Abstract
The outcome of adults with acute lymphocytic leukemia (ALL) who achieve a complete response (CR) on salvage therapy is thought to be poor, but not previously analyzed. To define the course of adult ALL post CR on salvage therapy and the effects of pretreatment factors on prognosis. One hundred seventy-two adults with ALL who achieved a second or third CR on salvage therapy were reviewed. Prognostic factors affecting survival were analyzed by multivariate analysis. The median survival post achieving CR for the entire group was 10 months. The estimated 1-year survival rate was 42%. Forty-three patients underwent stem cell transplant in subsequent CR: their median survival was 12 months and the 3-year survival rate was 25%. Independent poor prognostic factors for survival were age >55 years, duration of first CR <12 months, and lactate dehydrogenase levels >1000 IU/L. This analysis defines the outcome of adult ALL in CR post salvage therapy and the prognostic factors influencing survival. These results could be used in assessing the efficacy of new treatments aimed at improving CR durations and survival post salvage therapy.
Keywords: Survival, post salvage, second CR
Introduction
Intensive chemotherapy programs in pediatric acute lymphocytic leukemia (ALL) consisting of induction, consolidations, maintenance, and central nervous system prophylaxis have increased the cure rates to 80% [1,2]. Similar programs in adult ALL have improved the complete response (CR) rates to 80–90%. However, long-term survival rates are only 30–50% [3–7]. The most common cause of failure in adult ALL is disease progression; hence, there is a need to discover active anti-ALL agents.
Patients with resistant or relapsed ALL have a poor prognosis. With salvage therapy, the CR rates range from 10 to 50%, depending on the duration of first CR, patient age, comorbid conditions, and other factors [8–11]. Patients able to achieve a subsequent CR have only one potentially curative option, allogeneic stem cell transplant (SCT). However, this approach is applicable to only a minority of patients (about 10–20%) based on historical experience [9]. Even then, the cure rates are low, ranging from 10 to 30%.
Given the rarity of the disease and high remission rates with frontline chemotherapy, regulatory approval of new anti-ALL agents in the de novo setting is challenging. Any approval strategy in the front-line setting requires a very large number of patients randomized to front-line standard regimens with or without the new agent. Therefore, regulatory approval of new agents in ALL has focused on studies in subsequent salvage situations. Examples include clofarabine for pediatric ALL in second salvage [12,13], nelarabine for T-cell ALL salvage [14], and tyrosine kinase inhibitors in Philadelphia chromosome (Ph)-positive ALL [15,16]. Another potential area of new drug development involves therapeutic strategies designed to maintain remission once CR is achieved after salvage therapy, given the propensity for disease recurrence. This is particularly relevant since several monoclonal antibodies directed at cellular surface components of lymphoblasts such as CD19, CD20, and CD22 have been developed, either in native forms or conjugated to toxins. Such investigations could include randomized trials of the new agent versus maintenance therapy or observation in post salvage remission. Alternatively, results of single arm trials of the new agent could be compared to well-defined historical control populations. Very few studies have detailed the post-remission outcome of adults with ALL who achieve CR in second or subsequent CR. The aim of this analysis is to define the precise outcome of such patients, against which the results of new agents or other therapeutic strategies could be compared.
Study group and methods
Study group
The study group comprised adults with a diagnosis of ALL receiving salvage therapy for ALL and who achieved CR with either first or second ALL salvage attempts. Patients achieving CR after a third salvage attempt or beyond were not included in the analysis because of the rarity of the event, and because such patients were often accounted for in the subset of patients in CR after the first or second salvage treatment. The analysis included patients referred to the Leukemia Department at the M. D. Anderson Cancer Center recurring after treatment with front-line therapy, or patients referred from outside institutions following failure of front-line therapy. Only patients treated since 1990 were reviewed, in order to minimize potential bias related to improvements in the treatment regimens or in supportive care measures that might affect the outcome of subsequent remission duration (CRD) compared to studies conducted prior to 1990. Selection of the salvage treatment regimen generally depended on study period, prior induction therapy response, and timing of relapse. The use of allogeneic SCT following subsequent remission was also evaluated.
