Abstract
The identification of aberrant cellular pathways and dysfunctional molecules important in neoplastic transformation has begun to provide us with a number of targets for drug development. It is likely that many of these agents will be incorporated into our existing treatment strategies that include cytotoxic agents. Sorafenib, a multi-kinase inhibitor has been approved in the United States for the treatment of renal cell carcinoma as well as hepatocellular cancer. Its potential role in hematological malignancies, particularly acute myeloid leukemia (AML) is under evaluation. Here we describe the biological pathways in AML that are the potential targets of sorafenib action and discuss the early clinical data with the agent in solid tumors and AML.
Keywords: AML, FLT3 mutations, MAP kinase signalling, sorafenib
Introduction
Every year, ~11,000 new patients with acute myelogenous leukemia (AML) are diagnosed. AML is a heterogeneous hematopoietic stem cell (HSC) neoplasm with a median age at presentation of ~65 years. The incidence of both AML and myelodysplastic syndrome (MDS) is on the rise [1]. AML develops de novo, from other hematopoeitic/bone marrow disorders, or following chemotherapy and radiation. Despite significant advances in the treatment of AML, the long term outcome in most patients with the disease is unsatisfactory and new therapeutic strategies are badly needed. Progress in understanding the biology of the disease has led to the identification of a number of disordered cellular pathways that can be the targets for drug development.
Sorafenib is a small molecule oral kinase inhibitor, initially developed to target Raf kinase. Subsequently, it was shown to inhibit vascular endothelial growth factor receptor (VEGFR), platelet derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR), FMS-like tyrosine kinase 3 (FLT3), c-KIT and RET proteins. Its effectiveness in cancer therapy was proven in the ‘Treatment Approaches in Renal Cancer Global Evaluation Trial (TARGET)’ where clear activity in patients with advanced renal cell carcinoma (RCC) was demonstrated. As a result of this trial, sorafenib was recently approved by the US Food and Drug Administration for the treatment of renal cell carcinoma. Similar promising data have been noted in hepatocellular carcinoma (HCC). Phase III randomised trials in HCC have recently shown that there was a statistically significant improvement in survival of 3 months [2].
With the identification of its ability to inhibit several receptor kinases, preclinical and clinical studies in leukemias including AML were initiated. In this article, we review the putative mechanisms of action of this multi-kinase inhibitor, particularly as related to the signalling pathways involved in leukemogenesis and evaluate its potential as an agent for treating patients with AML.
Potential targets of sorafenib as applied to AML
Ras/Raf/Mek/Erk signalling pathway
The mitogen-activated protein Kinase (MAPK) signalling pathway is constitutively activated in ~50% of patients with AML [3]. The Ras/Raf/Mek/Erk signalling cascade includes a number of serine/threonine kinases, which are activated in response to extracellular stimuli and mediate cellular processes such as proliferation, differentiation and inhibition of apoptosis (Figure 1). Through a series of phosphorylation reactions and in combination with several other signalling pathways, these MAPKs can alter the activation status of a number of transcription factors. Normally, these pathways are intricately regulated but when these processes go awry oncogenesis ensues.
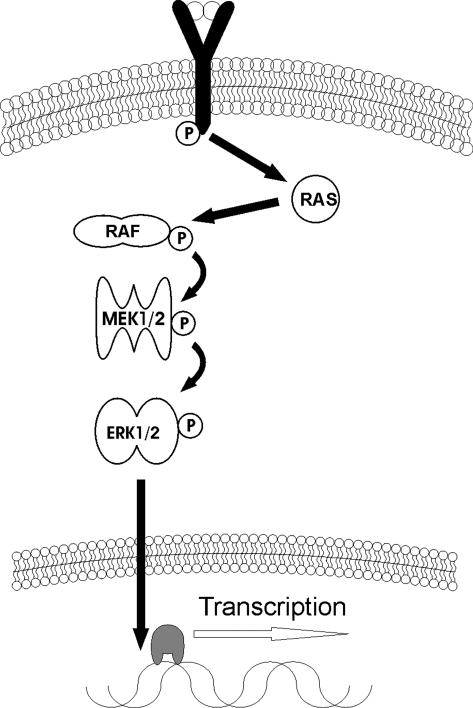
Figure 1.
The Ras/Raf/Mek/Erk (MAPK) pathway. Ligand-mediated activation of RTK via G-protein coupled mechanism activates RAS. Through a series of phosphorylations and dephosphorylations ultimately resulting in ERK activation, the survival signal results in differentiation and proliferation. Over activation, however, leads to oncogenesis in conjunction with G1 escape via amplification or de-regulation of nuclear transcription factor targets such as myc and AP-1.
The role of the MAPK signalling pathway in cellular processes has been well studied [4]. Once bound to its receptor causing its dimerisation at the cell surface, which in turn causes autophosphorylation of cytoplasmic portions exposing the binding site for Grb/Sos protein. The latter then binds and activates Ras protein by exchanging a GTP for GDP. Activated Ras binds and activates Raf (a dual specificity serine-threonine kinase), which then phosphorylates and activates MEK 1 and MEK 2 at two sites on serine218 and serine222. Activated MEK 1 and MEK 2 go on to phosphorylate and activate their respective substrates ERK 1 (p44MAPK) and ERK2 (p42MAPK). Once ERK is activated, the protein traverses the nuclear membrane and phosphorylates many targets including transcription factors necessary for progression through the cell cycle (Figure 1). Additionally, ERKs inhibit proapoptotic proteins Bim, Bax, Bak and phosphorylates Bcl-2, ensuring smooth progression of cell division (Figure 2). The importance of this pathway is underscored by the findings that ~25% of patients with AML display mutations in the Ras gene and further that constitutively activated (phosphorylated) ERK is associated with a poor prognosis in AML patients [1,5–9].
