Abstract
Cramer et al. (Reports, 23 March 2012: 1503-1506) (1) demonstrated that treatment of APP/PS1ΔE9 mice with bexarotene decreased Aβ pathology and ameliorated memory deficits. We confirm the reversal of memory deficits in APP/PS1ΔE9 mice expressing human APOE3 or APOE4 to the levels of their non-transgenic controls and the significant decrease interstitial fluid Aβ, but not the effects on amyloid deposition.
The inheritance of Apolipoprotein (APOE) ε4 allele is the only established genetic risk factor for late-onset Alzheimer Disease (AD) (2, 3).
Liver X receptors α and β (LXRs) are transcription factors that act as heterodimers with retinoid X receptors (RXR) to activate the transcription of their target genes. LXR and RXR agonists were shown to ameliorate memory deficits and decrease amyloid β (Aβ) in AD mouse models presumably through up-regulation of Abca1 and Apoe (1, 4-6).
Recently Cramer et al. (1) demonstrated that treatment with FDA approved RXR agonist bexarotene in APP/PS1ΔE9 mice significantly decreased interstitial fluid (ISF) Aβ, amyloid plaques and soluble and insoluble Aβ in the brain. Importantly, they demonstrated a rapid reversal of cognitive, social and olfactory deficits. The authors postulated that RXR activation stimulates normal Aβ clearance processes and concluded that bexarotene facilitates APOE-dependent clearance of soluble Aβ from ISF which correlates to the improvement of behavior.
At the time the report was available online, we had been working with APP/PS1ΔE9 mice expressing human APOE3 and APOE4 isoforms (referred to as APP/E3 and APP/E4 mice) (7). 7 months old mice were treated by oral gavage with 100 mg/kg Targretin® and controls with vehicle (0.2mg/kg glycerol) for 15 days. Cognitive deficits were evaluated first by Radial Arm Water maze task (RWM) (7) at 10 days of treatment and by novel object recognition (8) (12th day of treatment). The mice were perfused (7) after completion of the behavioral testing (15th day of treatment). One hemibrain was fixed, sectioned and stained with X-34 and anti-Aβ antibody 6E10 (7). The other hemibrain was homogenized for WB, to extract Aβ for ELISA (4, 9) or soluble oligomers for dot blotting (10, 11). For microdialysis experiments (4, 7) 3.4 month old APP/E3 and APP/E4 mice (male and female) were treated with 100 mg/kg bexarotene or vehicle for 48 hours; ISF Aβ40 and Aβ42 were determined by ELISA (4, 7).
Bexarotene treatment did not affect weight or general behavior of treated mice compared to controls. Target engagement was confirmed by the increased protein levels of known RXR targets: ABCA1 (2-fold), APOE (1.3-fold), APOA-I (1.5-fold) in brain and HDL in plasma.
RWM testing showed that bexarotene restored spatial memory deficits in both APP/E3 (Fig.1A) and APP/E4 (Fig.1B) mice – i.e., there was no statistical difference between bexarotene treated APP transgenic mice and their respective non-transgenic controls. Novel object recognition testing of long-term memory demonstrated that bexarotene restored memory deficits and both APP/E3 and APP/E4 treated mice did not differ from their non-transgenic controls (Fig. 1C). Bexarotene treatment was equally effective in both genders of APP/E3 and APP/E4 mice. We conclude that bexarotene (should we delete treatment?) significantly improves memory functions in APP mice expressing both APOE isoforms and restores cognitive performance to that of non-transgenic controls, which is in agreement with Cramer et al. (1).
Figure 1. Bexarotene restores cognitive function and decreases ISF Aβ level in APP/E3 and APP/E4 mice.