Statistical considerations
Criteria for response were standard. A CR was defined as a bone marrow blast count of ≤5% in a cellular marrow with normalization of peripheral counts, including a granulocyte count ≥109/L and a platelet count ≥100 × 109/L. Patients achieving CR without recovery of the platelet count to 100 × 109/L (CRi) were also included in the analysis. Survival was measured from the start of the first salvage therapy. Response duration was measured from the date of response until evidence of ALL recurrence. Patients achieving more than one subsequent CR were analyzed in the category of the first CR documented at our institution. Survival (OS) post achievement of response (i.e. survival in subsequent CR) was calculated from the date of achievement of response until death from any cause. Multivariate analyses to define prognostic factors for survival were conducted by established methods [17,18].
Results
A total of 172 patients achieving subsequent CR (n = 164) or CRi (n = 8) after first (n = 148) or second (n = 24) salvage therapy were analyzed. Thirty-two patients (19%) had been refractory to front-line therapy, whereas 43 (25%) recurred after a first CRD of over 1 year. Details of patient characteristics are shown in Table I. Their median age was 32 years (range 16–81 years); 20 patients (12%) were 60 years or older. The salvage regimens are listed in Table II. The majority of patients (86%) were treated with combination therapy.
Table I.
Characteristics of the Study Group (n = 172).
| Characteristics | Category | n (%) |
|---|---|---|
| Age (years) | Median [range] | 32 [16–81] |
| ≥60 | 20 (12) | |
| Response to frontline therapy | CR | 140 (81) |
| No CR | 32 (19) | |
| Duration of first CR (months) | 0 | 32 (18) |
| 1–11 | 63 (37) | |
| 12–36 | 43 (25) | |
| Karyotype | Diploid | 64 (37) |
| Philadelphia-positive | 27 (16) | |
| Other | 44 (26) | |
| Insufficient metaphases/not done | 30 (17)/7 (4) | |
| Hemoglobin (g/dL) | Median [range] | 11 [6–36.6] |
| <10 | 49 (29) | |
| WBC (× 109/L) | Median [range] | 5.4 [0.2–292.5] |
| ≥5 | 93 (55) | |
| Platelets (× 109/L) | Median [range] | 104 [8–418] |
| <25 | 19 (11) | |
| 25–100 | 63 (37) | |
| >100 | 88 (52) | |
| Albumin (g/L) | Median | 3.8 [1.7–4.9] |
| <3.8 | 75 (46) | |
| Year of study | 1990–2000 | 104 (60) |
| 2001–2007 | 68 (40) | |
| Marrow blast % | Median [range] | 58 [0–99] |
| 20–50 | 29 (17) | |
| >50 | 91 (54) | |
| Peripheral blast % | Median [range] | 1 [0–98] |
| 1 | 4 (2) | |
| >1 | 82 (49) | |
| LDH (IU/L) | Median [range] | 672 [180–15 779] |
| >672 | 86 (50) |
CR, complete response; WBC, white blood cell count; LDH, lactate dehydrogenase.
Table II.
Salvage therapy categories (n = 172).
| Therapy | n (%) |
|---|---|
| VAD–Hyper CVAD | 113 (66) |
| Cytarabine combinations | 20 (12) |
| Allogeneic stem cell transplant | 14 (8) |
| Methotrexate-asparaginase | 5 (3) |
| Other combinations | 9 (5) |
| Single agent | 11 (6) |
VAD, vincristine, doxorubicin, and dexamethasone; Hyper-CVAD, fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone.