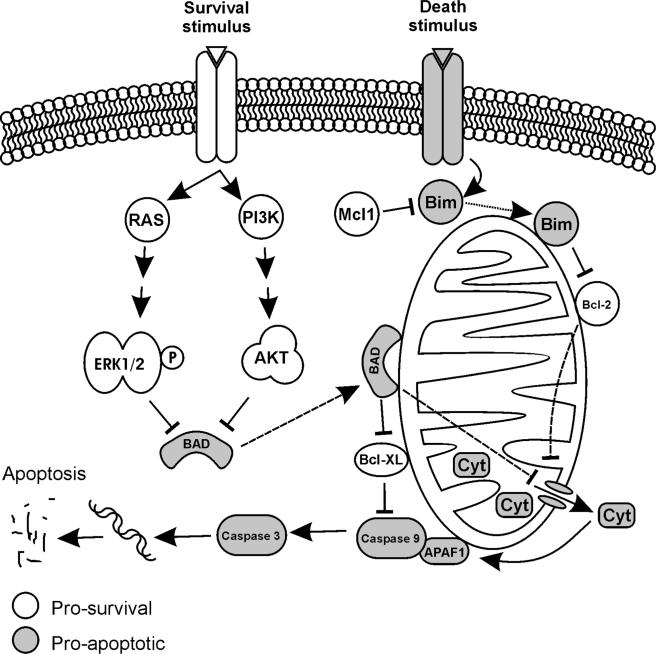
Figure 2.
Interaction of RAS/AKT proteins with apoptotic and survival signals. The pro-survival Bcl-2 proteins prevent mitochondrial mediated release of cytochrome c and thus activation of caspases whereas the pro-death proteins including Bim and Bad, Bax and Bak release apoptotic factors from the mitochondria. Bad in the non-phosphorylated state associates with Bcl-2 or Bcl-XL, promoting apoptosis. Akt and ERK phosphorylate Bad and allow its sequestration and thus inhibition of apoptosis. MCL-1 is a negative regulator of apoptosis, which acts by directly binding with BH3-only proteins such as Bim and inhibiting cytochrome c release and subsequent activation of caspases and the apoptotic cascade.
FMS-like tyrosine kinase 3 signalling
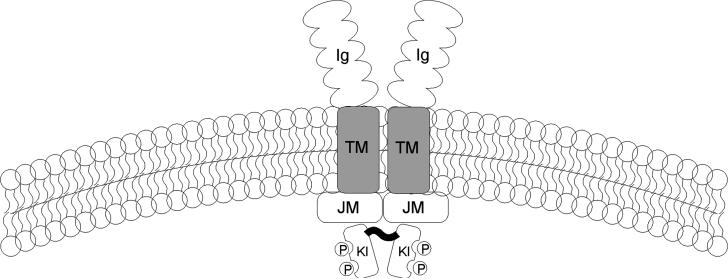
In addition to MAPK deregulation, AML blasts display other abnormalities in cell signalling. The FLT3 gene encodes a membrane-bound receptor, which when activated by its ligand [FLT3 ligand (FL)], allows proliferation, differentiation and survival of HSCs [10]. FLT3 is a transmembrane protein with five extracellular immunoglobulin-like domains: a transmembrane domain, a juxtamembrane domain and two tyrosine-kinase domains linked together by a hydrophilic tyrosine-kinase insert. It shares signifi-cant homology to the PDGF, Kit (receptor for stem cell factor, SCF) and macrophage colony stimulating factor receptors (Figure 3).
Figure 3.
The structure of FLT3. FLT3 has 5 immunoglobulin-like chains that make up the extracellular ligand-binding motif, a transmembrane domain and a cytoplasmic portion composed of a kinase domain interrupted by a kinase insert. The juxtamembrane domain is where the ITDs occur.
Wild-type FLT3 is expressed primarily on CD34þ HSCs as well a small portion on CD347 cells [11]. It usually resides in monomeric, inactivated form on the cell surface until FL binds the receptor resulting in its dimerisation. Once dimerised, FLT3 proteins promote phosphorylation of the tyrosine-kinase domains, thereby activating the receptor and downstream effectors. The dimerised receptors are quickly internalised and degraded [12,13]. FLT3 is over-expressed in a number of leukemias including AML, acute lymphoblastic leukemia, and chronic myelogenous leukemia (CML) [10]. Two major classes of FLT3 mutations have been identified, which include internal tandem duplication (ITD) of the juxtamembrane domain (FLT3-ITD) and point mutations in the activating loop. These mutations allow for constitutive, ligand-independent activation of FLT3 resulting in the aberrant proliferation and survival of hematopoietic stem/progenitor cells [14].
The importance of FLT3 in leukemogenesis is underscored by the fact that it is one of the most frequently mutated genes in hematological malignancies occurring in ~25 to 30% of patients with AML.
Although the FLT3 signalling cascade has yet to be elucidated fully, it is well established that the signal transduction occurs via two main pathways, PI3 and MAPKs. PI3 kinase (PI3K) activity is likely regulated via the phosphorylated FLT3 and SH2-containing sequence proteins [15,16]. Activated PI3K stimulates downstream proteins resulting in the activation of p3-phosophinositide-dependent protein kinase, protein kinase B (Akt/PKB) and mammalian target of rapamycin (mTOR) [17]. The signalling protein mTOR, through the activation of p70-S6 kinase, initiates transcription and translation of crucial regulatory genes and blocks apoptosis by phosphor-ylation of the pro-apoptotic Bcl2-family protein Bad [18,19]. Additionally, activated FLT3 associates with GRB2 and activates Ras/Raf/Mek/Erk signalling pathway, stimulating downstream effectors mentioned above and providing a cross-talk between these two signal transduction pathways. The coordinated differentiation and proliferation of HSCs also involves the Janus tyrosine kinase (JAK)/signal transducer and activator of transcription (STAT) pathways. The JAK/STAT pathway is involved in cellular proliferation, differentiation and survival and modulated by FLT3 and MAPK signalling. The constitutive, unregulated activation of these pathways has shown to play an important role in oncogenesis [20]. C-kit, another receptor tyrosine kinase (RTK) III, has also been shown to cause leukemogenesis via JAK/STAT pathway [21].
