Abstract
Astrogliosis, whereby astrocytes in the central nervous system (CNS) become reactive in response to tissue damage, is a prominent process leading to the formation of the glial scar that inhibits axon regeneration after CNS injury. Upon becoming reactive, astrocytes undergo various molecular and morphological changes including upregulating their expression of GFAP and chondroitin sulfate proteoglycans (CSPGs) as well as other molecules that are inhibitory to axon growth. We have developed an in vitro model of reactive astrogliosis as a result of treating cultured astrocytes with transforming growth factor-β (TGF-β), which induces increased expression as well as secretion of CSPGs. These reactive astrocytes show inhibitory effects on neuron growth both in neuron-astrocyte coculture and in neurite guidance spot assay using astrocyte-conditioned medium. These reactive astrocytes provide a vehicle for testing substances that might overcome the glial scar and promote regeneration.
Keywords: Reactive astrogliosis, CSPGs, GAG chains, TGF-β, Chondroitinase ABC, Neuron-astrocyte coculture, Neurite outgrowth, Spot assay
1. Introduction
Astrocytes respond to various forms of central nervous system (CNS) insults, such as trauma, by the process of reactive astrogliosis. After CNS injury, astrocytes migrate, proliferate, and form a glial scar which represents a physical and biochemical barrier that contributes to the failure of axonal regeneration. Astrogliosis involves various molecular and morphological changes, including hypertrophy and upregulated expression of intermediate filament proteins such as glial fibrillary acidic protein (GFAP) and vimentin, as well as chondroitin sulfate proteoglycans (CSPGs) and other molecules that inhibit axonal growth (1). CSPGs produced by reactive astrocytes consist of a core protein decorated with one or more unbranched polysaccharide glycosaminoglycan (GAG) chains which contribute to their inhibitory actions. Removal of GAG chains by the enzyme chondroitinase ABC (cABC) reduces the inhibitory properties of CSPGs, suggesting that the active moieties are the chondroitin sulfate (CS) GAG chains (2). These GAG chains are composed of repeating disaccharide units of D-glucuronic acid (GlcA) and N-acetyl-D-galactasamine (GalNAC) which undergo extensive modification of sulfation at the C2 position of GlcA and/or the C4 or C6 position of GalNAC residues. Neither the length of the GAG chain nor the pattern of sulfation is genetically determined, leading to an enormous number of potential combinations. Our lab and others have shown that a specific sulfation pattern in CS GAG chains is essential for the repellant activity of CSPGs (3, 4).
These experiments have employed an in vitro model of reactive astrocytes that we created by treating astrocytes with TGF-β (4). Using cocultures of neurons and reactive astrocytes, we have demonstrated that TGF-β-treated astrocytes inhibit the neurite outgrowth of cerebellar granule neurons (CGNs). Conditioned medium derived from the reactive astrocytes also inhibits neurite outgrowth of CGNs and can have negative effects on neuronal guidance using a boundary assay that we developed. The conditioned medium from these reactive astrocytes induced by TGF-β contains high levels of CSPGs as demonstrated by both Western blotting and ELISA. These assays have allowed us to demonstrate that the inhibitory activity of CSPGs on both neurite outgrowth and guidance can be eliminated by cABC treatment. In addition, growth and guidance can be altered by treating either the astrocytes or the neurons with molecules or siRNAs that are directed against intracellular targets, demonstrating the utility of these assays to investigate fundamental properties of reactive astrocytes and the mechanisms within neurons that respond to them.
2. Materials
2.1. Primary Culture of Mouse Cerebral Cortical Astrocytes
Hank’s Balanced Salt Solution (HBSS).
0.25% trypsin solution and 0.25% trypsin solution with 1 mM ethylenediamine tetraactic acid (trypsin/EDTA) (see Note 1).
DNase I is dissolved at 1 mg/mL in DMEM and stored in single-use aliquots at −20°C.
Astrocyte culture medium (10% FBS/DMEM): Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (see Note 2) and Penicillin (50 U/mL)/Streptomycin (50 μg/mL).
Cell strainer (70 μm).
2.2. Reactive Astrocytes and Preparation of Conditioned Medium and Cell Lysates
Recombinant human transforming growth factor beta 1 (TGF-β1, R&D Systems, Minneapolis, MN): reconstitute at 1 μg/mL in 4 mM HCl containing 1 mg/mL bovine serum albumin (BSA) and freeze in aliquots at −20°C. Working concentration is 10 ng/mL.