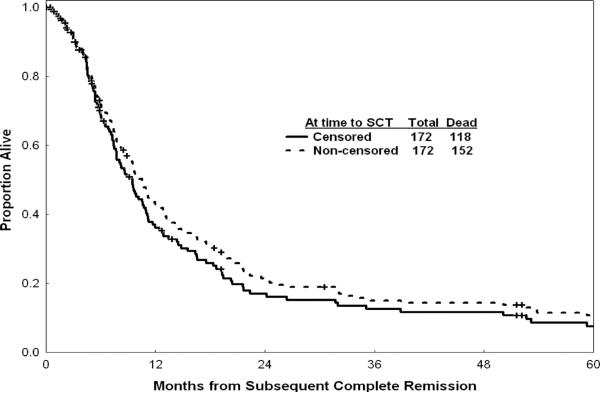
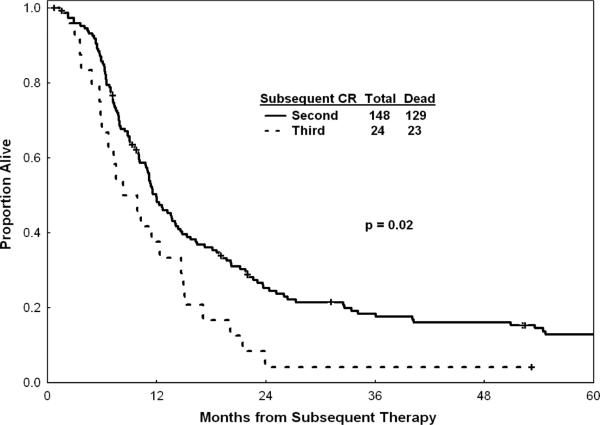
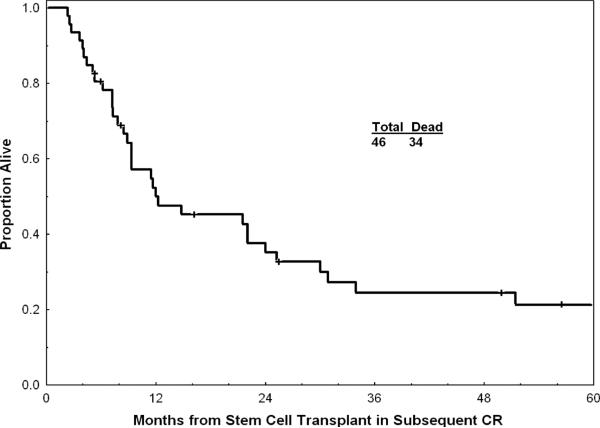
For the entire group, the median OS post achievement of response was 10 months; the estimated 1-year OS rate was 42% (Figure 1). Figure 1 shows survival with or without censoring of patients who underwent allogeneic SCT at the time of the procedure, and suggests that the benefit of allogeneic SCT (survival without censoring at the time of SCT) did not alter drastically the outcome of the total study group. Survival by pretreatment characteristics is detailed in Table III. Median OS was 14 months after first salvage therapy and 9 months after second salvage therapy (p = 0.02, Figure 2). In addition to the 14 patients in whom allogeneic SCT was offered as salvage therapy (Table II), 46 of the 172 patients in CR post salvage underwent SCT (44 allogeneic, two autologous) after second CR (n = 43) or after third CR (n = 3). Median OS of these 46 patients was 12 months after SCT; the 3-year OS rate was 25% (Figure 3). Preparative regimens for SCT included total body irradiation (TBI) in 20 patients and non-TBI regimens in 24 (two unknown). Matched sibling donors were used in 28 patients and matched unrelated donors in 12 (four unknown).
Figure 1.
Survival in subsequent CR with and without censoring for time to allogeneic stem cell transplant (dated from time of subsequent CR). The survival of patients without censoring at the time of allogeneic stem cell transplant is slightly but not significantly better (dotted curve) suggesting that the procedure did not change drastically the outcome of the total study group.
Table III.
Prognostic factors for survival post achievement of response.