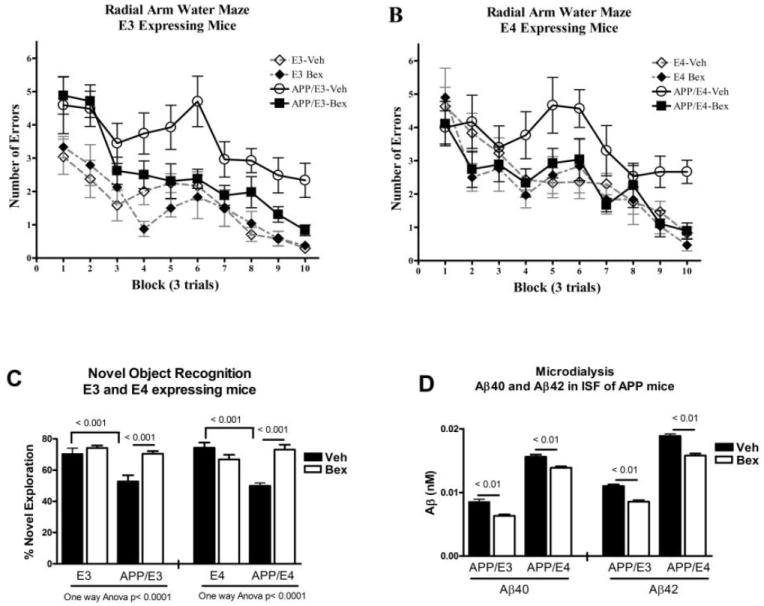
A, B and C, APP/E3 and APP/E4 mice (7 months old) and matched non-transgenic littermates (E3 and E4) were treated with bexarotene or vehicle. N=8-13 mice per group (male and female). RWM (7) was used to assess bexarotene effect on spatial learning deficits in APP/E3 (A) and APP/E4 mice (B). Analysis by two-way repeated measures ANOVA shows a significant effect on treatment (p<0.0001) and training (p<0.001) in both APOE isoforms. Tukey’s posttest shows that bexarotene treated APP mice were significantly different from vehicle treated APP mice (p < 0.05 for both APOE isoforms) but not different from their non-transgenic controls. C. Novel object recognition test was performed on the same mice following RWM. Analysis by one-way ANOVA and Tukey’s post hoc test shows that bexarotene treated APP mice were significantly different from vehicle treated APP mice (p < 0.001 for both isoforms) but not from their non-transgenic controls. D. Bexarotene treatment significantly decreases Aβ level in ISF. APP/E3 and APP/E4 mice (3.4 month old) were treated with 100 mg/kg bexarotene for 48 hours and in vivo microdialysis was performed in the hippocampus as in (7). ISF A β 40 and A β 42 were determined by ELISA. Analysis by t-test; N=5 male and female mice per group.
Using microdialysis (Fig. 1D), we found that bexarotene treatment decreased ISF Aβ in APP/E3 and APP/E4 mice approximately by the same factor as Cramer et al. reported. Bexarotene decreased Aβ40 in APP/E3 mice by 23% and Aβ42 by 26% (compared to APP/E3-vehicle). The treatment also caused a statistically significant decrease of Aβ in APP/E4 mice (compared to APP/E4-vehicle): 17% for Aβ40 and 12% for Aβ42.
We visualized the compact fibrillar amyloid plaques with X-34 and did not find bexarotene effect neither in APOE3 nor APOE4 expressing mice (Fig. 2A and representative picture on the right). Staining with anti-Aβ antibody did not show any significant difference in Aβ-plaques between treated and control mice of both genotypes (Fig. 2B). Soluble Aβ was extracted from cortices and hippocampi by TBS buffer followed by extraction of insoluble Aβ from the remaining pellets with formic acid, and Aβ levels determined by ELISA (7, 9). The results (Fig. 2C and D) demonstrate no effect of bexarotene on insoluble Aβ40 and Aβ42 in cortex and hippocampus respectively. Soluble Aβ40 and Aβ42 were also unchanged in cortex (Fig.2E) and hippocampus (not shown). Finally, we examined the level of soluble oligomers on dot blots using A11-antibody (11) and found that bexarotene decreased A11-positive oligomers in both APP/E3 and APP/E4 mice (Fig. 2F).
Figure 2. Bexarotene treatment does not affect amyloid deposition.