10× phosphate-buffered saline (PBS): dilute 1:10 with water for use.
2× Cell lysis buffer: 125 mM Tris, 4% sodium dodecyl sulfate (SDS), 20% glycerol, 2 mM EDTA, adjust pH to 6.8 with HCl, store at room temperature.
Protease inhibitor cocktail (Set V, Calbiochem, La Jolla, CA): reconstitute each vial with 1 mL H2O and freeze in aliquots at −20°C, add 10% (v/v) to the conditioned medium.
Centricon YM-30 (Millipore, Billerica, MA).
Chondroitinase ABC (Northstar BioProducts, Falmouth, MA).
BCA Protein Assay Kit.
2.3. Western Blotting
SDS-polyacrylamide gel electrophoresis (PAGE) separating buffer: 1.5 M Tris–HCl, pH 8.8, 0.4% SDS; stacking buffer: 0.5 M Tris–HCl, pH 6.8, 0.4% SDS; 30% acrylamide/bis solution; ammonium persulfate; TEMED.
Tris/Glycine/SDS running buffer (10×, Bio-Rad Laboratories, Hercules, CA): dilute 50 mL with 450 mL water for use.
β-mercaptoethanol: add 5% (v/v) to each protein sample.
Prestained molecular weight markers.
Tris/Glycine transfer buffer (10×, KD Medical): dilute 50 mL with 400 mL water and 50 mL methanol (10%, v/v) for use (see Note 3).
PBS with 0.1% Tween (PBS-Tween).
Blocking and antibody dilution buffer: 5% nonfat dry milk in PBS-Tween.
Primary antibodies: mouse monoclonal IgM anti-chondroitin sulfate antibody (clone CS-56, Sigma), mouse monoclonal IgG anti-ΔDi-4S antibody (clone 2-B-6, Northstar BioProducts), and mouse monoclonal IgM anti-ΔDi-6S antibody (clone 3-B-3, Northstar BioProducts) (see Note 4).
Secondary antibodies: HRP-conjugated goat anti-mouse IgM (Abcam, Cambridge, MA).
Enhanced chemiluminescent (ECL) reagents.
2.4. ELISA
Immulon 4HBX 96-well immunoassay microplates (Thermo Scientific, Milford, MA).
Blocking buffer and antibody dilution buffer: 2.5% (w/v) BSA in PBS-Tween.
Primary antibody: anti-chondroitin sulfate antibody (clone CS-56, Sigma).
Secondary antibody: HRP-conjugated goat anti-mouse IgM, F (ab’)2 (Abcam).
SureBlue TMB microwell peroxidase substrate (KPL, Gaithersburg, MD): warm to room temperature before use.
1 M HCl.
Chicken CSPGs (Millipore).
2.5. Live Cell Immunocytochemistry for CSPGs and GFAP Expression on Reactive Astrocytes
Primary antibody: anti-chondroitin sulfate antibody (CS-56, Sigma); rabbit polyclonal anti-GFAP antibody (Dako Denmark A/S, Glostrup, DK).
Secondary antibody: FITC-conjugated goat anti-mouse IgM antibody and rhodamine-conjugated goat anti-rabbit IgG antibody (Jackson ImmunoResearch Laboratories, PA).
Acetic acid/ethanol (5/95%, v/v).
PBS with 0.1% (v/v) Triton X-100 (PBS/Triton).
Vectashield containing DAPI (Vector laboratories, CA).
2.6. Isolation of Mouse Cerebellar Granular Neurons (CGNs)
HBSS, 0.25% trypsin solution, DNase I, and 10% FBS/DMEM (see Subheading 2.1).
CGN medium (Neurobasal-A/B27): Neurobasal-A medium (GIBCO) supplemented with B27 (1:50; v/v), 2.5 mM L-Glutamine, 25 nM KCl, 50 U/mL Penicillin, and 50 μg/mL Streptomycin.
Cell Strainer (40 μm).