| Characteristic | Category | Median survival (months) | 1-year survival (%) | p-value |
|---|---|---|---|---|
| Age (years) | <32 (median) | 11 | 49 | 0.07 |
| 32–49 | 10 | 42 | (0.02 at cut-off 50 years) | |
| 50–59 | 5 | 27 | ||
| ≥60 | 8 | 35 | ||
| Response to salvage | S1-CR | 11 | 45 | 0.01 |
| S2-CR | 7 | 33 | ||
| Duration of first CR (months) | 0 | 10 | 44 | 0.004 |
| 1–11 | 7 | 25 | ||
| 12–36 | 11 | 51 | ||
| >36 | 20 | 65 | ||
| Karyotype | Diploid | 11 | 44 | 0.008 |
| Philadelphia-positive | 8 | 30 | ||
| Other (including IM) | 11 | 47 | ||
| Not done | 8 | 43 | ||
| Hemoglobin (g/dL) | <10 | 9 | 38 | 0.02 |
| 10–11.9 | 8 | 36 | ||
| ≥12.0 | 12 | 51 | ||
| WBC (× 109/L) | <5.0 | 10 | 46 | 0.0004 |
| 5–9.9 | 16 | 60 | ||
| ≥10 | 7 | 24 | ||
| Platelets (× 109/L) | <25 | 6 | 31 | 0.046 |
| 25–49 | 11 | 48 | ||
| 50–100 | 8 | 34 | ||
| >100 | 11 | 46 | ||
| Albumin (g/L) | <3.8 | 9 | 39 | 0.007 |
| ≥3.8 | 11 | 46 | ||
| Year of study | 1990–2000 | 11 | 46 | 0.24 |
| 2001–2007 | 8 | 38 | ||
| Salvage producing remission | VAD–Hyper-CVAD | 11 | 47 | 0.01 |
| Cytarabine combination | 8 | 30 | ||
| Allogeneic transplant | 9 | 36 | ||
| Other | 9 | 40 | ||
| Marrow blast (%) | <20 | 11 | 51 | 0.01 |
| 20–50 | 7 | 21 | ||
| >50 | 11 | 45 | ||
| Peripheral blast (%) | ≤1 | 11 | 47 | 0.08 |
| >1 | 9 | 38 | ||
| LDH (IU/L) | ≤672 | 12 | 52 | 0.03 |
| >672 | 9 | 33 |
S1 = salvage 1; S2 = salvage 2; CR, complete response; WBC, white blood cell count; LDH, lactate dehydrogenase; IM, insufficient metaphases; VAD, vincristine, doxorubicin, and dexamethasone; Hyper-CVAD, fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone.
Figure 2.
Survival of patients achieving second or third remission (dated from subsequent treatment).
Figure 3.
Survival post allogeneic stem cell transplant in second or subsequent remission (dated from stem cell transplant).
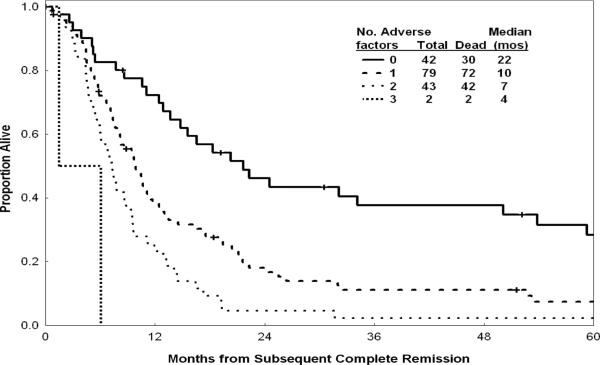
Prognostic factors significantly associated with survival after achieving subsequent CR were: duration of first CR, age, lactate dehydrogenase (LDH) levels, albumin levels, hemoglobin levels, platelet counts, karyotype, and peripheral blast percent. Multivariate analysis selected the following independent poor prognostic factors for survival: duration of first CR <12 months (p = 0.004); age >55 years (p < 0.001), LDH > 1000 IU/L (p < 0.001). Median and 1-year rates for OS/CRD declined progressively with increasing number of these adverse factors (none, one, two, or all three) (Figure 4, Table IV). We then studied the subsequent treatment effect on survival after accounting for other significant disease and patient characteristics. Interestingly, allogeneic SCT performed for active disease (n = 14; Table II) was not associated with a survival benefit, and may have negatively influenced OS compared with the other therapy regimens used to induce the subsequent CR (p = 0.046). However, among the 46 patients who received SCT at the time of CR, the 3-year survival rate was 25%, which is favorable (Figure 3).
Figure 4.
Survival by number of adverse factors.
Table IV.
Survival and remission duration by number of adverse factors.
| Survival |
Remission duration |
||||
|---|---|---|---|---|---|
| No. of adverse factors* | Patients (n) | Median (months) | 1-year (%) | Median (months) | 1-year (%) |
| 0 | 42 | 22 | 72 | 15 | 58 |
| 1 | 79 | 10 | 40 | 7 | 36 |
| 2 | 43 | 7 | 26 | 4 | 16 |
| 3 | 2 | 4 | 0 | 3 | 0 |
Adverse factors: age >55 years; duration of first complete response (CR) < 12 months; lactate dehydrogenase (LDH) > 1000 IU/L.
