deficits | Unknown | Harrison et al (2003)43 |
| Neurodegeneration with age | Unknown | Hu et al (2010)45 |
| Postnatal lethality, growth retardation | Unknown | Dominguez et al (2005)27 |
| Retinal abnormalities | FLT1 | Cai et al (2012)47 |
| Schizophrenia endophenotypes | NRG1 | Savonenko et al (2008)46 |
| Seizures | SCN2B | Kim et al (2007),51 Kobayashi et al (2008),40 Hu et al (2010),45 Hitt et al (2010)48 |
| Spine density reduction | NRG1 | Savonenko et al (2008)46 |
|
| ||
| BACE2 knockout mouse phenotypes | ||
|
| ||
| Normal | .. | Dominguez et al (2005)27 |
| Pancreatic β-cell increase | TMEM27 | Esterhazy et al (2009)52 |
| Pigmentation abnormalities | PMEL | Rochin et al (2013)53 |
|
| ||
| BACE1/2 knockout mouse phenotypes | ||
|
| ||
| Similar to BACE1 knockout, except postnatal lethality is enhanced | .. | Dominguez et al (2005)27 |
BACE1 substrates
BACE1−/− phenotypes result from deficient β-secretase processing of BACE1 substrates (figure 2B). Proteomic studies have identified many putative BACE1 substrates potentially involved in neuronal functions,17,54 in accordance with BACE1 neuronal expression and BACE1 null neurological phenotypes. BACE1 substrates are primarily type 1 membrane proteins like APP, but other BACE1 substrates have complex membrane topology. BACE1 processing releases an extracellular fragment of a given substrate from the cell, which can then interact with another molecule on the same (autocrine) or an adjacent (paracrine) cell to either reduce or enhance signal transduction or cell–cell interactions. For example, BACE1 cleavage of type 3 NRG1 liberates a fragment containing an epidermal growth factor-like domain that interacts with EGFR receptors on Schwann cells to initiate the signal for myelination.37,38,55,56 In BACE1−/− mice, reduced shedding of NRG1 decreases instructive signals to myelinating cells and causes hypomyelination. A second example is BACE1 processing of CHL1, a type 1 membrane protein that plays a part in axon outgrowth and neuronal survival.57,58 BACE1 cleavage of CHL1 releases a soluble ectodomain fragment that might interact with neuropilin 1 and semaphorin 3A to affect axon guidance, thus explaining axon mistargeting in BACE1 null mice.17,36,54 Although reduced cleavage of many BACE1 substrates impairs their function, deficient cleavage of other substrates might facilitate function. For example, JAG1 ligand, which activates the Notch receptor to regulate differentiation of many cell types, is also a BACE1 substrate.44 Deficient BACE1 cleavage of JAG1 in BACE1 knockout mice elevates cell surface concentrations of JAG1, which in turn leads to enhanced Notch activity in neighbouring cells and increased JAG1–Notch signalling.59 During early development, enhanced JAG1-Notch activity in radial glial neural stem cells favours astrogenesis by reducing neurogenesis.44 As further BACE1 substrates and functions are elucidated, the molecular basis of BACE1−/− phenotypes and their relevance to potential mechanism-based toxic effects of BACE1 inhibition will become better understood.
Many BACE1 substrates undergo a process called ectodomain shedding, wherein they are also cleaved by proteases in the so-called a disintegrin and metalloproteinase domain (ADAM) family. The degree to which a given substrate is processed by BACE1 compared with an ADAM protease differs depending on the protein (figure 2B). Some substrates are almost exclusively cleaved by BACE1 (eg, SEZ6, APLP1), whereas others are predominantly processed by ADAMs (eg, APP, neuroligin 1).17,54 Therefore, mechanism-based sideeffects associated with BACE1 inhibition might involve substrates that primarily undergo ectodomain shedding by BACE1. Conversely, BACE1 inhibition might have less effect on the processing and function of other substrates primarily cleaved by ADAM proteases, thus mitigating potential toxic effects.
BACE2 knockout mice
The homology between BACE1 and BACE2 has raised concerns that BACE1 inhibitors might cross-inhibit BACE2. Therefore, BACE2−/− mice were generated to clarify the physiological functions of BACE2 and investigate potential mechanism-based toxic effects of BACE2 cross-inhibition. Like BACE1 knockouts, BACE2−/− mice were initially reported to exhibit a normal phenotype.27 Additionally, BACE1−/−/BACE2−/− double knockout mice did not have a more serious phenotype than did BACE1−/− single knockouts, except that early postnatal lethality was increased.27 These results suggested that cross-inhibition of BACE2 could be tolerated, at least in adult individuals.
Recent investigations have shown new BACE2 functions and null phenotypes. BACE2 is expressed in pancreatic β cells, and BACE2−/− mice exhibit increased β-cell mass and improved glucose regulation because of elevated insulin concentrations.52 BACE2 was identified as the sheddase that cleaves the proproliferative type 1 transmembrane protein TMEM27 in β cells, thus providing a molecular mechanism for increased β-cell mass in BACE2−/− mice. These results support BACE2 inhibition as a treatment strategy for type 2 diabetes; however, further research is necessary to prove this hypothesis. BACE2−/− mice also display a silvery hypopigmented coat compared with the dark coat of wildtype C57BL/6 littermates. BACE2 processing of the melanocyte protein PMEL, expressed in pigment cells of the skin and eye, generates a proteolytic fragment that forms a matrix of amyloid fibrils onto which melanin is deposited in melanosomes.53 Deficient BACE2 cleavage of PMEL in BACE2−/− mice thus leads to abnormal melanosome formation and hypopigmentation. These results imply possible hypopigmentation if BACE1 inhibitors cross-inhibit BACE2.
BACE1 inhibitor drugs for Alzheimer’s disease
In view of the strong in-vivo and in-vitro validation of BACE1 as the major β-secretase enzyme in the brain, intense efforts are underway in both academia and industry to develop small-molecule inhibitors of BACE1. Initial inhibitors were non-cleavable peptide-based transition state analogues modelled after the β-secretase cleavage site of APP.14,60 In vitro, these sizable peptidomimetic molecules are potent BACE1 inhibitors, mainly because the large open BACE1 active site evolved to bind polypeptide substrates. However, peptidomimetic BACE1 inhibitors do not possess optimum drug-like properties in vivo, such as oral bioavailability, long serum half-life, or blood–brain barrier penetration. It has proven challenging to develop non-peptidic BACE1 inhibitors that are large enough to make sufficient contacts and bind with high affinity to the active site, yet small enough to have satisfactory pharmacokinetics and achieve adequate brain penetration. Additionally, BACE1 inhibitors must be lipophilic enough to cross the plasma and endosomal membranes to reach the luminal BACE1 active site.
