Abstract
This study assessed physiological measures to study emotional dysregulation associated with borderline personality disorder (BPD). Two patient groups, individuals with BPD-only (n = 16) and individuals with BPD and co-occurring Substance Use Disorders (SUDs; n = 35), and a group of healthy controls (n = 45) were shown standardized pictures of varying valance and arousal levels while the affective modification of the startle eye-blink response, heart rate, facial electromyography (EMG; corrugator and zygomatic activity), and skin conductance responses were collected during picture presentation and during a brief recovery period. Startle data during picture presentation indicated a trend for the expected increase in startle magnitude to negative stimuli to be moderated by group status, with patients with BPD-SUD showing a lack of affective modification while the BPD-only group showing similar affective modification as controls. Heart rate data suggested lower reactivity to negative pictures for both patient groups. Differences in facial EMG responses did not provide a clear pattern and skin conductance responses were not significantly different between groups. The data did not suggest differences between groups in the recovery from the emotional stimuli. The startle and heart rate data suggest a possible hyporeactivity to emotional stimuli in BPD.
Keywords: Borderline Personality Disorder, Emotional dysregulation, Startle Reflex, Substance use, Psychophysiology
Borderline personality disorder (BPD), listed as an Axis II personality disorder in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; American Psychological Association, 1994), is a disorder characterized by highly labile mood, impulsivity, and unstable interpersonal relationships. These characteristics are, in turn, associated with significant personal suffering (e.g., self-harm, suicide, strained relationships) and societal costs (e.g. health care costs, lost productivity; Bender et al., 2001; Hall, Caleo, Stevenson, & Meares, 2001; van Asselt, Dirksen, Arntz, & Severens, 2007). Understanding the core psychological processes that underlie the features of BPD that lead to these negative outcomes is important for mitigating the negative outcomes associated with the disorder.
One of the core processes thought to underlie many of the negative consequences of BPD is the concept of emotional dysregulation (Sanislow et al., 2002). A commonly used definition of emotional dysregulation, posited by Linehan (1993), is that of emotional vulnerability characterized by three components, the tendency to respond to environmental stimuli with 1.) heightened sensitivity (quickly), 2.) heightened reactivity (high level of arousal), and 3.) a delayed recovery to baseline arousal. Though emotional dysregulation is considered one of the main components of BPD, this understanding has come mainly from self-report trait measures.
To better understand the role of emotional dysregulation in BPD beyond self-report measures, researchers have begun to utilize psychophysiological measures of emotion. Measures such as heart rate and skin conductance responses are used to assess reactions to the general arousal level of stimuli (Lang, Bradley, & Cuthbert, 1990) while electromyography (EMG) of the zygomatic and corrugator facial muscles can be used to assess positive and negative valence (respectively) of stimuli (Bradley, Codispoti, Cuthbert, & Lang, 2001). Another popular psychophysiological measure used to study emotion is the startle eye-blink reflex, measured by EMG. The startle reflex is a protective reaction of the body to sudden onset, high intensity stimuli and is moderated by the emotional state of the individual. Generally, positive affective states lead to an attenuation of the startle reflex, while negative affective states lead to facilitation of the startle reflex (Lang et al., 1990). In the laboratory, these affective states are typically induced with imagery or pictoral stimuli. This change in the startle reflex due to affective state is often referred to as affective modification of startle.
Previous research studies incorporating psychophysiological measures of emotion to examine emotional processing in BPD have demonstrated mixed findings (see Rosenthal et al., 2008 for review). Some studies have shown no increases in reactivity in startle, heart rate, or skin conductance responses in reaction to emotional stimuli (pictures, films, and scripts) between groups of BPD patients and controls (Herpertz, Kunert, Schwenger, & Sass, 1999; Herpertz et al., 2001; Kuo & Linehan, 2009; Schmahl et al., 2004) failing to support the claim that BPD patients show increased emotional reactivity to negative stimuli. A few studies even suggest that patients with BPD may show hyporeactivity to emotional stimuli as evidenced by lower skin conductance responses in BPD participants to emotional stimuli compared to control participants (Herpertz et al., 1999; Kuo & Linehan, 2009; Schmahl et al., 2004). Some studies have found support for hyper-reactivity among BPD patients with one study reporting greater startle reactivity during tones among BPD patients compared to controls (Ebner-Priemer et al., 2005) and another study reporting larger startle eyeblink responses to unpleasant, compared to neutral, words presented on a computer screen (Hazlett et al., 2007), and one study found greater cortical reactivity to negative pictures (Marissen, Meuleman, & Franken, 2010).
Like previous work, this study attempted to assess emotional reactivity to pictoral stimuli using physiological measures of facial EMG, skin conductance, heart rate, and startle eyeblink responses. It differs from earlier studies in that it attempts to assess the role of stimulus intensity, recovery from reactivity to baseline, and the impact of substance misuse on emotional processing in BPD. It may be that understanding the role of these three factors may lead to a better understanding of emotional reactivity in BPD and help explain the mixed findings in the literature.
The role of stimulus intensity may be an important factor in understanding emotional processing in BPD. It is possible that high intensity stimuli may be so arousing that individuals with BPD have no problems processing the information and reacting in a similar manner as comparison samples. It may be that the problem in emotional reactivity may lie in the processing of mid to low level intensity stimuli as these stimuli may be more ambiguous to individuals with BPD and thus lead to exaggerated responses. The current study attempted to address this issue by testing differences in psychophysiological reactivity to pictoral stimuli of three different arousal levels (low, medium, high; see Cuthbert, Bradley, & Lang, 1996). We predicted that BPD patients, compared to control participants, would show similar patterns of physiological responding to high arousal stimuli and greater levels of physiological responding to low and medium arousal stimuli.
Impaired recovery to baseline is one aspect of Linehan's model of emotional dysregulation as mentioned above. Previous studies of physiological reactivity to emotional cues among BPD patients have not focused much on the recovery from arousal back to baseline. It may be that recovery of the physiological responses is slower among BPD patients compared to control samples. This impairment in recovery may lead to self-reports of exaggerated emotional reactivity often reported in the literature. To assess recovery to exposure to emotional stimuli in this study, we continued to collect physiological data beyond the offset of each stimulus. To the extent that individuals with BPD show impaired recovery we predicted that physiological responses would continue to be elevated post stimulus offset in the BPD sample but not the control sample.