FMS-like tyrosine kinase 3 mutations
Activating mutations in the FLT3 protein were first identified a decade ago [22]. FLT3 gene is located on chromosome 13q12 [23]. Although predominantly a hematopoietic protein, FLT3 can also be identified in the thymus, lymph node, testis, brain and placenta. As the hematopoietic cells mature and differentiate, FLT3 protein expression and their ability to repopu-late myeloid cells is lost [24]. The ligand for FLT3 (FL) is a 20 kD ubiquitously expressed protein produced by bone marrow stromal cells, which upon binding to its receptor and in high synergy with other growth factors results in expansion of HSCs [25]. Knockout mice with the inability to express both FLT3 and FL have been developed. These mice show normal life-expectancy with no hematological abnormalities with the exception of reduced B-cell, reduced dendritic cell (DC) and natural killer cell numbers [26,27]. This suggests that signal transduction through FLT3 is redundant. Addition of FL in vitro allows the proliferation and differentiation of monocytic and DC progenitors, as well as early B-lymphoid precursors (only in the presence of lineage specific cytokines). In contrast, FL alone can only induce early hematopoeitic monocytic differentiation [28,29]. The transplantation of FLT3 deficient cells resulted in a decrease in myeloid and lymphoid re-population but the effect was significantly more exaggerated in the former population [27]. Thus it appears that the role of FL/FLT3 in normal hematopoiesis involves the recruitment of myeloid and lymphoid progenitor cells.
FLT3 is highly expressed on the surface of AML blasts [11,23,30]. The first report of mutant FLT3 in patients with AML identified duplications in the juxtamembrane domain of the protein, coined the (ITD) mutation. These repeating sequences were of varying lengths (4–68 amino acids), which could involve new sequences or repeats of native sequences. The presence of these repeats change the length of the JM domain, which is important not only for the auto-inhibition of the RTK but also for accessibility of the activation loop to kinases [22]. The activation loop domain point mutations are mainly confined to single amino acid substitution of Asp835 [31].This results in sustained activity of the receptor without the presence of its ligand [32].
The crystal structure of FLT3 has revealed that the JM domain and the activation loop bring about auto-inhibition of the protein by preventing ATP binding to the activation centre. This is thought to occur by the dephosphorylation of tyrosine residues in both segments resulting in conformational change in the protein and the exposure of the activation centre [32]. The current theory suggests that the mutations in the ITD and the activation loop remove the inhibitory conformation allowing for unrestricted access to the activation loop. Recently, activating ITDs have been identified in non-JM domain in ~25% of patients with AML [33].
The ITD mutations occur predominantly in patients with normal karyotype. Their incidence varies among the FAB subtypes; they occur more frequently in patients with acute promyelocytic leukemia and are associated with leukocytosis and increased bone marrow blasts. They occur least frequently in the M2 and M6 phenotypes [34]. There is inter-mediate expression on other subtypes [34–36]. The presence of ITD mutations is associated with poor disease-free survival [37]. It is important to note that AML blasts express both wild-type and ITD-mutant alleles and that the expression can be varied in the course of disease. However, it appears that higher ratio of mutant to wild-type allele expression correlates directly with poorer overall prognosis [31].
The tumorigenicity of FLT3/ITD mutations has been well established in vitro by a number of laboratories showing that transfection of mutants into tissue culture cells resulted in gain of function of activity through constitutive auto-phosphorylation [36,38]. Mechanistically, transfection of mutated FL into the pro-B cell line could induce tumorigenesis in syngeneic mice by induction of the JAK/STAT pathway [39]. Therefore, although the wild-type signalling is not essential for normal hematopoiesis, it appears to be important in leukemogenesis. It is interesting to note that although in its normal state ligand-activated FLT3 is a weak activator of STAT5, the mutant form is a strong and constitutive inducer of STAT5 in the presence of FL and irrespective of mitogenic signals [37]. When this signalling is disrupted by RTK inhibitors, the leukemic cells were unable to differentiate into myeloid cell lines [28].
Another protein involved in the pathogenesis of AML, CAAT/enhancer binding protein α (C/EBPα) should also be mentioned. C/EBPα is a leucine zipper transcription factor required for myeloid cell differentiation, which is expressed in early myeloid precursors and is up-regulated during granulocytic differentiation [40–42]. In AML, the expression and/or activity of C/EBPα is inhibited thus removing the initiator of differentiation into neutrophilic population [41,43]. More importantly, the re-expression of functional C/EBPα into leukemic cells could restore their differentiation into granulocytes. C/EBPα is directly phosphorylated by ERK1/2 on Serine 21, which affects the ability of C/EBPα to induce differentiation [41]. In FLT3 mutant myeloid cells, the C/EBPα protein activity is inhibited by post-translational modification (phosphorylation of the Serine 21). Elegant work has shown that by removing the ability of C/EBPα to be phosphorylated, the differentiation block can be removed [40] (Figure 3).
Sorafenib
Sorafenib is an oral small molecule bi-aryl urea multi-kinase inhibitor, which was first shown to inhibit Raf-1 of the MAPK pathway resulting in diminished MEK and ERK phosphorylation [44]. It was later shown that the molecule was also capable of inhibiting endogenous MAPK activation in the cancer cell lines NIH 3T3 and HCT 116 [45]. The framework for the use of RTK inhibitors was originally laid out by the success of Imatinib (Gleevac) in the treatment of CML [46]. Sorafenib was developed by screening small molecule libraries for molecules capable of inhibiting Raf-1 [44]. In pre-clinical studies, the selective RTK inhibitor demonstrated significant dose dependent anti-tumor effects in human xenograft models of colon, ovarian, pancreatic, thyroid, melanoma and breast cancers [47]. It was later shown that sorafenib inhibits the auto-phosphorylating ability of several RTKs including FLT3, VEGFR1-3, PDGFR, c-KIT and RET as well downstream targets such as RAF. Interestingly, in some colon cancer and NSCLC xenografts, the anti-tumor activity was not through the MAPK pathway [45], which suggested other, yet to be identified signalling pathways are involved [48,49]. Because of its ability to target VEGF/EGF receptor pathway, it has been suggested that sorafenib could inhibit its anti-tumor effect by an anti-angiogenic mechanism [45,50]. This was supported by studies showing decreased microvessel density in xenograft human HCC models in sorafenib-treated animals [51]. Also, via inhibiting the MAPK signal transduction pathway, sorafenib exerted its anti-tumor effect by inducing apoptosis in several cancer cell lines [52].
Clinical development of sorafenib
Phase I clinical trials of sorafenib (as monotherapy) were initially conducted in patients with refractory solid tumors including colorectal, renal and HCC. In four clinical trials, 170 patients were treated with various doses of sorafenib. The 400 mg twice daily dose was identified as having the least toxicity and was later chosen for the Phase II trials. In Phase I trials, diarrhea, fatigue, skin lesions (rash) and hypertension were the most frequent adverse effects, although most were seen at the 600 mg and 800 mg dose levels (DL). Almost all patients showed mild to moderate side-effects manageable with dose-titration or discontinuation of treatment. In 11 patients with RCC, one patient had a sustained partial response lasting 104 days and two showed stable disease 42 years [52].