The x-ray cocrystal structure of BACE1 with a peptidomimetic BACE1 inhibitor showed crucial inhibitor–enzyme interactions and was a major advance in the development of BACE1 inhibitors.61 Soon thereafter, new classes of small-molecule BACE1 inhibitors were designed that possessed improved drug-like properties, including low molecular weight, plasma membrane permeability, and enhanced pharmacokinetics.62,63 Unfortunately, these second-generation BACE1 inhibitors were unable to achieve sufficiently high brain concentrations, because most were substrates of P-glycoprotein, the ATP-dependent drug efflux pump for xenobiotics in the blood–brain barrier.
Recently, poor blood–brain barrier penetration has been solved with the development of potent third-generation small-molecule BACE1 inhibitors that exhibit satisfactory pharmacokinetics and robust cerebral Aβ reduction in preclinical animal models.62,63 As a result, several BACE1 inhibitors have entered clinical trials in man (table 2). Most trials are in early phases and little data about them have been published. However, early clinical trial results for three compounds have been presented at recent meetings and are described below. Other promising treatment approaches for BACE1 inhibition, such as anti-BACE1 antibodies,64,65 are in preclinical phases and will not be described in the interest of brevity.
Table 2.
Small-molecule BACE1 inhibitors in clinical trials
| Phase | NCT trial number | |
|---|---|---|
| AZD3293 | Phase 1 | 01739647, 01795339 |
| CTS-21166 | Phase 1 | 00621010 |
| E2609 | Phase 1 | 01294540, 01511783, 01600859 |
| HPP854 | Phase 1 | 01482013 |
| LY2886721 | Phase 2* | 01227252, 01534273, 01561430 |
| MK-8931 | Phase 2/3 | 01496170, 01739348, 01953601 |
| PF-05297909 | Phase 1 | 01462851 |
| RG7129 | Phase 1† | Not available |
| TAK-070 | Phase 1 | Not available |
Terminated because of abnormal liver biochemistry.
Removed from pipeline.
MK-8931
In 2012, the results were presented of a two-part randomised, double-blind, placebo-controlled phase 1 clinical trial of the BACE1 inhibitor MK-8931 in 88 healthy individuals (18–45 years old).66 Safety, tolerability, pharmacokinetics, and pharmacodynamics of single and multiple (daily for 14 days) doses were evaluated. MK-8931 seems to be generally well tolerated, and no serious adverse events were reported. A major goal of this study was to determine whether MK-8931 was able to enter the brain and block β secretase. To monitor this, biomarkers of BACE1 activity in the CSF were measured, including Aβ40 and Aβ42, as was soluble peptide APP (sAPPβ), a direct product of BACE1 cleavage of APP. MK-8931 significantly reduced CSF Aβ concentrations in a sustained and dose-dependent manner. At 36 h post-dose, a single dose of 100 mg reduced CSF Aβ40 concentrations by 75% and a single dose of 550 mg by 92%. Similar reductions of CSF concentrations of Aβ42 and sAPPβ, the BACE1-cleaved ectodomain of APP, were also observed. Multiple oral dosing of MK-8931 also achieved more than 90% reduction of Aβ concentration in the CSF. Notably, the plasma half-life of MK-8931 was roughly 20 h, showing that a single daily dose would be sufficient to maintain drug concentrations in vivo.
A randomised, double-blind, placebo-controlled phase 1b trial of MK-8931 for safety, tolerability, pharmacokinetics, and pharmacodynamics was also done in 32 patients with mild-to-moderate Alzheimer’s disease (mean age 73 years; mean Mini Mental State Examination score 22).67 One of three doses (12 mg, 40 mg, or 60 mg) of MK-8931 or placebo was orally administered once daily for 7 days and concentrations of CSF Aβ40, Aβ42, and sAPPβ were measured. Similar for the healthy individuals, MK-8931 administration resulted in robust reduction of CSF Aβ concentrations in a sustained and dose-dependent manner. Daily dosing of 12 mg, 40 mg, or 60 mg resulted in 57%, 79%, or 84% reductions of CSF Aβ40, respectively, and similar reductions for CSF Aβ42 and sAPPβ. No serious adverse events related to MK-8931 administration were reported. The MK-8931 phase 1b results are important, particularly because they show that the pharmacokinetic and pharmacodynamic properties of a BACE1 inhibitor are not substantially changed by the presence of high cerebral amyloid concentrations in Alzheimer’s disease.
The positive results of the MK-8931 phase 1a and 1b studies led to a phase 2/3 clinical trial in late 2012. The EPOCH study (NCT01739348) is a 78-week, randomised, placebo-controlled, parallel-group, double-blind clinical trial to evaluate the safety and efficacy of 12 mg, 40 mg, or 60 mg per day oral dosing of MK-8931 versus placebo in 200 patients with mild-to-moderate Alzheimer’s disease. Primary efficacy outcomes of MK-8931 administration will consist of the changes from baseline in the Alzheimer’s Disease Assessment Scale Cognitive Subscale (ADAS-Cog) and the Alzheimer’s Disease Cooperative Study- Activities of Daily Living (ADCS-ADL) scores.
The results of the interim safety analysis in 200 patients treated with MK-8931 for at least 3 months have been announced and enrolment in the trial will continue. Up to 1960 patients are expected to be enrolled for phase 3. Additionally, a new trial of MK-8931 has been initiated, the APECS study (NCT01953601), which is a 104 week randomised, placebo-controlled, parallel-group, double-blind phase 3 clinical trial to evaluate the safety and efficacy of 12 mg or 40 mg per day oral dosing of MK-8931 versus placebo in 1500 patients with prodromal Alzheimer’s disease, also known as amnestic mild cognitive impairment. The primary efficacy outcome is the change from baseline in the Clinical Dementia Rating Scale-Sum of Boxes (CDR-SB) score. Both EPOCH and APECS include secondary outcome substudies to measure Alzheimer’s disease biomarkers, including cortical amyloid load, CSF Aβ and tau, and hippocampal volume. The phase 3 efficacy studies for EPOCH and APECS are expected to conclude in 2017 and 2018, respectively.