Finally, many studies utilizing BPD samples exclude participants for drug use or dependence. This may lead to discrepancies between laboratory based psychophysiological research findings and clinical based findings in emotional dysregulation in that the majority of patients presenting with BPD typically have a history of drug abuse or dependence (Trull, Sher, Minks-Brown, Durbin, & Burr, 2000). In fact it has been suggested that BPD should not be studied in isolation from its commonly co-occurring disorders (Skodol et al., 2002). Comorbid substance use disorders (SUDs) are important to consider when studying BPD as substance use may be an attempt to self-medicate emotional dysregulation associated with the disorder. This study includes three groups of participants: two groups with BPD, one with SUDs and one without, and a comparison group of participants with no history of SUD or BPD. We predicted that to the extent comorbidity is associated with more severe BPD psychophysiological responses, both during picture viewing and recovery, responsivity would be greatest among the BPD-SUD participants.
Method
Participants
Participants included individuals with borderline personality disorder without a current or past substance use disorder (BPD; n = 16), borderline personality disorder with substance use disorder (BPD-SUD; n = 35), and healthy controls (Control; n = 45). Groups did not differ in age or racial composition. Demographic data is summarized in Table 1 for each group. Participants were recruited from the community via newspaper ads, fliers, and from patient clinics and referrals from within a large academic medical center. Participants were excluded from the study if they met criteria for psychotic disorder or were experiencing an active manic episode. Participants were also excluded if they met criteria for current or past substance abuse or dependence (excluding nicotine) but did not meet criteria for BPD. Participants were compensated monetarily for their time.
Table 1.
Demographic variables for borderline personality disorder (BPD), borderline personality disorder with substance use disorder (BPD-SUD), and a control group (Control).
| Variable | BPD (n = 16) |
BPD-SUD (n = 35) |
Control (n = 45) |
|---|---|---|---|
| Age | 34.8 (12.5) | 38.0 (9.9) | 34.2 (13.0) |
|
| |||
| Sex (% female) | 81 | 80 | 84 |
|
| |||
| Race (%) | |||
| White | 88 | 74 | 67 |
| African-American | 13 | 17 | 24 |
| Other | 0 | 9 | 9 |
|
| |||
| Gross Domestic Income | $20,341 (12,888) | $16,030 (15,262) | 36,962 (31,422) |
|
| |||
| Education (%) | |||
| Less than high school | 0 | 14 | 0 |
| High school diploma/GED | 25 | 29 | 2 |
| Some college | 19 | 23 | 9 |
| College graduate | 44 | 21 | 51 |
| Attended/graduated graduate school | 13 | 3 | 38 |
|
| |||
| Employment (%) | |||
| Unemployed | 50 | 69 | 2 |
| Employed part-time or student | 31 | 14 | 45 |
| Employed full-time | 6 | 17 | 42 |
| Keeping house | 13 | 0 | 9 |
| Retired | 0 | 0 | 2 |
|
| |||
| Marital status (%) | |||
| Married or co-habiting | 38 | 14 | 38 |
| Widowed/divorced/separated | 31 | 54 | 13 |
| Never married | 31 | 31 | 49 |
|
| |||
| Psychiatric comorbidity (%) | |||
| Anxiety disorder (1 or more) | 81 | 80 | 0 |
|
| |||
| Mood disorder | 69 | 74 | 2 |
| Substance use disorder (past or present) | 0 | 100 | 0 |
Diagnostic Measures
The Structured Interview for DSM-IV Personality (SIDP-IV; (Pfohl, Blum, & Zimmerman, 1994) was used to diagnose BPD and other Cluster B personality disorders. The SIDP-IV has been shown to have good psychometric properties (Damen, De Jong, & Van der Kroft, 2004).
Substance use disorders were diagnosed using the Diagnostic Interview Schedule-Computerized for DSM-IV (CDIS; (Robins et al., 2000). The DIS has satisfactory psychometric properties (Vandiver & Sher, 1991) and the computerized version has been shown to be as reliable as the non-computerized version (Greist et al., 1987).
The Mini-International Neuropsychiatric Interview (MINI; (Sheehan et al., 1998), a short structured interview was used to diagnose other Axis I disorders. Axis I disorders assessed in this study included current major depression, bipolar disorder, panic disorder, generalized anxiety disorder, social phobia, posttraumatic stress disorder, obsessive-compulsive disorder, anorexia nervosa, and bulimia nervosa. Diagnostic status of the sample is presented in Table 1.
The SIDP-IV and MINI were administered by pre-doctoral clinical psychology interns or postdoctoral clinical psychology research fellows. The CDIS was administered by Masters- and bachelors-level research assistants. Each interviewer was trained and supervised by senior study personnel (SFC and JAS), both licensed clinical psychologists.
Affective Stimuli
Affective stimuli included 84 pictures from the International Affective Picture System (IAPS; (Lang, Bradley, & Cuthbert, 2008). See Appendix A for IAPS picture numbers. Pictures were selected for greater representation of interpersonal significance (e.g. social interactions) across 7 picture types based on arousal and valence (i.e., low, medium, high arousal negative pictures, neutral pictures, and low, medium and high arousal positive pictures). Picture trials were randomized in blocks of 21 with the following constraints; 1.) pictures of the same valence × arousal condition could not be shown consecutively, 2.) ≤ 3 consecutive picture trials of a particular valence or arousal condition was allowed, and 3.) the startle probe (see below) could not be presented (either during the picture or during recovery) during more than 2 consecutive trials. Four of the high arousal, positive pictures had alternative pictures selected to match picture content with the sexual orientation of the participant (i.e. four pictures with female models and four pictures with male models).
Appendix A.
| High Arousal, Negative |
Medium Arousal, Negative |
Low Arousal, Negative |
Neutral | Low Arousal, Positive |
Medium Arousal, Positive |
High Arousal, Positive (female image/male image) |
|---|---|---|---|---|---|---|
| 3000 | 2053 | 2205 | 1935 | 1460 | 1463 | 4002/4510 |
| 3005 | 2095 | 2455 | 2191 | 1750 | 1710 | 4180/4531 |
| 3010 | 2141 | 2490 | 2385 | 2170 | 1811 | 4220/4538 |
| 3030 | 2800 | 2750 | 7002 | 2360 | 2160 | 4250/4572 |
| 3068 | 2900 | 9000 | 7009 | 2370 | 2209 | 5621 |
| 3071 | 3101 | 9001 | 7037 | 2530 | 2303 | 8030 |
| 3170 | 3160 | 9220 | 7140 | 2540 | 7230 | 8080 |
| 3500 | 3230 | 9265 | 7550 | 5000 | 7270 | 8185 |
| 3530 | 3301 | 9280 | 7595 | 5200 | 8210 | 8190 |
| 6230 | 3350 | 9290 | 7820 | 5760 | 8380 | 8200 |
| 9410 | 9140 | 9330 | 7830 | 5780 | 8420 | 8370 |
| 9635 | 9920 | 9331 | 9070 | 8330 | 8500 | 8490 |
IAPS picture number by arousal and affect condition.