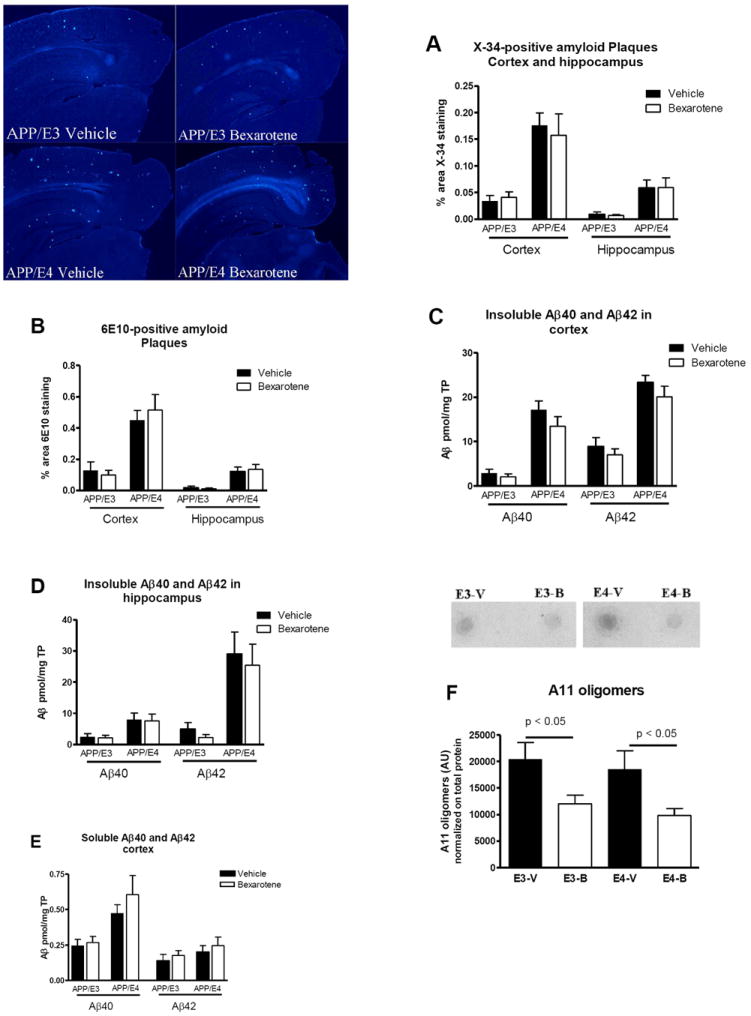
Following the behavior tests the levels of amyloid plaques, soluble and insoluble Aβ and soluble oligomers were compared in 7 month old APP/E3 and APP/E4 (comparable number of male and female). A. Results of X-34 staining of compact fibrillar amyloid plaques in cortex and hippocampus (7). Representative pictures for X-34 (20X) are shown on the right. B. Results of anti-A β antibody staining of brain sections. For A and B, N=8-12 mice per group. C, D and E, ELISA results for insoluble Aβ40 and Aβ42 in cortex (C) and hippocampus (D), and soluble Aβ in cortex (E). N=9-17 mice per group. F. Bexarotene treatment significantly decreased A11-positive oligomers (4). Intensity of the dots were quantified and normalized on the total protein measured on dot blots stained with coomassie blue. N=7-9 mice. For all panels analysis is by t-test.
In summary our study shows that bexarotene significantly improved cognitive deficits in APP/PS1ΔE9 mice expressing human APOE3 and APOE4. We also found a significant decrease of ISF Aβ in both APOE isoforms. However, we could not confirm bexarotene effect on Aβ or amyloid plaques in cortex and hippocampus. It is possible that bexarotene exerts its effect on memory not by modifying amyloid plaques but by reducing soluble oligomers in the brain or by non-Aβ related mechanism. Finally, it is important to note that the effect of bexarotene on memory was equally beneficial in mice expressing human APOE4 and APOE3 isoform. Regardless of the discrepancy, we consider the data relevant to an unique therapeutic approach in AD, targeting APOE at transcriptional level with the possibility to modulate LXR/RXR-ABCA1-APOE regulatory axis and functional aspects of APOE in brain that have been revealed during the last decade (3, 12-14).
Acknowledgments
This work was supported by NIH/NIA Grants R01AG037481, R01AG037919, R21ES021243 and F32AG034031. We are grateful for the excellent technical support of Sai Pratyusha Kancherla and Alexis Carter. A11 antibody was kindly provided by Dr. Charles Glabe, University of California, Irvine, CA.
References and Notes
- 1.Cramer PE, Cirrito JR, et al. Science. 2012;335:1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corder EH, Saunders AM, et al. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 3.Kim J, Basak JM, et al. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitz NF, Cronican A, et al. J Neurosci. 2010;30:6862–6872. doi: 10.1523/JNEUROSCI.1051-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang Q, Lee CYD, et al. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koldamova RP, Lefterov IM, et al. J Biol Chem. 2005;280:4079–4088. doi: 10.1074/jbc.M411420200. [DOI] [PubMed] [Google Scholar]
- 7.Fitz NF, Cronican AA, et al. J Neurosci. 2012;32:13125–13136. doi: 10.1523/JNEUROSCI.1937-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dere E, Huston JP, et al. Neurosci Biobehav Rev. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Lefterov I, Fitz NF, et al. J Biol Chem. 2010;285:36945–36957. doi: 10.1074/jbc.M110.127738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kayed R, Head E, et al. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 11.Lefterov I, Fitz NF, et al. ASN Neuro. 2009;1 doi: 10.1042/AN20090015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koldamova R, Fitz NF, et al. Biochim Biophys Acta. 2010;1801:824–830. doi: 10.1016/j.bbalip.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koldamova R, Lefterov I. Curr Alzheimer Res. 2007;4:171–178. doi: 10.2174/156720507780362227. [DOI] [PubMed] [Google Scholar]
- 14.Verghese PB, Castellano JM, et al. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