LY2886721
The oral non-peptidic small-molecule BACE1 inhibitor LY2811376 (figure 3) exhibited satisfactory pharmacokinetic and pharmacodynamic properties in animal models that translated to studies in man in a phase 1 clinical trial.68 However, clinical development of this molecule was discontinued after a chronic toxicology study in rats showed non-clinical non-target-associated pathology in the retina and brain. Although LY2811376 was not pursued further, this compound showed the feasibility of designing a potent blood– brain barrier-penetrant, orally available small-molecule BACE1 inhibitor and was the first reported translation of reduced CSF biomarkers of BACE1 activity from preclinical animal models to man.
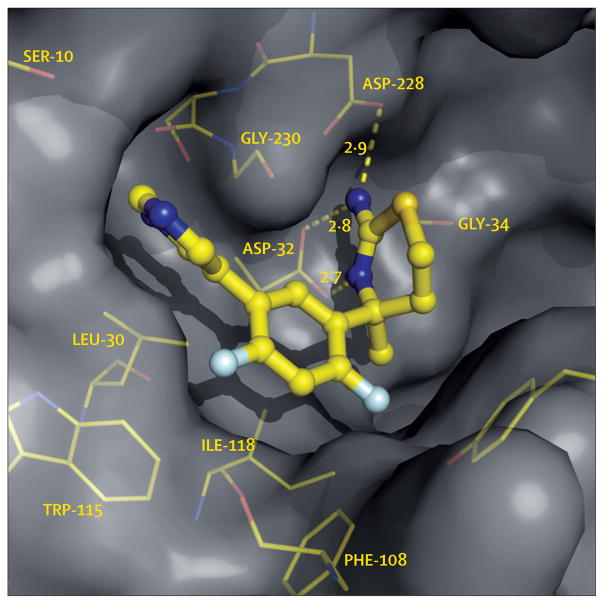
Figure 3. BACE1 inhibitor LY2811376 bound within the active site of BACE1.
In this x-ray cocrystal structure, LY2811376 is observed to hydrogen bond (dashed yellow lines) to both of the active site aspartic acid residues, here labelled ASP-32 and ASP-228, thus inhibiting the catalytic activity of the enzyme. Numbers represent lengths of hydrogen bonds in Angstroms. Other residues in the active site that interact with LY2811376 are shown in yellow. Adapted from May and colleagues,68 by permission of the Society for Neuroscience.
A next-generation compound, LY2886721, advanced into phase 1 and 2 clinical trials to determine its pharmacokinetic and pharmacodynamic effects. Like LY2811376, LY2886721 proved to be a potent orally available BACE1 inhibitor that produced robust reduction of cerebral Aβ concentrations in preclinical animal models. However, unlike LY2811376, LY2886721 did not cause pathology in the retina and brain. In phase 1 trials, 47 healthy individuals were orally administered either LY2886721 or placebo daily for 14 days.69 Two phase 1 study designs were done, consisting of either a multiple ascending dose (5 mg, 15 mg, and 35 mg) or a single dose (70 mg) followed by multiple ascending doses. LY2886721 was reported to be safe and well tolerated during the course of the 14-day study. Plasma half-life of the compound was roughly 12 h, allowing once-daily dosing. LY2886721 administration resulted in dose-dependent decreases of both plasma and CSF Aβ40 concentrations. In the CSF, Aβ40 concentrations were reduced up to 74% with the highest dose. Similar decreases in concentrations of CSF Aβ42 and sAPPβ were also observed, and an increase in the CSF α-secretase cleavage product sAPPα;70 this is consistent with BACE1 inhibition, because β and α secretases compete for cleavage of APP.
Based on these positive results, a 6-month phase 2 trial of LY2886721 (35 mg and 70 mg, once-daily oral dosing) was started in 130 patients with mild cognitive impairment or mild Alzheimer’s disease.71 However, the phase 2 trial was recently voluntarily terminated because of a small number of cases of abnormal liver biochemical tests associated with LY2886721 administration. The drug-induced abnormal liver function did not seem to be related to the BACE1 mechanism, a conclusion supported by the observation that BACE1 knockout mice have normal liver phenotypes. Abnormal liver function is not an uncommon non-target-related side-effect of many therapeutic small molecules in clinical development for diverse indications. Therefore, the termination of LY2886721 should not suggest that BACE1 is not a viable drug target.
E2609
E2609 is an orally available small-molecule BACE1 inhibitor that has shown robust cerebral Aβ reduction in preclinical studies. E2609 advanced to a randomised, double-blind, placebo-controlled phase 1 clinical trial in healthy individuals.72–74 Two separate clinical trials comprising a single oral ascending dose study (73 participants) and a 14-day multiple oral ascending dose study (50 participants) were conducted. The single oral ascending dose study assessed plasma Aβ concentrations in response to E2609 doses that ranged from 5 mg to 800 mg (nine cohorts), whereas the multiple oral ascending dose study assessed both plasma and CSF Aβ concentrations in response to E2609 doses that ranged from 25 mg to 400 mg (five cohorts). Plasma half-life of E2609 was measured to be 12–16 h, allowing once-daily dosing. Both studies showed robust dose-dependent reductions of Aβ concentrations in CSF, plasma, or both. At the highest dose of E2609 in the multiple oral ascending dose study (400 mg), CSF Aβ concentrations were reduced up to 85%. Concentrations of CSF sAPPβ exhibited similar reductions, whereas sAPPα was increased. E2609 seemed to be well tolerated and no serious adverse events were reported in either study. Additionally, a single oral dose phase 1 trial of E2609 in patients with mild cognitive impairment or mild Alzheimer’s disease has recently been completed (NCT01600859).
Outstanding questions
The long-awaited initiation of clinical trials for BACE1 inhibitors is a promising development and raises hopes that disease-modifying therapies involving BACE1 inhibition for Alzheimer’s disease are within reach. However, several crucial questions concerning therapeutic goals and outcomes of these trials remain.