2.7. Neurite Outgrowth Assay with Cocultures of CGNS and Astrocytes
4% Paraformaldehyde (PFA) fixation buffer. Prepare the following: (1) 500 mL 2× PHEM buffer (18.14 g PIPES, 6.5 g HEPES, 3.8 g EGTA, 0.99 g MgSO4), adjust pH to 7.0 with 10 M KOH (will not go into solution until pH approaches 7), store in aliquots at −20°C; (2) 5 mL 37% PFA (1.85 g PFA, 3.5 mL dH2O, 10μL 10 M KOH), mix together in a 50-mL Falcon tube. Boil water in a glass beaker on hot plate and place tube into boiling water with the cap loose for no longer than 5 min; swirl frequently to mix until the PFA goes into solution, make fresh every time; (3) 50 mL fixation buffer (25 mL 2× PHEM buffer, 5 mL 37% PFA, 20 mL dH2O).
PBS with 0.1% (v/v) Triton X-100 (PBS/Triton).
Primary antibody: Mouse monoclonal anti-β-III tubulin antibody (Sigma); rabbit polyclonal anti-GFAP antibody (Dako).
Secondary antibody: FITC-conjugated goat anti-mouse IgG and rhodamine-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories).
Vectashield containing DAPI (Vector laboratories).
2.8. Neurite Guidance Spot Assay
Poly-L-Lysine (PLL, mol wt ≤150,000; Sigma) is dissolved at 1 mg/mL in milli-Q water and stored in single-use aliquots at −20°C. Working solution (200 μg/mL) is prepared by dilution in milli-Q water.
Tx-Red (Invitrogen) is dissolved in DMSO at 5 mg/mL stock solution, pipet up and down to completely dissolve the contents, protect from light, and store in aliquots at −20°C. Working solution is prepared by dilution in HBSS.
CSPG (1,000 μg/mL, Millipore): Store in aliquots at −20°C, working solution is prepared by dilution in HBSS.
4% PFA fixation buffer.
PBS with 0.1% (v/v) Triton X-100 (PBS/Triton).
Primary antibody: Monoclonal mouse anti-β-III tubulin antibody (Sigma).
Secondary antibody: FITC-goat anti-mouse IgG (Jackson ImmunoResearch Laboratories).
Vectashield containing DAPI (Vector laboratories).
3. Methods
Reactive astrocytes upregulate expression of inhibitory CSPGs which can be detected by Western blot or ELISA, using commercially available antibodies. To assess an in vitro astrogliosis model, it is not sufficient to evaluate only the molecular and morphological changes, but it is ultimately essential to determine whether the reactive astrocytes are inhibitory to neuron growth. This can be determined by employing a neurite outgrowth assay either using astrocyte-neuron cocultures or astrocyte-conditioned medium. In addition, since neurite growth and guidance may be controlled independently, guidance should be monitored through the use of a boundary assay. Though astrocytes produce CSPGs, they primarily support neuron growth by expressing many growth-promoting molecules such as L1, laminin and fibronectin. The key to the coculture assay is to compare neurons growing on reactive astrocytes with those on naive astrocytes. For the boundary assay, in addition to using the commercially available CSPGs as a positive control, the enzyme cABC provides a useful tool to further confirm that the inhibitory activity actually results specifically from CSPG GAG chains (see data figures later), as some proteoglycans, such as NG2, can also be inhibitory through their core proteins (5, 6).
3.1. Primary Culture of Mouse Cerebral Cortical Astrocytes
Alcohol spray new born pup (P0–3), rapidly cut off the head into an empty dish using sharp scissors, use another empty dish for body parts; grasp a pup head at the nose with curved forceps, insert spring scissors into the foramen magnum at the base of the skull where the medulla and spinal cord extend; cut from one side laterally, then anteriorly through the ear, and curve toward the midline to the nose; reinsert scissors into the foramen magnum, and repeat the cutting procedure on the other side. Lift the skin and skull up to expose the brain and remove the entire brain from the skull using spring scissors. Place in 6-cm dishes containing 6 mL of cold HBSS (three brains per dish).
Under a dissection microscope, separate the cerebral cortex from the brain, carefully strip away the meninges with fine forceps, remove the hippocampus from the underlying cortex, and place the clean cortex into a new 6-cm dish containing 3 mL of HBSS.
Chop the tissue into small pieces (about 1 mm3) with fine scissors, add 3 mL of 0.25% trypsin into the dish in a final volume of 6 mL, and incubate the dish at 37°C for 15 min (see Note 5).