Psychophysiological Measures
Psychophysiological responses were sampled and stimuli were presented using VPM software (Cook, Atkinson, & Lang, 1987). All measures used Ag/AgCl electrodes. Startle eyeblink responses were elicited by auditory startle probes (50 ms, 101 dB white noise with instantaneous rise/fall times) presented via headphones. Startle eyeblink responses where measured by electromyography (EMG) via electrodes attached over the obicularis oculi muscle of each eye. The amplified EMG signal (30-300 Hz) was sampled at 1000 Hz from 50 ms pre-probe onset to 300 ms post. Facial EMG (30-300 Hz) was measured from the left zygomatic and corrugator muscles. A modified lead II configuration was used to record EKG to calculate heart rate (HR). The EKG signal was amplified and filtered (1-300 Hz) and R-waves were detected with a Schmitt trigger. Skin conductance responses, sampled at 10 Hz, were recorded from two electrodes placed on the hypothenar eminences of the non-dominant hand. Facial EMG, HR, and SCR data were sampled from 2 sec prior to stimulus onset until the end of the trial.
Procedure
All procedures were approved by an Institutional Review Board. Participants who met initial criteria for study participation were scheduled for three data collection sessions. The first session consisted of the assessment battery. During this session participants completed questionnaires and structured clinical interviews. Participants who met inclusion/exclusion criteria for one of the participant groups were scheduled for the affective processing task reported here. Results from an additional experimental session are reported elsewhere (Coffey, Schumacher, Baschnagel, Hawk, & Holloman, 2011). Sessions were typically scheduled within one week of each other.
Participants were asked to abstain from illicit drug and alcohol use for four days prior to the laboratory session. Prior to the laboratory session, participants completed a urine drug screen (OnTrak Testcup II – 5, Varian, Inc., Lake Forest, CA), an alcohol breathalyzer test (Alco-sensor IV, Intoximeters, Inc., St. Louis, MO), and self-reported drug use was reported. Participants who tested positive for metabolites of cocaine, opioids, amphetamines, or PCP or whose blood alcohol level was greater than 0.01 or who reported drug use (including alcohol) in the past 4 days were rescheduled.
For the affective processing task participants were asked to sit in a comfortable chair in a sound attenuated, electrically shielded, dimly lit room (IAC, Bronx, NY). Electrodes were attached and the participants were presented 2 test startle probes, instructed on the task, and asked to practice the ratings. At task onset one habituation startle probe was presented. The task consisted of a series of pictures presented on an 18 ¾ × 11 ¾ inch monitor placed 32 inches away from the participant. Following a variable intertrial interval (averaging 30 sec) each picture was displayed for 6 sec followed by a 6 sec recovery period. Startle probes were presented over headphones (Telephonics Co. TDH-49P, Farmingdale, NY) during either the picture or recovery portion on 66% of the trials with onsets equally distributed across 4 stimulus onset asynchronies (SOAs; from picture onset to probe onset, 3000, 5000, 7000, 9000 ms). Participants were instructed to view each picture while it was on the screen and to disregard the noises presented over the headphones. At the end of the recovery period, participants were prompted to rate the pictures on dimensions of interest, valence, and arousal using computer line ratings and the Self-Assessment Manikin (SAM; (Hodes, Cook, & Lang, 1985). At the end of the picture presentation, electrodes were removed and participants were reminded of their next scheduled session. All participants were debriefed at the end of the last session in the overall study.
Data Analysis
ANOVAs were conducted to compare reactivity to positive and negative pictures. Reactivity during picture viewing and during recovery was analyzed separately. Mean startle eyeblink magnitude was computed for each 3 Group (BPD, BPD-SUD, Control) × 2 Affect (positive vs. negative) × 3 Arousal (low, medium, high) condition. Mean change from 2 sec baseline was computed for facial EMG and HR for each 3 Group (BPD, BPD-SUD, Control) × 2 Affect (positive vs. negative) × 3 Arousal (low, medium, high) condition with data from non-startle trials. SCR data was reduced as follows: Ln (Max SCR value – mean baseline) + 1 (Venables& Christie, 1980). These scores were then averaged to create means for each 3 Group (BPD, BPD-SUD, Control) × 2 Affect (positive vs. negative) × 3 Arousal (low, medium, high) condition with data from non-startle trials. Mean interest, valence and arousal rating scores were computed for each 3 Group (BPD, BPD-SUD, Control) × 2 Affect (positive vs. negative) × 3 Arousal (low, medium, high) condition.
Results
Startle Eyeblink Responses
Startle responses during picture viewing
Startle responses to neutral pictures were not significantly different between groups, F (2, 93) = 1.7, p = .18, η2 = .02 (M μV [SE]: BPD = 33.6 [6.0], BPD-SUD = 19.6 [4.1], Control = 22.8 [3.6]). When looking at affective modification of startle (difference in startle between negative and positive pictures) during picture viewing there were significant main effects for affect, F (1, 93) = 6.6, p < .05, η2 = .01, and arousal, F (1, 93) = 5.2, p < .05, η2 = .006, which were qualified by a significant Affect × Arousal linear and quadratic interaction, F (1, 93) = 15.1, p < .01, η2 = .03 and F (1, 93) = 6.0, p < .05, η2 = .01, respectively. There was a trend for this interaction to be moderated by group, Group × Affect × linear Arousal interaction, F (2, 93) = 2.9, p = .06, η2 = .01 (see Figure 1). There was no main effect of group, F (2,93) = 1.2, p > .3, η2 = .01. Startle responses during picture viewing are presented in Figure 1.
Figure 1.