The choice of RCC for Phase II and III trial was based on the fact that RCC is highly chemotherapy resistant. In RCC, Von-Hipple–Lindau (VHL) is a tumor suppressor gene, which is induced in response to tissue hypoxia. It functions by binding hypoxiainducible factor (HIF), a transcription factor, which promotes cell growth and survival via VEGF, TGF-α and PDGF-receptor and targets the cells for destruction. In RCC, the regulatory activity of VHL is lost and HIF can function at normal oxygen tension for angiogenesis and uncontrolled growth [53]. Sorafenib was deemed attractive for its anti-angiogenic effects.
After successful completion of Phase I trials, a Phase II study in advanced solid tumors was initiated [54]. Patients initially received oral sorafenib 400 mg twice daily. After 12 weeks, those with tumor regression of <25% were randomly assigned to sorafenib or placebo for an additional 12 weeks. Those with ≥25% tumor shrinkage continued open-label on sorafenib whereas non-responders, (those with ≥25% tumor growth) discontinued treatment. Among 202 patients treated during the run-in period, 73 patients had tumor shrinkage of ≥25% and 65 had stable disease at 12 weeks. The latter were randomised to sorafenib or placebo and followed for another 12 weeks. At 24 weeks, 50% of the sorafenib-treated group attained PFS versus 18% of the placebo group. Median PFS in sorafenib group was 24 weeks versus 6 weeks in the placebo group. Common adverse events were rash and fatigue. Responses included a 38% partial response and 28% stable disease in patients with RCC; there were no deaths related to the study drug [54].
The desirable safety profile of sorafenib and its ability to stabilise RCC led to the design of a Phase III, randomised, double blind, multi-centre trial: TARGET. In this intent-to-treat study, 903 patients with advanced clear cell RCC and Eastern Cooperative Oncology Group performance of < 2 with no brain metastasis were enrolled [55]. The patients were resistant to standard therapy and randomised to receive oral sorafenib (400 mg twice daily) or placebo with primary endpoint of overall survival. In the interim analysis, ~78% of patients had stable disease and as a result a crossover plan from placebo to sorafenib was instituted. There was also a trend to increased overall survival (19.3 versus 15.9 months), although not statistically significant. Partial responses were 10% of patients receiving sorafenib and 2% of those receiving placebo (p < 0.001). A similar toxicity profile to that in the Phase II study was observed.
Sorafenib and acute myeloid leukemia
We first reported the direct interaction of sorafenib with FLT3/ITD at an IC50 of 2 nM whereas the IC50 for FLT3wt cells was 3000 nM [56]. We further demonstrated inhibition of FLT3/ITD signalling in kinase assays associated with inhibition of ERK and subsequent cell cycle arrest and apoptosis in FLT3/ITD carrying cell lines [56]. The observations that sorafenib directly interacted with the mutant FLT3 protein, c-kit and inhibited Raf-1 in the MAPK (RAF/MEK/ERK) pathway led to its evaluation in AML. Approximately 90% of AML cells have abnormalities in c-kit and FLT3 and 50% of AML cells show over-expression of the MAPK signalling pathway.
By preventing the activation of ERK, sorafenib is able to inhibit AML blasts in a number of ways. In vitro evaluation of sorafenib against AML cell lines has confirmed the rationale for therapy. In vitro studies in leukemia cell lines MV4-11 (monocytic) and EOL-1 (eosinophlic) have shown that sorafenib inhibited FL3-dependent cell proliferation via cell cycle arrest and apoptosis [57]. In the same study, the xenograft transplantation of leukemic cells in NCr nu/nu mice and subsequent treatment with sorafenib administered orally for 14 days resulted in >60% complete responses. It is important to note that not all inhibitors of the MAPK pathway have similar mechanism of action. Some inhibitors have been shown to cause cell cycle inhibition without apoptosis, which may provide an opportunity for increased resistance [58,59].
Recently, specific anti-apoptotic pathways have been elucidated. We reported evidence for sorafenib-induced Bim-mediated activation of the apoptotic pathway [60]. Normally, activation of Raf/Mek/Erk pathway allows re-entry into the cell cycle. This is accomplished by inhibiting the pro-apoptotic BH3-only members of the Bcl-2 family Bim, Bad, Bax and Bak [61,62]. Sorafenib removes the negative regulatory effect on Bim in AML cell lines, which subsequently translocates to the mitochondria neutralising the effect of Bcl2 [60] [and results in Bim-mediated activation of caspase 3 and pro-apoptotic proteins (Bim, Bad, Bax and Bak)] leading to loss of mitochondrial membrane potential. Furthermore, sorafenib can also exert its effects by down regulating myeloid cell leukemia Sequence 1 (MCL-1), another anti-apoptotic protein member of the Bcl2 family [60,63] (Figure 3).
In a murine model of AML with FLT3/ITD mutation, sorafenib prevented proliferation and induced apoptosis of murine Ba/F3 leukemic cells, an effect, which was partially reversible by the addition of IL-3 [64]. In the same study, the therapeutic effect of sorafenib could only be demonstrated in FLT3/ITD mutants and not wild-type AML cells suggesting that it required the mutant protein for efficacy. Furthermore, sorafenib significantly lowered leukemic burden in the spleen and liver of a murine xenograft model. However, these effects were not as pronounced in as expected, suggesting that the micro-environment may play an important role in sorafenib efficacy [64].
Sorafenib may exert its apoptotic effect via PI3K signalling pathway. The latter is an inhibitor of the pro-apoptotic protein Bad. It is conceivable that sorafenib, due to its multi-kinase inhibitory ability, is able to induce apoptosis by targeting multiple redundant signalling pathways. Sorafenib can also mediate its anti-leukemic effect by inhibiting c-Kit activation of Erk [65]. Another possible mechanism of action of sorafenib may be that the inhibition of MAPK pathway eliminates ERK-mediated inactivation of C/EBPα thus allowing the leukemic cells to further differentiate into myeloid cells.