Stop trypsinization by transferring the tissue pieces to a 15-mL tube containing 6 mL of astrocyte culture medium.
Allow the tissue to settle to the bottom of the tube, aspirate the supernatant with a 10-mL serological pipette.
Add 3 mL DMEM containing 300 μL DNase I (see Note 6), triturate by pipetting up and down gently with a 5-mL serological pipette for up to 20 times until a homogenous cell suspension forms. Perform the last several triturations with a P-200 tip attached to the pipette.
Prewet a 70-μm cell strainer with DMEM, pass the cell suspension through the cell strainer to remove undissociated tissue chunks and collect into a 50-mL tube, flush the strainer with an additional 1 mL of DMEM.
Spin down at 500 rpm for 5 min.
Aspirate the supernatant and resuspend the cells in astrocyte culture medium (1 mL cell suspension per T75 flask), pipette up and down a couple of times to mix well, add the cell suspension into the T75 flasks containing 10 mL medium (1.5 brains/T75 flask). Keep cells in the incubator at 37°C, 5% CO2.
Feed the cultures with fresh astrocyte culture medium every 2–3 days.
When the cells become confluent (approximately 7–10 days after plating), shake the flasks to remove microglia, oligodendrocyte progenitors (OPCs) and neurons. Shake the flasks on a platform shaker at 200 rpm, 37°C overnight for 16–20 h.
Change the medium immediately after shaking. Aspirate off the medium, rinse once with fresh medium, and replace with fresh astrocyte medium.
3.2. Reactive Astrocytes and Preparation of Conditioned Medium and Cell Lysates
Harvest astrocytes from the T75 flask to provide experimental cultures on 6-well plates. Rinse cells 3× with warmed PBS; add 2 mL 0.25% trypsin/EDTA to each flask and incubate at 37°C for up to 2 min. Sharply tap the flask with your hand every 30 s to dislodge cells and check under the microscope. Stop the trypsinization by adding 8 mL astrocyte culture medium per flask, release the cells by repetitive pipetting several times and then transfer the cell suspension to a 15- or 50-mL tube. Pellet the cells at 500 rpm for 5 min; resuspend the cells in astrocyte culture medium. Mix 10 μL cell suspension with 10 μL trypan blue and determine the cell density and viability using a hemocytometer. Plate 2 × 105 cell/well of a 6-well plate in 2 mL medium.
After the cells become confluent (usually within 2–3 days), rinse the cells with DMEM (without serum) and incubate for a further 24 h in 2-mL DMEM per well (without serum).
Add 20 μL per well of 1 μg/mL TGF-β1, mix by shaking the plate back and forth and culture for an additional 5 days. Treat 3 wells with TGF-β1 and leave another 3 wells untreated as a control. Reactive astrocytes are characterized by abundant GFAP expression, increased production of CSPG, and compromised neurite growth in both the neurite guidance and neurite outgrowth assays described later in this section.
5 days after TGF-β treatment, collect the astrocyte-conditioned medium and concentrate with Centricon YM-30 in the presence of the protease inhibitor cocktail to 1/10 of the initial volume (see Note 7).
In some cases, concentrated conditioned medium is treated with 0.5 U/mL of cABC at 37°C for 3 h (see Note 8).
To prepare cell lysates, after removing the conditioned medium, rinse the cells 3 times with PBS, add 500 μL 2× cell lysis buffer and collect the cell lysate into a 1.5-mL tube, heat denature at 95°C for 10 min. Determine the protein concentrations in cell lysates using the BCA protein assay.
3.3. Quantification of CSPG Production in Astrocyte Cell Lysates and Conditioned Medium
Determine CSPG levels in the samples using SDS-PAGE using a 1-mm thick 6% stacking gel under reducing conditions (see Note 9). To compare CSPG levels in conditioned medium, use equal volumes of the conditioned medium; for cell lysates, load equal amounts of protein from each sample.
Wet the PVDF membrane in methanol for 10 s, then soak into 1× transfer buffer, leave on the shaker until the SDS-PAGE electrophoresis is complete. Soak two pieces of sponges and four pieces of blotting paper in transfer buffer.