What level of BACE1 inhibition will be necessary for efficacy?
The Ala673Thr mutation in APP suggests that life-long reduction of cerebral Aβ production by roughly 20% might protect against Alzheimer’s disease.11 The BACE1 inhibitors in clinical trials at present are capable of achieving this slight reduction in Aβ concentration. Modelling 50% therapeutic BACE1 inhibition by genetically decreasing the level of BACE1 by 50% (heterozygous BACE1+/−) in APP transgenic mice lowers Aβ production by nearly 20%.19,31 Because BACE1+/− mice do not have major BACE1 null phenotypes, 50% BACE1 inhibition might provide enough Aβ reduction, yet preserve sufficient BACE1 activity to avoid serious mechanism-based side-effects. In analogy with the Ala673Thr mutation, a therapeutic strategy targeting roughly 50% BACE1 inhibition and roughly 20% Aβ reduction would probably need to start before pronounced amyloid deposition, and be maintained for life to prevent or delay the onset of Alzheimer’s disease. Alternatively, greater than 50% BACE1 inhibition might be necessary if pronounced amyloid plaque burden is already present at the start of treatment. However, these arguments are speculative because the levels of BACE1 inhibition and Aβ reduction needed for efficacy in human beings are so far unknown; therefore, these are questions that might be answered by the outcomes of ongoing clinical trials, at least in part.
BACE1 concentration is increased roughly two-fold in the brain of someone with Alzheimer’s disease compared with a healthy non-demented brain.75–78 Both BACE1 and APP accumulate in swollen dystrophic neurites that surround amyloid plaques,32,79,80 suggesting that periplaque Aβ production might be elevated, thus exacerbating amyloid deposition and establishing a vicious pathogenic cycle. If so, normalisation of BACE1 activity in periplaque regions of the Alzheimer’s disease brain via BACE1 inhibition might represent a small but potentially efficacious therapeutic goal.
At what stage of Alzheimer’s disease will BACE1 inhibition be most effective?
Mutations in familial Alzheimer’s disease show that Aβ accumulation plays an early part in Alzheimer’s disease pathogenesis.6 Moreover, recent histopathology and amyloid imaging results suggest that amyloid deposition might begin more than a decade before the appearance of cognitive deficits and diagnosis of Alzheimer’s disease.81–83 In analogy to cholesterol-lowering statin drugs for the prevention of heart disease, Aβ-lowering BACE1 inhibitors might be most effective when administered early in the course of Alzheimer’s disease, before pronounced accumulation of cerebral amyloid. However, trials of Alzheimer’s disease prevention would take years and incur enormous costs. As a result, trials of Alzheimer’s disease prevention might be most feasible in the context of joint government–industry collaborations, such as those being done or planned by the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease (A4) trial (NCT02008357), Alzheimer’s Prevention Initiative (API; NCT01998841), and Dominantly Inherited Alzheimer Network Trials Unit (DIAN TU; NCT01760005), which considered the BACE1 inhibitor LY2886721 for a prevention trial to treat asymptomatic carriers of autosomal-dominant familial Alzheimer’s disease mutations, before this compound was terminated.
The BACE1 inhibitor trials in progress are being done with patients who have mild-to-moderate Alzheimer’s disease or mild cognitive impairment—this progresses to Alzheimer’s disease at a rate of roughly 10–15% per year.84 CSF Aβ and amyloid-imaging biomarker data will be collected to monitor target engagement and disease progression. Cognitive performance will also be tested because this measure is the gold standard for efficacy used in previous clinical trials in Alzheimer’s disease for palliative drugs that treat cognitive symptoms. However, as noted, amyloid pathology might begin years before memory deficits can be measured with present cognitive tests. Thus, the ability of BACE1 inhibitors to modify the course of Alzheimer’s disease could be challenging once amyloid is deposited, at least in terms of reducing cognitive decline.
Although the levels of BACE1 inhibition and Aβ reduction required for disease modification are unknown, they could be deduced from data collected in the clinical trials in progress. Future pharmacodynamic models developed from these data could enable estimation of the level of BACE1 inhibition needed to achieve the level of Aβ reduction for a given cerebral amyloid load and level of cognitive impairment. Such modelling might eventually lead to strategies for primary and secondary prevention of Alzheimer’s disease in presymptomatic individuals. However, at present, the relation between BACE1 inhibition, Aβ reduction, amyloid load, and cognitive status are not sufficiently understood to develop accurate pharmacodynamic models for determining the levels of BACE1 inhibition needed at a given stage of asymptomatic or symptomatic Alzheimer’s disease.
Will BACE1 inhibition cause mechanism-based side-effects?
Although BACE1−/− mice were initially reported to be free of negative phenotypes, subsequent investigations identified more than a dozen BACE1 null abnormalities and substantially more BACE1 substrates (table 1, figure 2B), suggesting that BACE1 inhibitors might produce mechanism-based side-effects. However, it is unclear to what extent BACE1 null phenotypes in mice will be representative of BACE1 inhibitor side-effects in human beings. BACE1−/− mice have no BACE1 from the moment of conception, such that BACE1−/− phenotypes could relate to functions of BACE1 either during development or in adulthood. For example, myelination is completed by adulthood,85 implying that NRG1-related hypomyelination in BACE1−/− mice is a developmental phenotype. Thus, therapeutic BACE1 inhibition in the adult might not affect myelination, unless remyelination becomes necessary. By contrast, axon guidance and neurogenesis are ongoing processes in specific neuronal systems that regenerate throughout life,36,44 suggesting that BACE1 null abnormalities in axon targeting and neurogenesis are adult phenotypes. As a result, treatment with BACE1 inhibitors in adults might lead to axon mistargeting events and deficient neurogenesis. Additionally, the possibility exists that developmental compensation from other proteases mitigates BACE1 deficiency, in which case treatment with BACE1 inhibitors could have more severe side-effects in the adult than those implied by BACE1−/− mice. In view of these considerations, comprehensive analyses of BACE1−/− mice should help to parse developmental versus adult BACE1 null phenotypes for the evaluation of risks of BACE1 inhibitors.