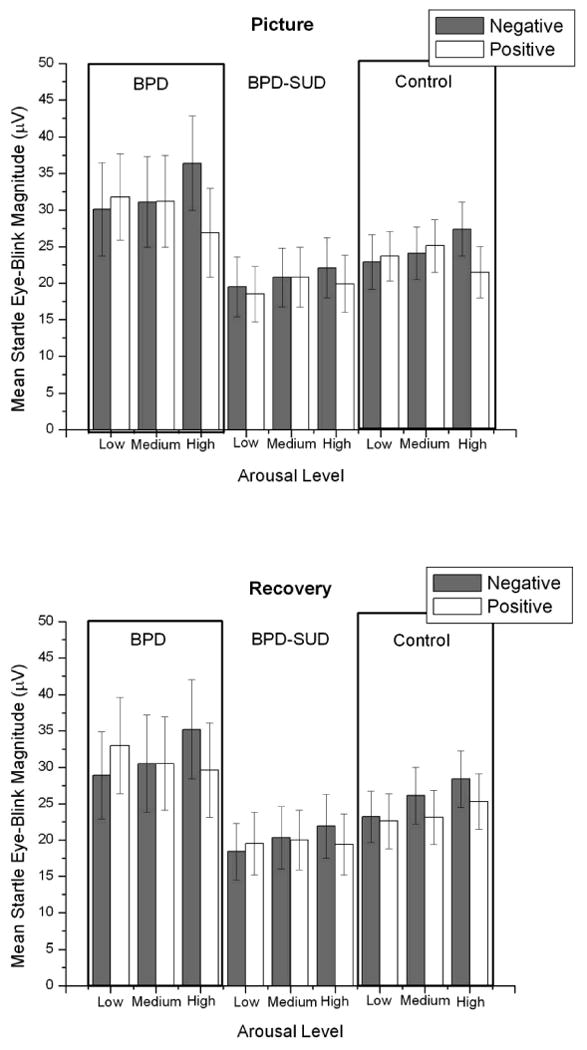
Mean startle magnitudes during picture and recovery periods by arousal and valence condition for borderline personality disorder (BPD; n = 16), borderline personality disorder and substance use disorder (BPD-SUD; n = 35), and control (n = 45) groups. Bars represent standard error.
Startle responses during picture recovery
Startle responses during recovery period after viewing neutral pictures did not significantly differ between groups, F < 1, η2 = .01, (M μV [SE]: BPD = 28.6 [6.0], BPD-SUD = 19.3 [4.1], Control = 23.6 [3.6]). Analysis of affective modification of startle during the recovery period (see Figure 1) indicated that there was significant linear main effect for arousal, F (1, 93) = 9.9, p < .01, η2 = .02 which was qualified by an Affect × linear Arousal interaction, F (1, 93) = 16.1, p < .001, η2 = .03. Pairwise comparisons indicated that startle responses were significantly greater after offset of negative pictures compared to positive pictures for high arousal pictures, p = .001, but not for low or medium arousal pictures, ps > .10. This interaction was not moderated by group, Group × Affect × Arousal interaction, F (2, 93) = 2.5, p = .09, η2 = .01. There was no main effect of group, F (2,93) = 1.3, p > .2, η2 = .01.
Facial EMG
Five participants from the startle sample were missing facial EMG data, one from the BPD group, two from the BPD-SUD group, and two from the Control group.
Corrugator
Means for each Group × Affect × Arousal condition are presented in Figure 2. Analysis of corrugator EMG responses during pictures resulted in significant main effects for affect, F (1, 88) = 23.0, p <.001, η2 = .08, and arousal, linear F (1,88) = 9.1, p < .01, η2 = .02. Mean corrugator responses were greater for negative pictures compared to positive pictures and increased linearly across low to high arousal pictures. These effects were not moderated by group, Fs < 1.2, ps > .20.
Figure 2.
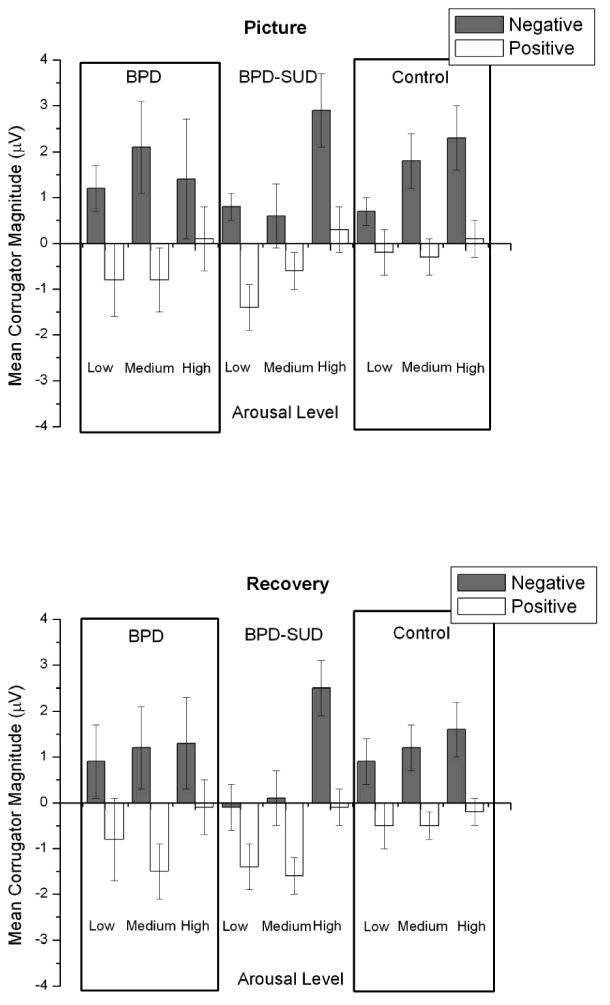
Mean corrugator magnitudes during picture and recovery periods by arousal and valence condition for borderline personality disorder (BPD; n = 16), borderline personality disorder and substance use disorder (BPD-SUD; n = 35), and control (n = 45) groups. Bars represent standard error.
Analysis of corrugator activity during picture recovery resulted in main effects for affect, F (1,88) = 26.9, p < .001, η2 = .09 and arousal, linear F (1,88) = 6.0, p <.05, η2 = .02 and quadratic F (1,88) = 9.5, p <.01, η2 = .01. As during picture viewing, mean corrugator responses were greater for negative pictures compared to positive pictures. Pairwise comparisons indicated that corrugator responses were significantly higher during high arousal pictures compared to low and medium arousal pictures (ps < .05) but did not differ between low and medium arousal pictures. There was no effect of group on corrugator responses during recovery, Fs < 1.8, ps > .18.