Electro-blot the SDS-PAGE-separated samples onto the PVDF membrane using a Bio-rad transfer device. Once the transfer is complete, block the membrane in blocking buffer at room temperature for 1 h or overnight at 4°C on the shaker.
Remove the blocking buffer and incubate with primary antibody, either CS-56 (1:500), 3-B-3, or 2-B-6 (1:200) in antibody dilution buffer for 1–2 h at room temperature or overnight at 4°C, on the shaker. Make sure the membrane is covered by the antibody.
Remove the primary antibody and wash the membrane 3 times for 10 min each with PBS-Tween.
Incubate with HRP-conjugated goat anti-mouse IgM secondary antibody (1:5,000) at room temperature for 45 min.
Remove secondary antibody and wash the membrane 6 times for 5 min with PBS-Tween.
Detect the signals using chemiluminescent methods. Add ECL reagents to the membrane and incubate at room temperature for 5 min.
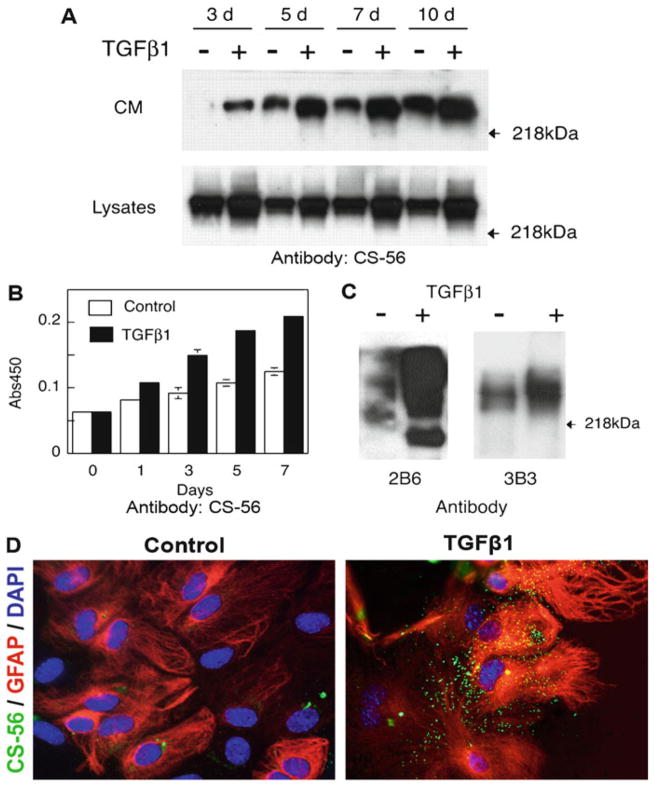
Remove membrane from ECL reagents, seal into a plastic bag and then place in an X-ray film cassette. Develop the film with optimized exposure time in the dark room. Examples of the signals for CS-56 and 3-B-3 are shown in Fig. 1a, c.
Fig. 1.
Reactive astrocytes induced by TGF-β1 produce more CSPGs. (a) Conditioned medium (CM) and cell lysates collected from control or TGF-β1-treated astrocytes at indicated time points were subject to western blotting with the CS-56 antibody. TGF-β 1-treated astrocytes showed increased production of CSPGs detected in CM as well as cell lysates; (b) CM from control or TGF-β1-treated astrocytes was processed for ELISA using antibody CS-56; (c) CM from control or TGF-β1-treated astrocytes was concentrated and digested with cABC followed by western blotting with 2B6 or 3B3 antibody; (d) TGF-β1-treated astrocytes showed increased immunoreactivity with CS-56 antibody as compared to untreated control astrocytes ((a–c) From Wang et al. (4)).
3.4. ELISA Measurement of CSPG Production in Reactive Astrocyte-Conditioned Medium
Coat 96-well microplates with conditioned medium (30 μL/well) or CSPG as positive control overnight at 4°C, then wash 5 times with PBS-Tween (100 μL/well).
Add blocking buffer (100 μL/well) and incubate for 2 h at room temperature, wash 5 times with PBS-Tween.
Add CS-56 primary antibody (1:500, 50 μL/well) and incubate for 2 h at room temperature, wash 5 times with PBS-Tween.
Add HRP-conjugated anti-mouse IgM secondary antibody (1:10,000, 50 μL/well) for 40 min at room temperature, wash 5 times with PBS-Tween.