Clinical experience with sorafenib in AML
A Phase I trial to evaluate the safety and efficacy of two different schedules of sorafenib was reported [66]. Twenty-one patients with refractory/relapsed AML (n = 20) and high risk MDS (n = 1) were enrolled. They were randomised to sorafenib for 5 days per week for 21 days (arm A, n = 11) or for 14 days for every 21 days (arm B, n = 10). In both arms the starting DL was 200 mg twice daily. Successive DLs were 600, 800 and 1200 mg daily. Peripheral blood and bone marrow samples were obtained for evaluation of FLT3 status, phosphorylated and total FLT3, and ERK expression. Median age was 62 years (range, 33–82), and the number of prior therapies 2 (range, 1–5). The median time from diagnosis to initiation of sorafenib was 9 months (range, 2–46), and median duration on study was 1.2 months (range, 0.1–3.4). Twenty patients were evaluable, 9/20 (45%) received ≤1 cycle of sorafenib because of disease progression (n = 6), self-discontinuation (n = 2) or no benefit (n = 1), of whom five (56%) were FLT3-ITD negative, three (33%) were FLT3-ITD positive and one (11%) was not tested. In contrast, 11/20 (55%) patients received >1 cycle of sorafenib, of whom eight (73%) were FLT3-ITD positive and three (27%) were FLT3-ITD negative; reasons for discontinuation were disease progression (n = 5), self-discontinuation (n = 2), stem cell transplant (n = 2) or no benefit (n = 2). Sorafenib was well tolerated with 1 patient achieving a DLT of Grade 3 hyperbilirubinemia at the 800 mg daily dose in arm B, but the MTD has not been reached. The only other Grade 3 toxicity was pleural effusion at the 600 mg daily dose in arm A, not considered as a DLT because it occurred during Cycle 2. A ≥50% reduction in peripheral blood or bone marrow blasts was achieved in 11/20 (55%) patients, 9/11 (82%) patients harboured the FLT3-ITD mutation and had a median duration of response of 42 days (range, 15–87). In these nine patients, the median peripheral blood absolute blast count at baseline and after maximal response to sorafenib were 10.3 (range, 0.2–18.7) and 0 (range, 0–1)(p = 0.008). The median bone marrow blast percentage at baseline and after maximal response to sorafenib were 72% (range, 14–96) and 42% (range, 12–58) (p = 0.002), with 1 pt achieving a morphologic complete remission in the bone marrow. Serial determinations of phosphorylation status following sorafenib (at 0, 2, 24 and 120 h) in patients with the FLT3-ITD mutation demonstrated inhibition of phospho-FLT3 in 3/3 and phospho-ERK in 5/5 patients. The authors concluded that sorafenib was safe in AML and appears to preferentially target the FLT3-ITD mutation. Other reports of activity of single agent sorafenib in AML have been recently published [67]. Molecular remission after therapy with sorafenib was reported in a patient with FLT3-ITD+ AML and extra-medullary disease who had relapsed following an allogeneic transplant. A plausible hypothesis for potential improvement in the efficacy of sorafenib after transplantation may be the absence of leukemic cell rescue by stromal cells. It is well established that quantitative and qualitative changes in the bone marrow micro-environment occur after transplantation [68,69]. Such suboptimal stromal environment may not furnish protective growth factors for leukemic cells and enhance their sensitivity to agents like sorafenib. Indeed, in Ba/F3-ITD leukemic cell lines transfected with a FL3/ITD mutant vector, the inhibitory effects of sorafenib was completely reversed by presence of IL-3 [64]. We reported that co-culture of AML cells with mesenchymal stromal cells protected AML cells and stem cells from chemotherapy [70]. Therefore, it may be important to reduce the protective effects of the hematopoietic microenvironment by mobilisation of blast cells from the bone marrow to ensure effective targeting resulting in leukemic cell apoptosis.
Generally, the use of RTK inhibitors as mono-therapy of AML has been disappointing. For example, only a transient partial remission in a small number of patients with AML was reported with SU11248 (an inhibitor of FLT3, c-Kit, VEGFR and PDGFR) [71,72]. For this reason, combination of sorafenib with traditional cytotoxic chemotherapy in AML is currently under evaluation. Cytarabine (Ara-C) induces MAPK activity in AML cells, and this attenuates its cytotoxic effects [73]. It is possible that sorafenib can potentiate the effect of Ara-C by counteracting the MAPK induction. Several studies are examining the role of sorafenib in combination with standard induction regimens in frontline therapy of AML, its role in elderly patients with AML and its potential activity in MDSs, chronic lymphocytic leukemia and other hematological malignancies.
The heterogeneous nature of AML poses problem for RTK inhibitors such as sorafenib. For instance, not all leukemic cells display RTK mutations [5]. Another challenge is the presence of quiescent leukemic stem cells that do not respond to chemotherapy and contribute to treatment failure and resistance [74]. Relevant to sorafenib, the presence of FLT3/ITD in AML stem cells has recently been reported [75]. It remains unclear whether RTK inhibitors are able to effectively target these quiescent cells. Another important point for consideration using sorafenib or other RTK inhibitors is the presence or development of resistance. Inherent resistance of mutant FLT3 proteins to MLN518 and PKC412 have already been reported [76,77].
Conclusion
The development of imatinib mesylate has introduced a new era in leukemia therapy. Identification of molecular aberrations in the cellular proteins involved in differentiation, growth and survival has provided us with new targets for the development of effective therapeutic strategies. Although in CML, single agent tyrosine kinase inhibitors are effective therapies, it is clear that in acute leukemias and in more advanced phases of CML agents with efficacy against multiple targets, perhaps in combination with traditional cytotoxic chemotherapy are needed. The identification of FLT3 mutations as well as other aberrant signalling pathways has re-defined the potential future therapy in AML. It remains important to further elucidate the pathogenic mechanisms involved in the development of the disease to identify other targets for therapy. In addition, it may be important to identify environmental and mitogenic factors that play a role in the pathogenesis of the disease and protection of leukemic blasts.
Footnotes
Declaration of interest: F. Ravandi received research funding from Bayer/Onyx.