Zygomatic
Means are presented in Figure 3 for each Group × Affect × Arousal condition. Analysis of zygomatic EMG responses to pictures indicated a significant Group × Affect × Arousal interaction, F (2, 88) = 4.8, p =.01, η2 = .02. Pairwise comparisons examining affect indicated that, for the BPD group there was no significant difference in zygomatic activity during positive compared to negative pictures (Fs <3.2, ps > .08). For the BPD-SUD group zygomatic activity was only significantly different between affect conditions for medium arousal pictures, F(1,88) = 8.7, p <.01, with positive medium arousing pictures eliciting more zygomatic activity compared to negative medium arousing pictures (low and high arousal Fs < 1). For the control group, zygomatic activity was significantly greater for positive compared to negative low arousal pictures, F (1,88) = 6.2, p <.02, with no significant differences for medium and high arousal pictures (Fs <3.1, ps > .08).
Figure 3.
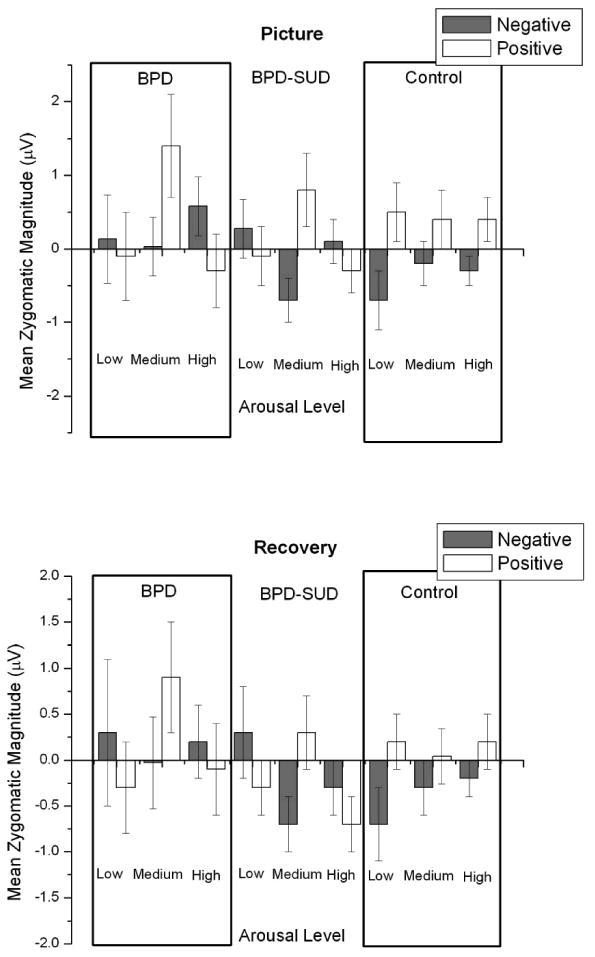
Mean zygomatic magnitudes during picture and recovery periods by arousal and valence condition for borderline personality disorder (BPD; n = 16), borderline personality disorder and substance use disorder (BPD-SUD; n = 35), and control (n = 45) groups. Bars represent standard error.
Analysis of zygomatic EMG responses during recovery from picture viewing indicated a significant Affect × Arousal interaction, F (1, 88) = 4.4, p < .04, η2 = .01, with a trend for this interaction to be moderated by group, Group × Affect × Arousal interaction F (2,88) = 3.0, p <.06, η2 = .02. All other comparisons Fs < 3.4, ps >.06).
Heart Rate
Mean heart rate responses are presented in Figure 4. Eighty-eight participants had complete heart rate data (BPD = 13, BPD-SUD = 32, Control = 43). Analysis of heart rate responses during picture viewing resulted in a significant main effect for Group, F(2,85) = 3.8, p <.05, η2 = .05, which was moderated by picture arousal condition, Group × quadratic Arousal interaction, F(1,85) = 4.1, p <.05, η2 = .01. Pairwise comparisons indicated that heart rate responses significantly differed between arousal conditions only for the control group, with mean heart rate decreasing from baseline more for low (p <.001) and high (p=.01) arousal pictures compared to medium arousal pictures in this group. Pairwise comparisons for the main effect of Group indicated that both BPD groups (ps <.05) showed significantly less heart rate change to the pictures than the control group but did not differ between each other (ps >.3).
Figure 4.
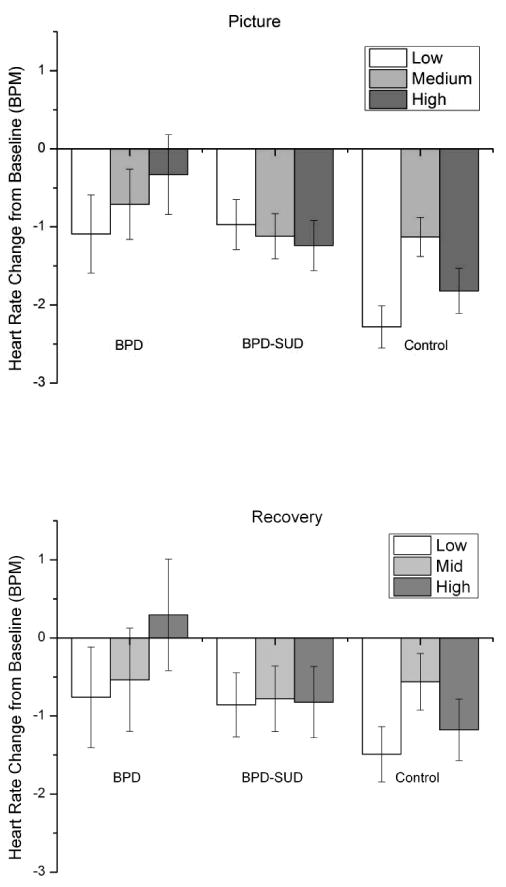
Mean heart rate change from baseline during picture and recovery periods by arousal condition for borderline personality disorder (BPD; n = 16), borderline personality disorder and substance use disorder (BPD-SUD; n = 35), and control (n = 45) groups. Bars represent standard error.
Analysis of heart rate responses during recovery period indicated a significant Group × quadratic Arousal interaction, F(1,85) = 3.3, p <.05, η2 = .01. Similar to heart rate during pictures, heart rate responses significantly differed only for the control group with mean heart rate decreasing more for low arousal pictures (p < .01) and marginally decreasing more for high arousal pictures (p = .06) compared to medium arousal pictures in this group.