Add 100 μL of TMB substrates to each well and incubate for 10 min at room temperature, stop the reaction by adding 100 μL of 1 M HCl to each well.
Measure the binding with a microplate reader at 450 nm wavelength. An example of these results is shown in Fig. 1b.
3.5. Live Cell Immunocytochemistry for CSPG Expression on Reactive Astrocytes
Harvest astrocytes from a T75 flask (see Subheading 3.2), plate astrocytes onto PLL-coated 12-mm glass coverslips in a 24-well plate at 5 × 104 cells/well in 0.5 mL astrocyte culture medium.
After cells become confluent (usually within 2–3 days), rinse the cells with DMEM (without serum) and incubate for a further 24 h in 0.5 mL DMEM (without serum) per well. Treat the cells with 5 μL TGF-β1 and culture for an additional 5-day period to create reactive astrocytes.
Rinse the cells with DMEM and incubate with 25 μL of primary antibody CS-56 (1:200 in DMEM), make sure the entire coverslip is covered with antibody solution. Incubate for 30 min at 4°C; wash the cells 3 times with DMEM followed by incubation with FITC-conjugated anti-mouse IgM antibody (1:200).
Fix the cells with cold acetic acid/ethanol (5/95%) for 5 min at −20°C.
Incubate the astrocytes with primary rabbit anti-GFAP antibody (1:500 in PBS/Triton) for 1 h at room temperature, rinse, followed by secondary rhodamine-conjugated goat-anti-rabbit IgG antibody (1:200 in PBS/Triton). Nonspecific binding of the secondary antibody is controlled by omitting the primary antibody in parallel cultures. Wash the stained cells and mount in Vectashield containing DAPI.
Acquire fluorescent images using an inverted microscope equipped with a CCD camera. Representative images are shown in Fig. 1d.
3.6. Isolation of Mouse CGNs
Alcohol spray mouse pups (P5–9); rapidly cut off the head into an empty dish using sharp scissors, use another empty dish for body parts; grasp a pup head at the nose with curved forceps, insert spring scissors into the foramen magnum; cut along the side of the skull through the ear, and curve toward the midline to the nose; repeat the process on the other side; carefully lift skin and skull up to expose brain, make sure that the cerebellum remains in the base of the skull; place the blades of the fine forceps under the cerebellum, lift it away from the remain of the brain, and place in a 6-cm dish containing 6 mL of cold HBSS.
Under the dissection microscope, use fine forceps to peel away the meninges or extraneous brain regions that are stuck to the cerebellum; place the clean cerebellum in a new 6-cm dish containing 3 mL of cold HBSS.
Chop the cerebellum tissue into small pieces (about 1 mm3) with fine scissors.
Add 3 mL 0.25% trypsin (final concentration: 0.125%) for up to 6 pups, incubate the dish at 37°C for 15 min.
Stop trypsinization by transferring the tissue pieces to a 15-mL tube containing 6 mL 10% FBS/DMEM.
Spin down at 500 rpm for 5 min.
Discard the supernatant, add 3 mL DMEM containing 300 μL DNase I (if there are more than six brains, increase the approximate amount of Trypsin and DNase for optimal digestion efficiency), triturate slowly using a 5-mL serological pipette for up to 20×. Perform the last several triturations with a sterile P-200 tip attached to the pipette until cells are a uniform suspension.
Pass through a 40-μm cell strainer into a 15-mL centrifuge tube.
Spin down at 500 rpm for 5 min.
Resuspend the cells in CGN medium; determine the cell number and viability using trypan blue and a hemocytometer and plate the cells at the desired density: for a neurite outgrowth assay cocultured on astrocytes (1–2 × 104 cell/well) or a neurite guidance spot assay (4–10 × 104 cells/well).
3.7. Neurite Outgrowth Assay with Neuron-Astrocyte Coculture
Plate astrocytes onto PLL-coated 12-mm glass coverslips in a 24-well plate at 5 × 104 cell/well in 0.5 mL astrocyte culture medium.
After the cells become confluent (usually within 2–3 days), rinse the cells with DMEM and incubate for a further 24 h in 0.5 mL DMEM (without serum) per well. Add 5 μL TGF-β1 and culture for 5 days to create reactive astrocytes.