The risk of BACE1 mechanism-based toxic effects might depend on the level of therapeutic BACE1 inhibition. At one extreme, the BACE1−/− mice model 100% BACE1 inhibition, but this level will not (and should not) be achieved in human beings treated with BACE1 inhibitors, thus partially mitigating the risk of side-effects. However, the chance of adverse events caused by BACE1 inhibition might be increased in frail, elderly patients with Alzheimer’s disease compared with healthy, young individuals. Additionally, BACE1 inhibitors will be given to patients chronically, requiring a high degree of safety. Ultimately, the clinical trials of BACE1 inhibitors in progress will answer these questions. The hope is that a therapeutic window can be achieved in which the dose range of the BACE1 inhibitor can be empirically determined, and balances tolerable mechanism-based toxic effects with sufficiently efficacious cerebral Aβ reduction.
Perhaps a framework example of the clinical development of BACE1 inhibitors is the case of statins, in which clinical trials determined a therapeutic dose window of HMG Co-A reductase inhibitor that sufficiently reduced serum cholesterol concentrations to prevent heart disease with minimum serious adverse events. We are now in the early phases of this framework for BACE1 inhibitors.
Conclusions and future developments
As the enzyme that initiates Aβ production, BACE1 is a key therapeutic target for Alzheimer’s disease. The Ala673Thr mutation and BACE1 gene knockout reduce Aβ generation, strongly suggesting that BACE1 inhibition should prove effective for Alzheimer’s disease. Although BACE1−/− mice are viable and fertile, they display many complex neurological phenotypes (table 1), which imply that BACE1 inhibitor drugs could cause mechanism-based side-effects, such as hypomyelination, seizures, axon guidance defects, memory deficits, neurogenesis abnormalities, and neurodegeneration. These side-effects could result from deficient BACE1 processing of a growing list of BACE1 substrates in neurons. A challenge for the future clinical development of BACE1 inhibitor drugs is to determine which, if any, of the BACE1−/− mouse phenotypes might mimic BACE1 inhibitor side-effects in human beings.
The development of BACE1 inhibitor drugs has been challenging, but the recent introduction of several BACE1 inhibitors into clinical trials has refocused attention on this promising treatment approach for Alzheimer’s disease. Thus far, MK-8931 has advanced the farthest and is in phase 2/3, whereas the other drugs are in phase 1 and nearing phase 2. These compounds are potent and can achieve up to 90% reduction in CSF Aβ levels. Additionally, they seem to be well tolerated for the most part, although two BACE1 inhibitor trials have recently been terminated because of toxicity concerns.
Perhaps the most challenging questions for the clinical development of BACE1 inhibitors concern the level of BACE1 inhibition and the stage of Alzheimer’s disease at which to treat for optimum efficacy. Theoretical arguments based on the Ala673Thr mutation and BACE1+/− mice suggest that roughly 50% BACE1 inhibition could achieve about 20% Aβ reduction, which might prevent Alzheimer’s disease if started before major amyloid pathology. However, it is unclear what, if any, level of BACE1 inhibition would be effective in the presence of pronounced amyloid deposition. Pathology, imaging, and biomarker studies suggest that amyloid deposition might begin years, even decades, before the clinical diagnosis of dementia. Furthermore, the relation between amyloid load and cognitive decline is not sufficiently understood to determine the appropriate stage of Alzheimer’s disease at which to treat with BACE1 inhibitors. Ongoing biomarker and imaging studies, future treatment and prevention trial results, and pharmacodynamic modelling are expected to help to determine the appropriate BACE1 inhibition level and Alzheimer’s disease stage for optimum efficacy. Ultimately, the hope is that a therapeutic dose window of BACE1 inhibitor could be achieved that reduces cerebral Aβ levels enough for efficacy, yet allows sufficient levels of active BACE1 to avoid side-effects. The results of the current BACE1 inhibitor clinical trials will contribute significantly towards solving these important questions. As such, this is a crucial juncture in BACE1 inhibitor drug development, and the question of the therapeutic potential of BACE1 inhibition for Alzheimer’s disease will be definitively answered in the near future.
Search strategy and selection criteria.
The sources of information in this Review were mainly peer-reviewed primary research journal articles and secondary review articles that were identified on PubMed with the search terms “beta-secretase and Alzheimer’s disease”, “BACE and Alzheimer’s disease”, “BACE inhibitor”, and “BACE inhibitor clinical trial” from Jan 1, 1990, to Oct 24, 2013. In a few instances, information about the status of BACE1 inhibitor clinical trials was obtained from statements on company websites. Criteria used to include or exclude information were based on the relevance and significance of a given study to therapeutic BACE1 inhibition for Alzheimer’s disease. Only sources in English were reviewed.
Acknowledgments
We thank Dr Patty Kandalepas for generating figures 1 and 2, and Drs Bruce Albala and Patrick May for reading and making useful comments on the Eisai and Lilly BACE1 inhibitor clinical trial sections, respectively.
Footnotes
Contributors
RV planned the Review, searched the primary sources for information, and wrote the first draft. RY edited and rewrote the first draft. RY and RV finalised the text.
Conflicts of interest
RY declares that he has no conflicts of interest. RY receives funding from NIH (grant numbers R01NS074256 and R01AG025493). RV is a consultant for Eisai, Lilly, and Vitae Pharmaceuticals. RV receives funding from NIH (grant numbers R01AG022560, R01AG030142), Cure Alzheimer’s Fund, Bright Focus Foundation, and the Alzheimer’s Association.