References
- 1.Jabbour EJ, Estey E, Kantarjian HM. Adult acute myeloid leukemia. Mayo Clin Proc. 2006;81:247–260. doi: 10.4065/81.2.247. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 3.Ricciardi MR, McQueen T, Chism D, Milella M, Estey E, Kaldjian E, et al. Quantitative single cell determination of ERK phosphorylation and regulation in relapsed and refractory primary acute myeloid leukemia. Leukemia. 2005;19:1543–1549. doi: 10.1038/sj.leu.2403859. [DOI] [PubMed] [Google Scholar]
- 4.Ravandi F, Talpaz M, Estrov Z. Modulation of cellular signaling pathways: prospects for targeted therapy in hematological malignancies. Clin Cancer Res. 2003;9:535–550. [PubMed] [Google Scholar]
- 5.Doepfner KT, Boller D, Arcaro A. Targeting receptor tyrosine kinase signaling in acute myeloid leukemia. Crit Rev Oncol Hematol. 2007;63:215–230. doi: 10.1016/j.critrevonc.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Milella M, Kornblau SM, Estrov Z, Carter BZ, Lapillonne H, Harris D, et al. Therapeutic targeting of the MEK/MAPK signal transduction module in acute myeloid leukemia. J Clin Invest. 2001;108:851–859. doi: 10.1172/JCI12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milella M, Estrov Z, Kornblau SM, Carter BZ, Konopleva M, Tari A, et al. Synergistic induction of apoptosis by simultaneous disruption of the Bcl-2 and MEK/MAPK pathways in acute myelogenous leukemia. Blood. 2002;99:3461–3464. doi: 10.1182/blood.v99.9.3461. [DOI] [PubMed] [Google Scholar]
- 8.Milella M, Konopleva M, Precupanu CM, Tabe Y, Ricciardi MR, Gregorj C, et al. MEK blockade converts AML differentiating response to retinoids into extensive apoptosis. Blood. 2007;109:2121–2129. doi: 10.1182/blood-2006-05-024679. [DOI] [PubMed] [Google Scholar]
- 9.Kornblau SM, Womble M, Qiu YH, Jackson CE, Chen W, Konopleva M, et al. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood. 2006;108:2358–2365. doi: 10.1182/blood-2006-02-003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100:1532–1542. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 11.Drexler HG. Expression of FLT3 receptor and response to FLT3 ligand by leukemic cells. Leukemia. 1996;10:588–599. [PubMed] [Google Scholar]
- 12.Lyman SD. Biologic effects and potential clinical applications of FLT3 ligand. Curr Opin Hematol. 1998;5:192–196. doi: 10.1097/00062752-199805000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Small D, Levenstein M, Kim E, Carow C, Amin S, Rockwell P, et al. STK-1, the human homolog of Flk-2/Flt-3, is selectively expressed in CD34+ human bone marrow cells and is involved in the proliferation of early progenitor/stem cells. Proc Natl Acad Sci USA. 1994;91:459–463. doi: 10.1073/pnas.91.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly LM, Yu JC, Boulton CL, Apatira M, Li J, Sullivan CM, et al. CT53518, a novel selective FLT3 antagonist for the treatment of acute myelogenous leukemia (AML). Cancer Cell. 2002;1:421–432. doi: 10.1016/s1535-6108(02)00070-3. [DOI] [PubMed] [Google Scholar]
- 15.Dosil M, Wang S, Lemischka IR. Mitogenic signalling and substrate specificity of the Flk2/FLT3 receptor tyrosine kinase in fibroblasts and interleukin 3-dependent hematopoietic cells. Mol Cell Biol. 1993;13:6572–6585. doi: 10.1128/mcb.13.10.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lisovsky M, Braun SE, Ge Y, Takahira H, Lu L, Savchenko VG, et al. FLT3-ligand production by human bone marrow stromal cells. Leukemia. 1996;10:1012–1018. [PubMed] [Google Scholar]
- 17.Lyman SD, Seaberg M, Hanna R, Zappone J, Brasel K, Abkowitz JL, et al. Plasma/serum levels of FLT3 ligand are low in normal individuals and highly elevated in patients with Fanconi anemia and acquired aplastic anemia. Blood. 1995;86:4091–4096. [PubMed] [Google Scholar]
- 18.Martelli AM, Tazzari PL, Evangelisti C, Chiarini F, Blalock WL, Billi AM, et al. Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin module for acute myelogenous leukemia therapy: from bench to bedside. Curr Med Chem. 2007;14:2009–2023. doi: 10.2174/092986707781368423. [DOI] [PubMed] [Google Scholar]
- 19.Zeng Z, Sarbassov dos D, Samudio IJ, Yee KW, Munsell MF, Ellen Jackson C, et al. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood. 2007;109:3509–3512. doi: 10.1182/blood-2006-06-030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 21.Ning ZQ, Li J, Arceci RJ. Signal transducer and activator of transcription 3 activation is required for Asp(816) mutant c-Kit-mediated cytokine-independent survival and proliferation in human leukemia cells. Blood. 2001;97:3559–3567. doi: 10.1182/blood.v97.11.3559. [DOI] [PubMed] [Google Scholar]
- 22.Nakao M, Janssen JW, Erz D, Seriu T, Bartram CR. Tandem duplication of the FLT3 gene in acute lymphoblastic leukemia: a marker for the monitoring of minimal residual disease. Leukemia. 2000;14:522–524. doi: 10.1038/sj.leu.2401695. [DOI] [PubMed] [Google Scholar]
- 23.Carow CE, Kim E, Hawkins AL, Webb HD, Griffin CA, Jabs EW, et al. Localization of the human stem cell tyrosine kinase-1 gene (FLT3) to 13q12–>q13. Cytogenet Cell Genet. 1995;70:255–257. doi: 10.1159/000134046. [DOI] [PubMed] [Google Scholar]
- 24.Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. FLT3 ligand regulates dendritic cell development from FLT3+ lymphoid and myeloid-committed progenitors to FLT3+ dendritic cells in vivo. J Exp Med. 2003;198:305–313. doi: 10.1084/jem.20030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kusadasi N, Koevoet JL, van Soest PL, Ploemacher RE. Stromal support augments extended long-term ex vivo expansion of hemopoietic progenitor cells. Leukemia. 2001;15:1347–1358. doi: 10.1038/sj.leu.2402204. [DOI] [PubMed] [Google Scholar]