5 day after TGF-β1 treatment, remove the medium and plate 1×104 neurons per well in 1 mL CGN medium on the confluent monolayer of astrocytes and coculture for 24 h.
Fix cells with 4% PFA for 15 min, rinse twice with PBS.
Label neurons and astrocytes by immunofluorecence staining. Firstly, block the cells with 10% normal goat serum in PBS/Triton for 1 h at room temperature; incubate with a mixture of primary mouse anti-β-III tubulin antibody (1:1,000 in PBS/Triton) and rabbit anti-GFAP polyclonal antibody (1:500 in PBS/Triton) for 1 h at room temperature; rinse with PBS and then incubate with secondary FITC-goat anti-mouse IgG (1:200 in PBS/Triton) and rhodamine-conjugated goat anti-rabbit IgG antibodies (1:200 in PBS/Triton); rinse with PBS and mount the coverslips on glass slides with Vectashield containing DAPI.
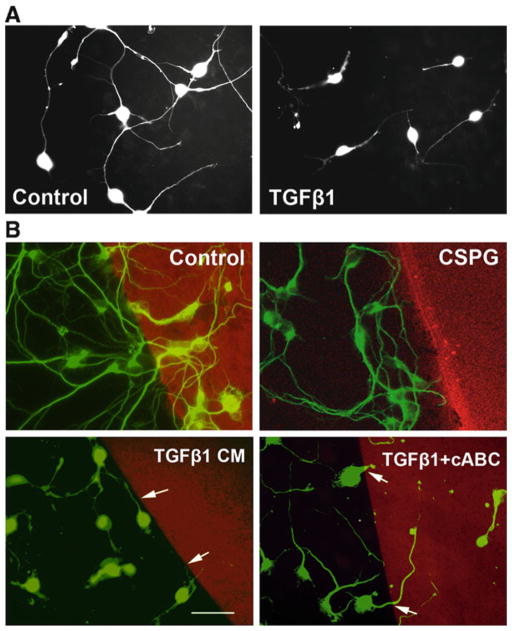
Obtain images from randomly selected fields of the cultures. Perform the neurite length analysis on these images using the NeuronJ plug-in for the ImageJ program (available from http://rsb.info.nih.gov/ij/). Alternatively, use an unbiased stereological method to measure relative changes in neurite length (7). The stereological method gives equivalent results as the direct measurement of neurite length, but is much more efficient. Representative images of neurons on astrocyte monolayers are shown in Fig. 2a.
Fig. 2.
Increased produce of CSPGs by reactive astrocytes is responsible for reduced neuron growth. (a) CGNs grown on a monolayer of TGF-β1-treated astrocytes showed a decrease in neurite length compared to that on untreated control astrocytes; (b) CM from control astrocytes, or CM from TGF-β 1-treated astrocytes with or without cABC treatment, or CSPGs (positive control) was immobilized on PLL-coated coverslips for the neurite guidance spot assay. Like CSPGs, TGF-β1-CM formed a strong boundary to neurite crossing, while cABC-treated TGF-β1-CM permitted increased crossing of neurites onto the spot.
3.8. Neurite Guidance Spot Assay
Coat coverslips in 24-well plates with 0.5 mL PLL (200 μg/mL) overnight at 4°C, rinse twice with water, and leave the plates inside the culture hood to dry completely.
An interface between astrocyte-conditioned medium and PLL is created by placing a 5-μL drop of concentrated conditioned medium (native or after cABC treatment) together with Texas Red. Chicken CSPGs (5 μg/mL) are used as a positive control. The mixture is placed in the center of a PLL-coated glass coverslip and allowed to coat at 37°C, 100% humidity for 1 h 30 min to 2 h (see Note 10). Rinse with HBSS and then the plate is ready for plating. Texas Red is used to visualize the interface and is used alone as a negative control (see Note 11).
Plate the dissociated CGNs on the coverslips in a 24-well plate at a density of 4–6 × 104 cell/well and culture for 48 h (see Note 12). Fix the cells with 4% PFA and stain with anti-β-III tubulin antibody (Sigma), wash then incubate with FITC-conjugated anti-mouse IgG antibody.
Obtain images of the cultures and determine an estimate of the boundary strength by measuring the percentage of neurites within 10 μm of the spot interface that cross onto the Texas Red spot. Count only single, nonfasciculated neurites growing toward the immobilized spot; do not include neurites whose soma is sitting at the interface. Example images of the spot assay are shown in Fig. 2b.