References
- 1.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–56. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Vassar R, Kovacs DM, Yan R, Wong PC. The beta-secretase enzyme BACE in health and Alzheimer’s disease: regulation, cell biology, function, and therapeutic potential. J Neurosci. 2009;29:12787–94. doi: 10.1523/JNEUROSCI.3657-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sisodia SS, St George-Hyslop PH. gamma-Secretase, Notch, Abeta and Alzheimer’s disease: where do the presenilins fit in? Nat Rev Neurosci. 2002;3:281–90. doi: 10.1038/nrn785. [DOI] [PubMed] [Google Scholar]
- 4.De Strooper B, Vassar R, Golde T. The secretases: enzymes with therapeutic potential in Alzheimer disease. Nat Rev Neurol. 2010;6:99–107. doi: 10.1038/nrneurol.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol. 2009;29:431–38. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanzi RE. The genetics of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006296. doi: 10.1101/cshperspect.a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullan M, Crawford F, Axelman K, et al. A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet. 1992;1:345–47. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- 8.Di Fede G, Catania M, Morbin M, et al. A recessive mutation in the APP gene with dominant-negative effect on amyloidogenesis. Science. 2009;323:1473–77. doi: 10.1126/science.1168979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhn PH, Wang H, Dislich B, et al. ADAM10 is the physiologically relevant, constitutive alpha-secretase of the amyloid precursor protein in primary neurons. EMBO J. 2010;29:3020–32. doi: 10.1038/emboj.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suh J, Choi SH, Romano DM, et al. ADAM10 missense mutations potentiate β-amyloid accumulation by impairing prodomain chaperone function. Neuron. 2013;80:385–401. doi: 10.1016/j.neuron.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonsson T, Atwal JK, Steinberg S, et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 12.Vassar R, Bennett BD, Babu-Khan S, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–41. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 13.Yan R, Bienkowski MJ, Shuck ME, et al. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature. 1999;402:533–37. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- 14.Sinha S, Anderson JP, Barbour R, et al. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–40. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- 15.Hussain I, Powell D, Howlett DR, et al. Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol Cell Neurosci. 1999;14:419–27. doi: 10.1006/mcne.1999.0811. [DOI] [PubMed] [Google Scholar]
- 16.Lin X, Koelsch G, Wu S, Downs D, Dashti A, Tang J. Human aspartic protease memapsin 2 cleaves the beta-secretase site of beta-amyloid precursor protein. Proc Natl Acad Sci USA. 2000;97:1456–60. doi: 10.1073/pnas.97.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhn PH, Koroniak K, Hogl S, et al. Secretome protein enrichment identifies physiological BACE1 protease substrates in neurons. EMBO J. 2012;31:3157–68. doi: 10.1038/emboj.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett BD, Babu-Khan S, Loeloff R, et al. Expression analysis of BACE2 in brain and peripheral tissues. J Biol Chem. 2000;275:20647–51. doi: 10.1074/jbc.M002688200. [DOI] [PubMed] [Google Scholar]
- 19.Laird FM, Cai H, Savonenko AV, et al. BACE1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J Neurosci. 2005;25:11693–709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farzan M, Schnitzler CE, Vasilieva N, Leung D, Choe H. BACE2, a beta-secretase homolog, cleaves at the beta site and within the amyloid-beta region of the amyloid-beta precursor protein. Proc Natl Acad Sci USA. 2000;97:9712–17. doi: 10.1073/pnas.160115697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan R, Munzner JB, Shuck ME, Bienkowski MJ. BACE2 functions as an alternative alpha-secretase in cells. J Biol Chem. 2001;276:34019–27. doi: 10.1074/jbc.M105583200. [DOI] [PubMed] [Google Scholar]
- 22.Fluhrer R, Capell A, Westmeyer G, et al. A non-amyloidogenic function of BACE-2 in the secretory pathway. J Neurochem. 2002;81:1011–20. doi: 10.1046/j.1471-4159.2002.00908.x. [DOI] [PubMed] [Google Scholar]
- 23.Basi G, Frigon N, Barbour R, et al. Antagonistic effects of beta-site amyloid precursor protein-cleaving enzymes 1 and 2 on betaamyloid peptide production in cells. J Biol Chem. 2003;278:31512–20. doi: 10.1074/jbc.M300169200. [DOI] [PubMed] [Google Scholar]
- 24.Luo Y, Bolon B, Kahn S, et al. Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat Neurosci. 2001;4:231–32. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- 25.Roberds SL, Anderson J, Basi G, et al. BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: implications for Alzheimer’s disease therapeutics. Hum Mol Genet. 2001;10:1317–24. doi: 10.1093/hmg/10.12.1317. [DOI] [PubMed] [Google Scholar]
- 26.Cai H, Wang Y, McCarthy D, et al. BACE1 is the major betasecretase for generation of Abeta peptides by neurons. Nat Neurosci. 2001;4:233–34. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- 27.Dominguez D, Tournoy J, Hartmann D, et al. Phenotypic and biochemical analyses of BACE1- and BACE2-deficient mice. J Biol Chem. 2005;280:30797–806. doi: 10.1074/jbc.M505249200. [DOI] [PubMed] [Google Scholar]
- 28.Ohno M, Sametsky EA, Younkin LH, et al. BACE1 deficiency rescues memory deficits and cholinergic dysfunction in a mouse model of Alzheimer’s disease. Neuron. 2004;41:27–33. doi: 10.1016/s0896-6273(03)00810-9. [DOI] [PubMed] [Google Scholar]
- 29.Luo Y, Bolon B, Damore MA, et al. BACE1 (beta-secretase) knockout mice do not acquire compensatory gene expression changes or develop neural lesions over time. Neurobiol Dis. 2003;14:81–88. doi: 10.1016/s0969-9961(03)00104-9. [DOI] [PubMed] [Google Scholar]
- 30.Ohno M, Cole SL, Yasvoina M, et al. BACE1 gene deletion prevents neuron loss and memory deficits in 5XFAD APP/PS1 transgenic mice. Neurobiol Dis. 2007;26:134–45. doi: 10.1016/j.nbd.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McConlogue L, Buttini M, Anderson JP, et al. Partial reduction of BACE1 has dramatic effects on Alzheimer plaque and synaptic pathology in APP Transgenic Mice. J Biol Chem. 2007;282:26326–34. doi: 10.1074/jbc.M611687200. [DOI] [PubMed] [Google Scholar]