Skin Conductance Responses (SCR)
One BPD, 1 BPD-SUD and 2 Control group participants were missing complete SCR data. Analyses indicated that SCR did not significantly differ between affect or arousal conditions or groups across picture conditions during picture presentation (Fs <1.4, ps > .24) or during recovery period (Fs < 1.6, ps > .21).
Picture Ratings
One participant from the BPD-SUD group was missing ratings data. Mean picture valence and arousal ratings are presented in Table 2. Valence ratings could range from -10, extremely negative to 10 extremely positive, with zero anchored as neutral. Arousal ratings could range from 0, completely un-arousing and calm, to 20, extremely arousing. Analysis of ratings of picture valence resulted in a significant main effects for affect, F (1, 92) = 637.7, p < .001, η2 = .80, and arousal, Linear F (1, 92) = 49.6, p < .001, η2 = .02, which were qualified by a significant Affect × Arousal interaction, linear F (1, 92) = 49.6, p < .001, η2 = .008 & quadratic F (1, 92) = 39.3, p < .001, η2 = .003. Pairwise comparisons indicated that, in general, negative pictures were rated as more negative with increasing arousal level and positive pictures were rated increasingly more positive with increasing arousal level except the high arousal positive pictures were rated significantly lower than the low and medium arousal conditions, all ps <.05. This interaction was not significantly moderated by group, Group × Affect × Arousal interaction, F (2, 92) = 2.6, p =.08, η2 = .0008. Affective ratings of neutral pictures did not differ between groups, F < 1.2.
Table 2.
Mean (SE) picture valence and arousal ratings by group and picture arousal level.
| Arousal Level | ||||
|---|---|---|---|---|
|
| ||||
| Low | Medium | High | ||
|
| ||||
| Valence | ||||
| Positive Pictures | ||||
| BPD | 4.6 (.6) | 4.9 (.6) | 3.3 (.7) | |
| BPD-SUD | 4.3 (.4) | 4.9 (.4) | 4.0 (.5) | |
| Control | 4.6 (.4) | 4.8 (.4) | 3.7 (.4) | |
| Negative Pictures | ||||
| BPD | -4.4 (.6) | -6.1 (.6) | -7.1 (.6) | |
| BPD-SUD | -3.2 (.4) | -5.9 (.4) | -7.0 (.4) | |
| Control | -3.8 (.3) | -6.0 (.4) | -7.5 (.4) | |
|
| ||||
| Arousal | ||||
| Positive Pictures | ||||
| BPD | 8.4 (1.0) | 10.1 (.9) | 11.2 (.9) | |
| BPD-SUD | 8.0 (.7) | 10.7 (.6) | 12.9 (.6) | |
| Control | 7.5 (.6) | 9.9 (.5) | 11.5 (.6) | |
| Negative Pictures | ||||
| BPD | 8.5 (.8) | 11.0 (.9) | 13.0 (1.1) | |
| BPD-SUD | 8.4 (.5) | 11.6 (.6) | 14.5 (.8) | |
| Control | 9.0 (.5) | 11.4 (.5) | 13.9 (.7) | |
Note: Borderline personality disorder (BPD; n = 16); borderline personality disorder and substance use disorder (BPD-SUD; n = 35); control (n = 45).
Results of the analysis of arousal ratings was similar with main effects for affect, F (1, 92) = 7.4, p < .01, η2 = .03 and arousal, linear F (1, 92) = 170.3, p < .01, η2 = .29 which were qualified by a significant Affect × Arousal interaction, F (1, 92) = 9.4, p < .01, η2 = .006. Arousal ratings generally increased linearly across arousal conditions and negative pictures were rated as significantly more arousing compared to positive pictures in the medium and high arousal conditions, ps < .05, but not in the low arousal condition p = .19. This interaction was not moderated by group, Group × Affect × Arousal interaction F < 1. Arousal ratings of neutral pictures did not differ between groups, F < 1.
Discussion
Clinical reports and current models of BPD suggest that BPD is characterized by difficulties in emotional regulation (Linehan, 1993; Sanislow et al., 2002; Zanarini & Frankenburg, 2007). Previous laboratory studies on emotional responding in BPD have resulted in mixed findings. This study assessed psychophysiological and self-report measures of emotional responding in patients with borderline personality disorder with and without comorbid substance use disorder and a comparison control group in an attempt to further the understanding of emotional responding in this clinical population. The aims of the study were to assess the impact of varying stimulus intensity on emotional responding between groups, possible differences in recovery of responses to baseline between groups, and the effect of SUD status on emotional responding in BPD.
The role of emotional intensity on emotional responding was assessed in this study by presenting pictures of varying arousal level to the participants. Previous psychophysiological research assessing emotional responding to affective stimuli has not directly assessed the effect of varying levels of arousal. As stated above, it may be that individuals with BPD respond similarly as controls to more intense stimuli where it is more clear what emotion should be experienced. It may be that the emotional dysregulation occurs for emotional stimuli that have the potential to be more ambiguous, such as stimuli of lower intensity.
Overall the startle reflex data indicated the expected affective modification effect but showed only a marginal difference in startle responding across groups for the high arousal pictures. This marginal effect suggests the BPD-SUD group had diminished affective modification of the startle reflex, indicating a potential hyporeactivity to the high arousal stimuli for this group. Looking at the pattern of startle responses (see Figure 1), it appears that the BPD group may have had an exaggerated startle response overall suggesting the expected hyper-reactivity response pattern. The lack of a significant difference for this pattern of responding may be due to the low sample size for the BPD group reducing the power of the analysis.
The facial EMG data did not clarify the results of the startle data. Corrugator responses did not differ across groups suggesting no differences in experience of negative emotion as indexed by this measure. Zygomatic responses did differ with the BPD-SUD group showing a significantly greater, difference in zygomatic activity for medium arousal positive pictures compared to negative pictures. The control group showed greater zygomatic responses for the low arousal positive pictures compared to negative pictures and the BPD group showed no significant differences in zygomatic activity. This suggests the BPD-SUD group was less sensitive to positive emotion stimuli at low and high arousal levels compared to controls, though this interpretation is complicated by the lack of a significant increase in zygomatic activity for the control group for the medium and high arousal conditions.