A total of 27 patients with Ph-positive ALL who achieved CR post salvage therapy were included in the analysis. Among them, seven patients had access to therapy with tyrosine kinase inhibitors. The median survival of patients treated without (n 20) or with tyrosine kinase inhibitors was 10.8 and 7.8 months, respectively (p = 0.21). The trend for worse outcome with tyrosine kinase inhibitors may be due to the selection of more refractory patients who relapsed after front-line combination regimens including chemotherapy and tyrosine kinase inhibitors. Five of 20 patients without tyrosine kinase inhibitor therapies proceeded to allogeneic SCT compared with none of seven patients who had access to tyrosine kinase inhibitor therapy, again possibly reflecting our capacity to transplant more of such patients in first CR because of their longer duration of CR with the combined modality therapies.
Discussion
In this analysis of 172 patients with ALL achieving CR post first or second salvage therapy, the median OS from the time of achievement of subsequent CR for the total study group was 10 months (Figure 1). A multivariate analysis of prognostic factors associated with survival showed that short duration of first CR, older age, and higher levels of LDH were independent adverse factors. Patients with at least one adverse factor (75%) had a median survival of 9 months and a 1-year survival rate of 34%.
This analysis confirmed the generally poor prognosis of adults with ALL who achieved CR post salvage therapy, and identified subsets with very poor survival. Exploration of novel single-agent or combination therapy strategies would clearly be justified for these patients. Promising approaches include novel formulations of standard chemotherapeutics (e.g. liposomal vincristine), agents which target pathways of resistance (e.g. anti-bcl-2), and monoclonal antibodies which target surface antigens such as CD19 (universally expressed in lymphoblasts), CD20 (expressed in 30–50% of adults with ALL), and CD22 (expressed in 80% of lymphoblasts). Potentially more potent CD20 monoclonal antibodies (ofatumumab) are now available. Other monoclonal antibodies include dual monoclonal antibodies (e.g. against CD19 and CD22) either as native antibodies or conjugated to toxins (e.g. diphtheria toxin); and CD22 targeted monoclonal antibodies attached to calicheamicin. Blinatumomab (MT103), a bispecific T-cell engaging single chain BiTE antibody, is showing favorable results in lymphoma and in pre-B ALL. Among 17 patients with pre-B ALL in morphologic CR but with positive minimal residual disease treated with blinatumomab, 13 had disappearance of residual ALL disease [19]. These monoclonal antibody therapies could be used in patients post salvage therapy who achieve CR. Their outcome can be now compared to the outcome of the patients analyzed in this study, to allow a preliminary assessment from phase II studies of whether these treatments, applied at the time of minimal residual disease, are indeed demonstrating favorable results, before large scale phase III randomized trials are embarked upon.
Two groups of patients underwent allogeneic SCT in this study. Fourteen patients underwent allogeneic SCT as salvage therapy for active ALL (Table II). Their outcome was not improved compared to other salvage therapies, suggesting the futility of this procedure in refractory active ALL. A significant number of the patients were able to undergo SCT in subsequent CR (46 of 172 patients: 27%), including 44 patients undergoing allogeneic SCT. Their estimated 3-year survival rate was 25%, which is favorable. This indicates the beneficial effect of allogeneic SCT for patients with ALL at the time of minimal residual disease, i.e. at the time of CR.