- 32.Kandalepas PC, Sadleir KR, Eimer WA, Zhao J, Nicholson DA, Vassar R. The Alzheimer’s β-secretase BACE1 localizes to normal presynaptic terminals and to dystrophic presynaptic terminals surrounding amyloid plaques. Acta Neuropathol. 2013;126:329–52. doi: 10.1007/s00401-013-1152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng M, He W, Tan Y, et al. Increased expression of reticulon 3 in neurons leads to reduced axonal transport of β site amyloid precursor protein-cleaving enzyme 1. J Biol Chem. 2013;288:30236–45. doi: 10.1074/jbc.M113.480079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajapaksha TW, Eimer WA, Bozza TC, Vassar R. The Alzheimer’s β-secretase enzyme BACE1 is required for accurate axon guidance of olfactory sensory neurons and normal glomerulus formation in the olfactory bulb. Mol Neurodegener. 2011;6:88. doi: 10.1186/1750-1326-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao L, Rickenbacher GT, Rodriguez S, Moulia TW, Albers MW. The precision of axon targeting of mouse olfactory sensory neurons requires the BACE1 protease. Sci Rep. 2012;2:231. doi: 10.1038/srep00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hitt B, Riordan SM, Kukreja L, Eimer WA, Rajapaksha TW, Vassar R. β-Site amyloid precursor protein (APP)-cleaving enzyme 1 (BACE1)-deficient mice exhibit a close homolog of L1 (CHL1) loss-of-function phenotype involving axon guidance defects. J Biol Chem. 2012;287:38408–25. doi: 10.1074/jbc.M112.415505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willem M, Garratt AN, Novak B, et al. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006;314:664–66. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- 38.Hu X, Hicks CW, He W, et al. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9:1520–25. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 39.Hu X, He W, Diaconu C, et al. Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves. FASEB J. 2008;22:2970–80. doi: 10.1096/fj.08-106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi D, Zeller M, Cole T, et al. BACE1 gene deletion: impact on behavioral function in a model of Alzheimer’s disease. Neurobiol Aging. 2008;29:861–73. doi: 10.1016/j.neurobiolaging.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Ohno M, Chang L, Tseng W, et al. Temporal memory deficits in Alzheimer’s mouse models: rescue by genetic deletion of BACE1. Eur J Neurosci. 2006;23:251–60. doi: 10.1111/j.1460-9568.2005.04551.x. [DOI] [PubMed] [Google Scholar]
- 42.Cheret C, Willem M, Fricker FR, et al. Bace1 and Neuregulin-1 cooperate to control formation and maintenance of muscle spindles. EMBO J. 2013;32:2015–28. doi: 10.1038/emboj.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrison SM, Harper AJ, Hawkins J, et al. BACE1 (beta-secretase) transgenic and knockout mice: identification of neurochemical deficits and behavioral changes. Mol Cell Neurosci. 2003;24:646–55. doi: 10.1016/s1044-7431(03)00227-6. [DOI] [PubMed] [Google Scholar]
- 44.Hu X, He W, Luo X, Tsubota KE, Yan R. BACE1 regulates hippocampal astrogenesis via the Jagged1-Notch pathway. Cell Rep. 2013;4:40–49. doi: 10.1016/j.celrep.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu X, Zhou X, He W, et al. BACE1 deficiency causes altered neuronal activity and neurodegeneration. J Neurosci. 2010;30:8819–29. doi: 10.1523/JNEUROSCI.1334-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Savonenko AV, Melnikova T, Laird FM, Stewart KA, Price DL, Wong PC. Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc Natl Acad Sci USA. 2008;105:5585–90. doi: 10.1073/pnas.0710373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai J, Qi X, Kociok N, et al. β-Secretase (BACE1) inhibition causes retinal pathology by vascular dysregulation and accumulation of age pigment. EMBO Mol Med. 2012;4:980–91. doi: 10.1002/emmm.201101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hitt BD, Jaramillo TC, Chetkovich DM, Vassar R. BACE1−/− mice exhibit seizure activity that does not correlate with sodium channel level or axonal localization. Mol Neurodegener. 2010;5:31. doi: 10.1186/1750-1326-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meakin PJ, Harper AJ, Hamilton DL, et al. Reduction in BACE1 decreases body weight, protects against diet-induced obesity and enhances insulin sensitivity in mice. Biochem J. 2012;441:285–96. doi: 10.1042/BJ20110512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoffmeister A, Tuennemann J, Sommerer I, et al. Genetic and biochemical evidence for a functional role of BACE1 in the regulation of insulin mRNA expression. Obesity (Silver Spring) 2013;21:626–33. doi: 10.1002/oby.20482. [DOI] [PubMed] [Google Scholar]
- 51.Kim DY, Carey BW, Wang H, et al. BACE1 regulates voltage-gated sodium channels and neuronal activity. Nat Cell Biol. 2007;9:755–64. doi: 10.1038/ncb1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Esterházy D, Stützer I, Wang H, et al. Bace2 is a β cell-enriched protease that regulates pancreatic β cell function and mass. Cell Metab. 2011;14:365–77. doi: 10.1016/j.cmet.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 53.Rochin L, Hurbain I, Serneels L, et al. BACE2 processes PMEL to form the melanosome amyloid matrix in pigment cells. Proc Natl Acad Sci USA. 2013;110:10658–63. doi: 10.1073/pnas.1220748110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou L, Barão S, Laga M, et al. The neural cell adhesion molecules L1 and CHL1 are cleaved by BACE1 protease in vivo. J Biol Chem. 2012;287:25927–40. doi: 10.1074/jbc.M112.377465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fleck D, van Bebber F, Colombo A, et al. Dual cleavage of neuregulin 1 type III by BACE1 and ADAM17 liberates its EGF-like domain and allows paracrine signaling. J Neurosci. 2013;33:7856–69. doi: 10.1523/JNEUROSCI.3372-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luo X, Prior M, He W, et al. Cleavage of neuregulin-1 by BACE1 or ADAM10 protein produces differential effects on myelination. J Biol Chem. 2011;286:23967–74. doi: 10.1074/jbc.M111.251538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heyden A, Angenstein F, Sallaz M, Seidenbecher C, Montag D. Abnormal axonal guidance and brain anatomy in mouse mutants for the cell recognition molecules close homolog of L1 and NgCAM-related cell adhesion molecule. Neuroscience. 2008;155:221–33. doi: 10.1016/j.neuroscience.2008.04.080. [DOI] [PubMed] [Google Scholar]