When looking at indices of autonomic arousal we see increased heart rate deceleration for low and high arousing stimuli among the control group but not for either patient group. During picture viewing heart rate typically decelerates, with greater deceleration during negatively valenced and high arousal positively valenced pictures compared to low and medium arousal positive pictures. This deceleration has been interpreted as an indicator of sensory processing (see Bradley and Lang, 2007). The effect found in this study suggests that the control participants showed more sensory processing of the low and high arousal pictures compared to the BPD groups. This may indicate a possible form of hypoarousal.
In terms of recovery, the data did not suggest differential recovery from emotional reaction to the pictures between groups. This is in contrast to Linehan's theory which posits that there would be impairment in recovery among individuals with BPD which we expected would have been represented in this study by higher rates of physiological arousal in the BPD groups during recovery. One possible reason for the lack of differences could be due to the lack of hyperarousal during the picture viewing period for the BPD groups, it would not be possible to see a longer recovery since there was no reaction from which to recover. Furthermore, it may be that our approach of operationalizing recovery, i.e. continued high levels of physiological arousal after stimulus offset, may not be the best way to assess the concept of emotional recovery. To our knowledge, no prior psychophysiological study of BPD has specifically looked at recovery.
Substance use disorders are often comorbid with BPD (Trull et al., 2000). The current study suggested a trend for BPD patients with co-occurring SUDs to show hypoarousal to highly arousing emotional stimuli as indexed by the affective startle modulation. The BPD only group showed similar startle responding as the control group. A possible explanation for this may be that continued substance use in the co-occurring SUD may either have resulted in changes to how these individuals react to emotional stimuli or that the substance use is, in part, used by these individuals to self-medicate this hypoarousal. Evidence of general hypoarousal but not differential affective modification in SUD patients is found in the literature. For example, in cocaine dependent individuals, reactions to the acoustic startle stimulus are decreased after one year of abstinence despite being similar immediately after the start of abstinence in relation to responses of control participants (Corcoran, Norrholm, Cuthbert, Sternberg, Hollis, & Duncan, 2011). In heroin addicts both with and without antisocial personality disorder, there is evidence of overall decreased startle reactivity but normal pattern of affective modification to picture stimuli compared to control participants (Walter, Degen, Treugut, Albrich, Oppel, Schulz, et al., 2011). In our study the BPD-SUD group did not show a difference in overall responses to the startle probes, though we did not present the startle probes independent of the picture stimuli. Research specifically assessing these differences in emotional responding in patients with comorbid SUD and psychiatric disorders would be useful.
This study is not the first to suggest affective hypoarousal in BPD. Previous studies have reported hypoarousal using various measures within BPD participants (Herpertz et al., 1999; Schmahl et al., 2004). The lack of the expected exaggerated affective response in the BPD groups to the stimuli did not support our hypotheses based upon current theories of BPD (e.g., Linehan, 1993; Zanarini & Frankenburg, 2007). Hazlett et al. (2007) did show a significant difference in startle responding to word cues that were selected to be BPD specific. Despite our attempt to use pictures that depicted or implied interpersonal interaction, a factor that may be important for the affective dysregulation reported clinically, it may be that the pictures were not salient enough to evoke an exaggerated physiological response. Perhaps standardized, static visual stimuli are not relevant enough to activate the processes associated with affective hyperarousal in BPD. Kuo and Linehan (2009) also found results suggestive of hypoarousal in BPD patients viewing a sad film. They suggest that this hypoarousal may actually be due to implicit emotion regulation in this group where the BPD participants used internal emotion regulation strategies to reduce their reactivity to the film despite reporting an increase in negative affect. The heart rate data in this study lends support to this idea in that the reduced deceleration suggests less processing of the emotional stimuli by the BPD groups. Additionally, Kuo and Linehan (2009) suggest that BPD may not be characterized by exaggerated emotional reactivity but rather high baseline affective intensity, that is high arousal levels independent of presentation of affective stimuli. This study was not designed to directly assess baseline affective intensity but the comparison of reactivity during neutral pictures did not suggest a difference between groups. Future studies should employee self-relevant stimuli to address the possibility that findings from the current study are a result of reduced reactivity to standardized, static visual stimuli rather than a lack of exaggerated affective response in individuals with BPD.
Another limitation of the current study is the small sample size for the BPD-only group. As mentioned above, the small n for this group may have hindered our ability to detect significant differences between this group and the control group. The small sample size for this group is reflective of our difficulty finding individuals with a BPD diagnosis without a history of comorbid substance use. An estimate from a review of studies reporting BPD-SUD comorbidity found that among individuals meeting diagnostic criteria for BPD, 48.8% met diagnostic criteria for current or lifetime alcohol use disorder and 38% met diagnostic criteria for current or lifetime drug use disorder (Trull et al, 2000). Another study found that 64.1% of participants meeting diagnostic criteria for BPD (n = 243) also met diagnostic criteria for at least one substance use disorder (Zanarini et al., 1998), a figure not too dissimilar from the makeup of the current study (i.e., 69% of the BPD sample met current or past SUD criteria). Future studies should strive to include a larger sample of patients with BPD without SUDs to further assess the role of SUDs in BPD symptomatology.
The results of this study, though limited, add to a growing amount of physiological studies assessing emotion dysregulation in BPD that suggest a hypoarousal response. Furthermore, despite the difficulty in finding BPD participants without a co-occurring SUD, this study highlights the need to assess BPD patients with and without SUDs.