- 26.McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, et al. Mice lacking FLT3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–3497. [PubMed] [Google Scholar]
- 27.Mackarehtschian K, Hardin JD, Moore KA, Boast S, Goff SP, Lemischka IR. Targeted disruption of the flk2/FLT3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity. 1995;3:147–161. doi: 10.1016/1074-7613(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 28.Zheng R, Friedman AD, Small D. Targeted inhibition of FLT3 overcomes the block to myeloid differentiation in 32Dcl3 cells caused by expression of FLT3/ITD mutations. Blood. 2002;100:4154–4161. doi: 10.1182/blood-2002-03-0936. [DOI] [PubMed] [Google Scholar]
- 29.Veiby OP, Jacobsen FW, Cui L, Lyman SD, Jacobsen SE. The FLT3 ligand promotes the survival of primitive hemopoietic progenitor cells with myeloid as well as B lymphoid potential. Suppression of apoptosis and counteraction by TNF-alpha and TGF-beta. J Immunol. 1996;157:2953–2960. [PubMed] [Google Scholar]
- 30.Rosnet O, Buhring HJ, Marchetto S, Rappold I, Lavagna C, Sainty D, et al. Human FLT3/FLK2 receptor tyrosine kinase is expressed at the surface of normal and malignant hematopoietic cells. Leukemia. 1996;10:238–248. [PubMed] [Google Scholar]
- 31.Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 32.Griffith J, Black J, Faerman C, Swenson L, Wynn M, Lu F, et al. The structural basis for autoinhibition of FLT3 by the juxtamembrane domain. Mol Cell. 2004;13:169–178. doi: 10.1016/s1097-2765(03)00505-7. [DOI] [PubMed] [Google Scholar]
- 33.Breitenbuecher F, Schnittger S, Grundler R, Markova B, Carius B, Brecht A, et al. Identification of a novel type of ITD mutations located in non-juxtamembrane domains of the FLT3 tyrosine kinase receptor. Blood. doi: 10.1182/blood-2007-11-125476. in press. [DOI] [PubMed] [Google Scholar]
- 34.Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 35.Yokota S, Kiyoi H, Nakao M, Iwai T, Misawa S, Okuda T, et al. Internal tandem duplication of the FLT3 gene is preferentially seen in acute myeloid leukemia and MDS among various hematological malignancies. A study on a large series of patients and cell lines. Leukemia. 1997;11:1605–1609. doi: 10.1038/sj.leu.2400812. [DOI] [PubMed] [Google Scholar]
- 36.Kiyoi H, Naoe T, Nakano Y, Yokota S, Minami S, Miyawaki S, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93:3074–3080. [PubMed] [Google Scholar]
- 37.Mizuki M, Fenski R, Halfter H, Matsumura I, Schmidt R, Muller C, et al. FLT3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood. 2000;96:3907–3914. [PubMed] [Google Scholar]
- 38.Fenski R, Flesch K, Serve S, Mizuki M, Oelmann E, Kratz-Albers K, et al. Constitutive activation of FLT3 in acute myeloid leukaemia and its consequences for growth of 32D cells. Br J Haematol. 2000;108:322–330. doi: 10.1046/j.1365-2141.2000.01831.x. [DOI] [PubMed] [Google Scholar]
- 39.Tse KF, Mukherjee G, Small D. Constitutive activation of FLT3 stimulates multiple intracellular signal transducers and results in transformation. Leukemia. 2000;14:1766–1776. doi: 10.1038/sj.leu.2401905. [DOI] [PubMed] [Google Scholar]
- 40.Radomska HS, Huettner CS, Zhang P, Cheng T, Scadden DT, Tenen DG. CCAAT/enhancer binding protein alpha is a regulatory switch sufficient for induction of granulocytic development from bipotential myeloid progenitors. Mol Cell Biol. 1998;18:4301–4314. doi: 10.1128/mcb.18.7.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ross SE, Radomska HS, Wu B, Zhang P, Winnay JN, Bajnok L, et al. Phosphorylation of C/EBP alpha inhibits granulopoiesis. Mol Cell Biol. 2004;24:675–686. doi: 10.1128/MCB.24.2.675-686.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Scott E, Sawyers CL, Friedman AD. C/EBP alpha bypasses granulocyte colony-stimulating factor signals to rapidly induce PU.1 gene expression, stimulate granulocytic differentiation, and limit proliferation in 32D cl3 myeloblasts. Blood. 1999;94:560–571. [PubMed] [Google Scholar]
- 43.Pabst T, Mueller BU, Zhang P, Radomska HS, Narravula S, Schnittger S, et al. Dominant-negative mutations of CEBPA, encoding CCAAT/enhancer binding protein-alpha (C/EBP alpha), in acute myeloid leukemia. Nat Genet. 2001;27:263–270. doi: 10.1038/85820. [DOI] [PubMed] [Google Scholar]
- 44.Wilhelm S, Chien DS. BAY 43-9006: preclinical data. Curr Pharm Des. 2002;8:2255–2257. doi: 10.2174/1381612023393026. [DOI] [PubMed] [Google Scholar]
- 45.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 46.Druker BJ. Imatinib mesylate in the treatment of chronic myeloid leukaemia. Expert Opin Pharmacother. 2003;4:963–971. doi: 10.1517/14656566.4.6.963. [DOI] [PubMed] [Google Scholar]
- 47.Lyons JF, Wilhelm S, Hibner B, Bollag G. Discovery of a novel Raf kinase inhibitor. Endocr Relat Cancer. 2001;8:219–225. doi: 10.1677/erc.0.0080219. [DOI] [PubMed] [Google Scholar]
- 48.Kang B, Hao C, Wang H, Zhang J, Xing R, Shao J, et al. Evaluation of hepatic-metastasis risk of colorectal cancer upon the protein signature of PI3K/AKT pathway. J Proteome Res. 2008;7:3507–3515. doi: 10.1021/pr800238p. [DOI] [PubMed] [Google Scholar]
- 49.Steelman LS, Stadelman KM, Chappell WH, Horn S, Basecke J, Cervello M, et al. Akt as a therapeutic target in cancer. Expert Opin Ther Targets. 2008;12:1139–1165. doi: 10.1517/14728222.12.9.1139. [DOI] [PubMed] [Google Scholar]
- 50.Sharma A, Trivedi NR, Zimmerman MA, Tuveson DA, Smith CD, Robertson GP. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res. 2005;65:2412–2421. doi: 10.1158/0008-5472.CAN-04-2423. [DOI] [PubMed] [Google Scholar]