- 58.Montag-Sallaz M, Schachner M, Montag D. Misguided axonal projections, neural cell adhesion molecule 180 mRNA upregulation, and altered behavior in mice deficient for the close homolog of L1. Mol Cell Biol. 2002;22:7967–81. doi: 10.1128/MCB.22.22.7967-7981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–33. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hong L, Turner RT, 3rd, Koelsch G, Shin D, Ghosh AK, Tang J. Crystal structure of memapsin 2 (beta-secretase) in complex with an inhibitor OM00-3. Biochemistry. 2002;41:10963–67. doi: 10.1021/bi026232n. [DOI] [PubMed] [Google Scholar]
- 61.Hong L, Koelsch G, Lin X, et al. Structure of the protease domain of memapsin 2 (beta-secretase) complexed with inhibitor. Science. 2000;290:150–53. doi: 10.1126/science.290.5489.150. [DOI] [PubMed] [Google Scholar]
- 62.Probst G, Xu YZ. Small-molecule BACE1 inhibitors: a patent literature review (2006–2011). Expert opinion on therapeutic patents. Expert Opin Ther Pat. 2012;22:511–40. doi: 10.1517/13543776.2012.681302. [DOI] [PubMed] [Google Scholar]
- 63.Evin G, Lessene G, Wilkins S. BACE inhibitors as potential drugs for the treatment of Alzheimer’s disease: focus on bioactivity. Recent Patents CNS Drug Discov. 2011;6:91–106. doi: 10.2174/157488911795933938. [DOI] [PubMed] [Google Scholar]
- 64.Atwal JK, Chen Y, Chiu C, et al. A therapeutic antibody targeting BACE1 inhibits amyloid-β production in vivo. Sci Transl Med. 2011;3:84ra43. doi: 10.1126/scitranslmed.3002254. [DOI] [PubMed] [Google Scholar]
- 65.Yu YJ, Zhang Y, Kenrick M, et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med. 2011;3:84ra44. doi: 10.1126/scitranslmed.3002230. [DOI] [PubMed] [Google Scholar]
- 66.Forman M, Palcza J, Tseng J, et al. The novel BACE inhibitor MK-8931 dramatically lowers cerebrospinal fluid Aβ peptides in healthy subjects following single- and multiple-dose administration. Alzheimers Dement. 2012;8:704. [Google Scholar]
- 67.Forman M, Kleijn H, Dockendorf M, et al. The novel BACE inhibitor MK-8931 dramatically lowers CSF beta-amyloid in patients with mild-to-moderate Alzheimer’s disease. Alzheimers Dement. 2013;9:139. [Google Scholar]
- 68.May PC, Dean RA, Lowe SL, et al. Robust central reduction of amyloid-β in humans with an orally available, non-peptidic β-secretase inhibitor. J Neurosci. 2011;31:16507–16. doi: 10.1523/JNEUROSCI.3647-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martenyi F, Dean RA, Lowe S, et al. BACE inhibitor LY2886721 safety and central and peripheral PK and PD in healthy subjects (HSs) Alzheimers Dement. 2012;8:583–84. [Google Scholar]
- 70.Willis B, Martenyi F, Dean R, et al. Central BACE1 inhibition by LY2886721 produces opposing effects on APP processing as reflected by cerebrospinal fluid sAPPalpha and sAPPbeta. Alzheimers Dement. 2012;8:582. [Google Scholar]
- 71.May PC, Mergott DJ, Cocke PJ, et al. Preclinical and phase I clinical characterization of LY2886721, a BACE inhibitor in Phase II development Alzheimer’s disease. The 11th International Conference On Alzheimer’s & Parkinson’s Diseases; Florence, Italy. March 6–10, 2013. [Google Scholar]
- 72.Lai R, Albala B, Kaplow JM, Aluri J, Yen M, Satlin A. First-in-human study of E2609, a novel BACE1 inhibitor, demonstrates prolonged reductions in plasma beta-amyloid levels after single dosing. Alzheimers Dement. 2012;8 (suppl):96. [Google Scholar]
- 73.Lai R, Albala B, Kaplow JM, et al. Novel BACE1 inhibitor E2609 reduces plasma and CSF amyloid in health subjects after 14 days oral administration. The 11th International Conference On Alzheimer’s & Parkinson’s Diseases; Florence, Italy. March 6–10, 2013. [Google Scholar]
- 74.Bernier F, Sato Y, Matijevic M, et al. Clinical study of E2609, a novel BACE1 inhibitor, demonstrates target engagement and inhibition of BACE1 activity in CSF. Alzheimers Dement. 2013;9:886. [Google Scholar]
- 75.Li R, Lindholm K, Yang LB, et al. Amyloid beta peptide load is correlated with increased beta-secretase activity in sporadic Alzheimer’s disease patients. Proc Natl Acad Sci USA. 2004;101:3632–37. doi: 10.1073/pnas.0205689101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang LB, Lindholm K, Yan R, et al. Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med. 2003;9:3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]
- 77.Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol. 2002;59:1381–89. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- 78.Holsinger RMD, McLean CA, Beyreuther K, Masters CL, Evin G. Increased expression of the amyloid precursor beta-secretase in Alzheimer’s disease. Ann Neurol. 2002;51:783–86. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- 79.Zhao J, Fu Y, Yasvoina M, et al. Beta-site amyloid precursor protein cleaving enzyme 1 levels become elevated in neurons around amyloid plaques: implications for Alzheimer’s disease pathogenesis. J Neurosci. 2007;27:3639–49. doi: 10.1523/JNEUROSCI.4396-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang XM, Cai Y, Xiong K, et al. Beta-secretase-1 elevation in transgenic mouse models of Alzheimer’s disease is associated with synaptic/axonal pathology and amyloidogenesis: implications for neuritic plaque development. Eur J Neurosci. 2009;30:2271–83. doi: 10.1111/j.1460-9568.2009.07017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jack CR., Jr Alzheimer disease: new concepts on its neurobiology and the clinical role imaging will play. Radiology. 2012;263:344–61. doi: 10.1148/radiol.12110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci Transl Med. 2011;3:77sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Musiek ES, Holtzman DM. Origins of Alzheimer’s disease: reconciling cerebrospinal fluid biomarker and neuropathology data regarding the temporal sequence of amyloid-beta and tau involvement. Curr Opin Neurol. 2012;25:715–20. doi: 10.1097/WCO.0b013e32835a30f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tarawneh R, Holtzman DM. The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment. Cold Spring Harb Perspect Med. 2012;2:a006148. doi: 10.1101/cshperspect.a006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Salzer JL. Axonal regulation of Schwann cell ensheathment and myelination. J Peripher Nerv Syst. 2012;17 (suppl 3):14–19. doi: 10.1111/j.1529-8027.2012.00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]