In summary, our experience in adult ALL in second or third CR emphasizes the urgent need to develop novel strategies in this setting, and establishes baseline expectations against which the efficacy of new approaches can be compared.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Pui CH, Evans WE. Acute lymphoblastic leukemia. N Engl J Med. 1998;339:605–615. doi: 10.1056/NEJM199808273390907. [DOI] [PubMed] [Google Scholar]
- 2.Nachman JB, Sather HN, Sensel MG, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338:1663–1671. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]
- 3.Kantarjian H, Thomas D, O'Brien S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101:2788–2801. doi: 10.1002/cncr.20668. [DOI] [PubMed] [Google Scholar]
- 4.Larson RA. Recent clinical trials in acute lymphocytic leukemia by the Cancer and Leukemia Group B. Hematol Oncol Clin North Am. 2000;14:1367–1379. doi: 10.1016/s0889-8588(05)70191-x. [DOI] [PubMed] [Google Scholar]
- 5.Durrant IJ, Richards SM, Prentice HG, Goldstone AH. The Medical Research Council trials in adult acute lymphocytic leukemia. Hematol Oncol Clin North Am. 2000;14:1327–1352. doi: 10.1016/s0889-8588(05)70189-1. [DOI] [PubMed] [Google Scholar]
- 6.Gökbuget N, Hoelzer D, Arnold R, et al. Treatment of Adult ALL according to protocols of the German Multicenter Study Group for Adult ALL (GMALL). Hematol Oncol Clin North Am. 2000;14:1307–1325. doi: 10.1016/s0889-8588(05)70188-x. [DOI] [PubMed] [Google Scholar]
- 7.Thiebaut A, Vernant JP, Degos L, et al. Adult acute lymphocytic leukemia study testing chemotherapy and autologous and allogeneic transplantation. A follow-up report of the French protocol LALA 87. Hematol Oncol Clin North Am. 2000;14:1353–1366. doi: 10.1016/s0889-8588(05)70190-8. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Manero G, Thomas DA. Salvage therapy for refractory or relapsed acute lymphocytic leukemia. Hematol Oncol Clin North Am. 2001;15:163–205. doi: 10.1016/s0889-8588(05)70204-5. [DOI] [PubMed] [Google Scholar]
- 9.Thomas DA, Kantarjian H, Smith TL, et al. Primary refractory and relapsed adult acute lymphoblastic leukemia: characteristics, treatment results, and prognosis with salvage therapy. Cancer. 1999;86:1216–1230. doi: 10.1002/(sici)1097-0142(19991001)86:7<1216::aid-cncr17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 10.Tavernier E, Boiron JM, Huguet F, et al. GET-LALA Group; Swiss Group for Clinical Cancer Research SAKK; Australasian Leukaemia and Lymphoma Group. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia. 2007;21:1907–1914. doi: 10.1038/sj.leu.2404824. [DOI] [PubMed] [Google Scholar]
- 11.Fielding AK, Richards SM, Chopra R, et al. Medical Research Council of the United Kingdom Adult ALL Working Party; Eastern Cooperative Oncology Group. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109:944–950. doi: 10.1182/blood-2006-05-018192. [DOI] [PubMed] [Google Scholar]
- 12.Jeha S, Kantarjian H. Clofarabine for the treatment of acute lymphoblastic leukemia. Expert Rev Anticancer Ther. 2007;7:113–118. doi: 10.1586/14737140.7.2.113. [DOI] [PubMed] [Google Scholar]
- 13.Jeha S, Gandhi V, Chan KW, et al. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood. 2004;103:784–789. doi: 10.1182/blood-2003-06-2122. [DOI] [PubMed] [Google Scholar]
- 14.DeAngelo DJ, Yu D, Johnson JL, et al. Nelarabine induces complete remissions in adults with relapsed or refractory T-lineage acute lymphoblastic leukemia or lymphoblastic lymphoma: Cancer and Leukemia Group B study 19801. Blood. 2007;109:5136–5142. doi: 10.1182/blood-2006-11-056754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ottmann OG, Druker BJ, Sawyers CL, et al. A phase 2 study of imatinib in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoid leukemias. Blood. 2002;100:1965–1971. doi: 10.1182/blood-2001-12-0181. [DOI] [PubMed] [Google Scholar]
- 16.Ottmann O, Dombret H, Martinelli G, et al. Dasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a phase 2 study. Blood. 2007;110:2309–2315. doi: 10.1182/blood-2007-02-073528. [DOI] [PubMed] [Google Scholar]
- 17.Cox DR, Snell EJ. The analysis of binary data. Chapman & Hall; London: 1970. pp. 33–43. [Google Scholar]
- 18.Cox DR. Regression models and life tables. J Stat Soc (B) 1972;34:187–220. [Google Scholar]
- 19.Topp MS, Goekbuget N, Kufer P, et al. Blinatumomab (anti-CD19 BiTE) for targeted therapy of minimal residual disease (MRD) in patients with B precursor acute lymphoblastic leukemia.. 14th Congress of European Society of Hematology; Berlin. 2009; (Abstract 430) [Google Scholar]