- 51.Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 52.Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 53.Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–2490. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 54.Ratain MJ, Eisen T, Stadler WM, Flaherty KT, Kaye SB, Rosner GL, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:2505–2512. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 55.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 56.Zhang W, Konopleva M, Shi YX, Harris D, Small D, Ling X, et al. Sorafenib (BAY 43-9006) directly targets FLT3-ITD in acute myelogenous leukemia. Blood. 2006:108. [Google Scholar]
- 57.Auclair D, Miller D, Yatsula V, Pickett W, Carter C, Chang Y, et al. Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia. 2007;21:439–445. doi: 10.1038/sj.leu.2404508. [DOI] [PubMed] [Google Scholar]
- 58.Baines P, Fisher J, Truran L, Davies E, Hallett M, Hoy T, et al. The MEK inhibitor, PD98059, reduces survival but does not block acute myeloid leukemia blast maturation in vitro. Eur J Haematol. 2000;64:211–218. doi: 10.1034/j.1600-0609.2000.90139.x. [DOI] [PubMed] [Google Scholar]
- 59.James JA, Smith MA, Court EL, Yip C, Ching Y, Willson C, et al. An investigation of the effects of the MEK inhibitor U0126 on apoptosis in acute leukemia. Hematol J. 2003;4:427–432. doi: 10.1038/sj.thj.6200327. [DOI] [PubMed] [Google Scholar]
- 60.Zhang W, Konopleva M, Ruvolo VR, McQueen T, Evans RL, Bornmann WG, et al. Sorafenib induces apoptosis of AML cells via Bim-mediated activation of the intrinsic apoptotic pathway. Leukemia. 2008;22:808–818. doi: 10.1038/sj.leu.2405098. [DOI] [PubMed] [Google Scholar]
- 61.Adams JM. Ways of dying: multiple pathways to apoptosis. Genes Dev. 2003;17:2481–2495. doi: 10.1101/gad.1126903. [DOI] [PubMed] [Google Scholar]
- 62.Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 63.Yu C, Bruzek LM, Meng XW, Gores GJ, Carter CA, Kaufmann SH, et al. The role of MCL-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene. 2005;24:6861–6869. doi: 10.1038/sj.onc.1208841. [DOI] [PubMed] [Google Scholar]
- 64.Zhang W, Konopleva M, Shi YX, McQueen T, Harris D, Ling X, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100:184–198. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 65.Tong FK, Chow S, Hedley D. Pharmacodynamic monitoring of BAY 43-9006 (Sorafenib) in phase I clinical trials involving solid tumor and AML/MDS patients, using flow cytometry to monitor activation of the ERK pathway in peripheral blood cells. Cytometry B Clin Cytom. 2006;70:107–114. doi: 10.1002/cyto.b.20092. [DOI] [PubMed] [Google Scholar]
- 66.Delmonte J. Update of a phase I study of Sorafenib in patients with refractory/relapsed acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood. 2007:110. [Google Scholar]
- 67.Safaian NN, Czibere A, Bruns I, Fenk R, Reinecke P, Dienst A, et al. Sorafenib (Nexavar((R))) induces molecular remission and regression of extramedullary disease in a patient with FLT3-ITD(+) acute myeloid leukemia. Leuk Res. doi: 10.1016/j.leukres.2008.04.017. in press. [DOI] [PubMed] [Google Scholar]
- 68.Fried W, Chamberlin W, Kedo A, Barone J. Effects of radiation on hematopoietic stroma. Exp Hematol. 1976;4:310–314. [PubMed] [Google Scholar]
- 69.Domenech J, Roingeard F, Binet C. The mechanisms involved in the impairment of hematopoiesis after autologous bone marrow transplantation. Leuk Lymphoma. 1997;24:239–256. doi: 10.3109/10428199709039012. [DOI] [PubMed] [Google Scholar]
- 70.Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16:1713–1724. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- 71.O'Farrell AM, Foran JM, Fiedler W, Serve H, Paquette RL, Cooper MA, et al. An innovative phase I clinical study demonstrates inhibition of FLT3 phosphorylation by SU11248 in acute myeloid leukemia patients. Clin Cancer Res. 2003;9:5465–5476. [PubMed] [Google Scholar]
- 72.Fiedler W, Serve H, Dohner H, Schwittay M, Ottmann OG, O'Farrell AM, et al. A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood. 2005;105:986–993. doi: 10.1182/blood-2004-05-1846. [DOI] [PubMed] [Google Scholar]
- 73.Jarvis WD, Fornari FA, Jr, Tombes RM, Erukulla RK, Bittman R, Schwartz GK, et al. Evidence for involvement of MAP kinase, rather than stress-activated protein kinase, in potentiation of 1-beta-D-arabinofuranosylcytosine-induced apoptosis by interruption of protein kinase C signaling. Mol Pharmacol. 1998;54:844–856. doi: 10.1124/mol.54.5.844. [DOI] [PubMed] [Google Scholar]
- 74.Ravandi F, Estrov Z. Eradication of leukemia stem cells as a new goal of therapy in leukemia. Clin Cancer Res. 2006;12:340–344. doi: 10.1158/1078-0432.CCR-05-1879. [DOI] [PubMed] [Google Scholar]
- 75.Li L, Piloto O, Kim KT, Ye Z, Nguyen HB, Yu X, et al. FLT3/ITD expression increases expansion, survival and entry into cell cycle of human haematopoietic stem/progenitor cells. Br J Haematol. 2007;137:64–75. doi: 10.1111/j.1365-2141.2007.06525.x. [DOI] [PubMed] [Google Scholar]
- 76.Clark JJ, Cools J, Curley DP, Yu JC, Lokker NA, Giese NA, et al. Variable sensitivity of FLT3 activation loop mutations to the small molecule tyrosine kinase inhibitor MLN518. Blood. 2004;104:2867–2872. doi: 10.1182/blood-2003-12-4446. [DOI] [PubMed] [Google Scholar]
- 77.Cools J, Mentens N, Furet P, Fabbro D, Clark JJ, Griffin JD, et al. Prediction of resistance to small molecule FLT3 inhibitors: implications for molecularly targeted therapy of acute leukemia. Cancer Res. 2004;64:6385–6349. doi: 10.1158/0008-5472.CAN-04-2148. [DOI] [PubMed] [Google Scholar]