References
- Diagnostic and Statistical Manual of Mental Disorders. 4th. American Psychological Association; Washington, DC: Author; 1994. [Google Scholar]
- Bender DS, Dolan RT, Skodol AE, Sanislow CA, Dyck IR, McGlashan TH. Treatment utilization by patients with personality disorders. American Journal of Psychiatry. 2001;158(2):295–302. doi: 10.1176/appi.ajp.158.2.295. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Emotion and motivation. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. New York: Cambridge University Press; 2007. [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1(3):276–298. [PubMed] [Google Scholar]
- Coffey SF, Schumacher JA, Baschnagel JS, Hawk LW, Holloman G. Impulsivity in borderline personality disorder with and without substance abuse. Personality Disorders: Theory, Research, and Treatment. Personality Disorders: Theory, Research and Treatment. 2011;2:128–141. doi: 10.1037/a0020574. [DOI] [PubMed] [Google Scholar]
- Cook EW, III, Atkinson LS, Lang KG. Stimulus control and data acquisition for IBM PCs and compatibles. Psychophysiology. 1987;24:726–727. [Google Scholar]
- Corcoran S, Norrholm SD, Cuthbert B, Sternberg M, Hollis J, Duncan E. Acoustic startle reduction in cocaine dependence persists for 1 year of abstinence. Psychopharmacology (Berl) 215(1):93–103. doi: 10.1007/s00213-010-2114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damen KF, De Jong CA, Van der Kroft PJ. Interrater reliability of the structured interview for DSM-IV personality in an opioid-dependent patient sample. European Addiction Research. 2004;10(3):99–104. doi: 10.1159/000077697. [DOI] [PubMed] [Google Scholar]
- Ebner-Priemer UW, Badeck S, Beckmann C, Wagner A, Feige B, Weiss I. Affective dysregulation and dissociative experience in female patients with borderline personality disorder: a startle response study. Journal of Psychiatric Research. 2005;39(1):85–92. doi: 10.1016/j.jpsychires.2004.05.001. S0022395604000676. [pii] [DOI] [PubMed] [Google Scholar]
- Greist JH, Klein MH, Erdman HP, Bires JK, Bass SM, Machtinger PE. Comparison of computer- and interviewer-administered versions of the Diagnostic Interview Schedule. Hospital and Community Psychiatry. 1987;38(12):1304–1311. doi: 10.1176/ps.38.12.1304. [DOI] [PubMed] [Google Scholar]
- Hall J, Caleo S, Stevenson J, Meares R. An Economic Analysis of Psychotherapy for Borderline Personality Disorder Patients. The Journal of Mental Health Policy and Economics. 2001;4(1):3–8. [PubMed] [Google Scholar]
- Hazlett EA, Speiser LJ, Goodman M, Roy M, Carrizal M, Wynn JK. Exaggerated affect-modulated startle during unpleasant stimuli in borderline personality disorder. Biological Psychiatry. 2007;62(3):250–255. doi: 10.1016/j.biopsych.2006.10.028. S0006-3223(06)01367-9. [pii] [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Kunert HJ, Schwenger UB, Sass H. Affective responsiveness in borderline personality disorder: a psychophysiological approach. American Journal of Psychiatry. 1999;156(10):1550–1556. doi: 10.1176/ajp.156.10.1550. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Werth U, Lukas G, Qunaibi M, Schuerkens A, Kunert HJ. Emotion in criminal offenders with psychopathy and borderline personality disorder. Archives of General Psychiatry. 2001;58(8):737–745. doi: 10.1001/archpsyc.58.8.737. yoa20015. [pii] [DOI] [PubMed] [Google Scholar]
- Hodes RL, Cook EW, 3rd, Lang PJ. Individual differences in autonomic response: conditioned association or conditioned fear? Psychophysiology. 1985;22(5):545–560. doi: 10.1111/j.1469-8986.1985.tb01649.x. [DOI] [PubMed] [Google Scholar]
- Kuo JR, Linehan MM. Disentangling emotion processes in borderline personality disorder: physiological and self-reported assessment of biological vulnerability, baseline intensity, and reactivity to emotionally evocative stimuli. Journal of Abnormal Psychology. 2009;118(3):531–544. doi: 10.1037/a0016392. 2009-12104-009. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97(3):377–395. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual Technical Report A-8. Gainsville, FL: University of Florida; 2008. [Google Scholar]
- Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York: Guilford; 1993. [Google Scholar]
- Pfohl B, Blum N, Zimmerman M. Structured interview for DSM-IV. University of Iowa Hospitals and Clinics; Iowa City: 1994. [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM, North CS, Rourke KM. Diagnostic Interview Schedule for the DSM-IV (DIS-IV) 2000 [Google Scholar]
- Rosenthal MZ, Gratz KL, Kosson DS, Cheavens JS, Lejuez CW, Lynch TR. Borderline personality disorder and emotional responding: a review of the research literature. Clinical Psychology Review. 2008;28(1):75–91. doi: 10.1016/j.cpr.2007.04.001. S0272-7358(07)00075-X [pii] 10.1016/j.cpr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Sanislow CA, Grilo CM, Morey LC, Bender DS, Skodol AE, Gunderson JG. Confirmatory factor analysis of DSM-IV criteria for borderline personality disorder: findings from the collaborative longitudinal personality disorders study. American Journal of Psychiatry. 2002;159(2):284–290. doi: 10.1176/appi.ajp.159.2.284. [DOI] [PubMed] [Google Scholar]
- Schmahl CG, Elzinga BM, Ebner UW, Simms T, Sanislow C, Vermetten E. Psychophysiological reactivity to traumatic and abandonment scripts in borderline personality and posttraumatic stress disorders: a preliminary report. Psychiatry Research. 2004;126(1):33–42. doi: 10.1016/j.psychres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. 34–57. quiz. [PubMed] [Google Scholar]
- Skodol AE, Gunderson JG, Pfohl B, Widiger TA, Livesley WJ, Siever LJ. The borderline diagnosis I: psychopathology, comorbidity, and personality structure. Biological Psychiatry. 2002;51(12):936–950. doi: 10.1016/s0006-3223(02)01324-0. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Sher KJ, Minks-Brown C, Durbin J, Burr R. Borderline personality disorder and substance use disorders: a review and integration. Clinical Psychology Review. 2000;20(2):235–253. doi: 10.1016/s0272-7358(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Van Asselt AD, Dirksen CD, Arntz A, Severens JL. The cost of borderline personality disorder: societal cost of illness in BPD-patients. European Psychiatry. 2007;22(6):354–361. doi: 10.1016/j.eurpsy.2007.04.001. S0924-9338(07)01313-2. [pii] [DOI] [PubMed] [Google Scholar]
- Vandiver T, Sher KJ. Temporal stability of the Diagnostic Interview Schedule. Psychological Assessment. 1991;3:277–287. [Google Scholar]
- Venables PH, Christie MJ. Electrodermal activity. In: Venables IMPH, editor. Techniques in Psychophysiology. Chichester, UK: Wiley; 1980. pp. 3–67. [Google Scholar]
- Walter M, Degen B, Treugut C, Albrich J, Oppel M, Schulz A, et al. Affective reactivity in heroin-dependent patients with antisocial personality disorder. Psychiatry Res. 2011;187(1-2):210–213. doi: 10.1016/j.psychres.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR, Dubo ED, Sickel AE, Trikha A, Levin A, Reynolds V. Axis I comorbidity of borderline personality disorder. American Journal of Psychiatry. 1998;155:1733–1739. doi: 10.1176/ajp.155.12.1733. [DOI] [PubMed] [Google Scholar]