Acknowledgments
This work was conducted in the NHLBI Intramural Research Program.
Footnotes
We use trypsin for primary isolation of cells from brain tissue and use trypsin/EDTA for passage of astrocytes.
The quality of FBS is important for cell growth. We always test different lots of FBS before purchasing.
Normally the concentration of methanol in transfer buffer is 20% (v/v). For high molecular weight proteins (MW > 100 kD), we decrease the methanol in the transfer buffer to 10% to facilitate the transfer of high molecular weight proteins. The molecular weight of CSPGs is >200 kD.
CS-56 antibody recognizes the GAG portion (chondroitin-4-sulfate and chondroitin-6-sulfate) of native CSPGs. 3-B-3 and 2-B-6 are “stub” antibodies and are respectively specific for chondroitin-6-sulfate and chondroitin-4-sulfate epitopes which are produced by cABC-digested CSPG GAG chains.
We do trypsin digestion in a dish instead of in a tube. When using a tube, occasionally swirling of the tube is required during trypsin digestion as the tissue will settle to the bottom. We found this step could be omitted when trypsinization is performed in a dish.
Use 3 mL of 0.25% trypsin and 300 μL DNase for up to six brains. If there are more than six brains, increase the amount of trypsin and DNase for optimal digestion.
We use an equal volume of conditioned medium before concentration. After concentration to about 1/10 of the initial volume, the concentrated conditioned media are diluted to an equal volume with DMEM.
There are 10 U/vial of cABC. 200 μL 0.1% BSA in water is used to reconstitute the enzyme, which gives 0.05 U/μL. We use 1/100 (v/v) of enzyme for digestion. For example, 1 μL of cABC is added to 99 μL of conditioned medium. This means 0.05 U/100 μL, i.e., 0.5 U/mL as a final concentration.
CSPGs are large molecules. We use a 6% stacking gel to facilitate the migration of large molecular weight proteins as well as the separation of proteins from each other in the gel and their transfer to the membrane. We also decrease the methanol in the transfer buffer to 10% to facilitate transfer.
The PLL-coated coverslips must be completely dried to promote surface tension to prevent the drop from spreading. We usually prepare the PLL-coated coverslips more than 3 days before the spot assay and leave them in the hood to dry completely. Spotting requires slow pipetting with the tip held vertically to ensure the uniformity of the spot and a sharp edge. It is noteworthy that during the incubation with CSPG spots, they are not permitted to dry, as we found that drying produces a nonuniform substrate such that the Texas Red alone repels neurite growth.
Using an optimized dilution of Texas Red is important. We found less Texas Red would make the spot invisible. However, if the concentration is too high, the Texas Red dye will diffuse outside from the border of the spot and blur the interface.
Plating too high a concentration of neurons promotes boundary crossing. For a 12-mm coverslip in a 24-well plate, we usually plate 2–10 × 104 cells.
References
- 1.Silver J, Miller JH. Regeneration beyond the glia scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 2.Bradbury EJ, Moon LD, Popat RJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 3.Laabs TL, Wang H, Katagiri Y, et al. Inhibiting glycosaminoglycan chain polymerization decreases the inhibitory activity of astrocyte-derived chondroitin sulfate proteoglycans. J Neurosci. 2007;27:14494–14501. doi: 10.1523/JNEUROSCI.2807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Katagiri Y, McCann TE, et al. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J Cell Sci. 2008;121:3083–3091. doi: 10.1242/jcs.032649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dou CL, Levine JM. Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J Neurosci. 1994;14:7616–7628. doi: 10.1523/JNEUROSCI.14-12-07616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ughrin YM, Chen ZJ, Levine JM. Multiple Region of the NG2 proteoglycan inhibit neurite growth and induce growth cone collapse. J Neurosci. 2003;23:175–186. doi: 10.1523/JNEUROSCI.23-01-00175.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rónn LC, Ralets I, Hartz BP, et al. A simple procedure for quantification of neurite outgrowth based on stereological principles. J Neurosci Methods. 2000;100:25–32. doi: 10.1016/s0165-0270(00)00228-4. [DOI] [PubMed] [Google Scholar]