Taxa from the genus Prosopis are widespread invasive aliens across the globe. Numerous species have contentious issues surrounding them as they provide both benefits and harm. Prosopis taxa are currently naturalised or invasive in 103 countries and are bioclimatically suitable for many more. There are numerous management practices available to control Prosopis invasions, each with their benefits and costs, however, in most areas management has had only limited success. There is need for more research to improve understanding and management success and for countries to develop strategic plants to guide managed in the future.
Keywords: Classification and regression tree, distribution, global review, impacts, logistic regression, management, mesquite, tree invasions.
Abstract
Invasive species cause ecological, economic and social impacts and are key drivers of global change. This is the case for the genus Prosopis (mesquite; Fabaceae) where several taxa are among the world's most damaging invasive species. Many contentious issues (‘conflicts of interest’) surround these taxa, and management interventions have not yet sustainably reduced the negative impacts. There is an urgent need to better understand the factors that drive invasions and shape management actions, and to compare the effectiveness of different management approaches. This paper presents a global review of Prosopis, focusing on its distribution, impacts, benefits and approaches to management. Prosopis was found to occur in a 129 countries globally and many more countries are climatically suitable. All areas with naturalized or invasive Prosopis species at present are suitable for more taxa and many Asian and Mediterranean countries with no records of Prosopis are bioclimatically suitable. Several Prosopis species have substantial impacts on biodiversity, ecosystem services, and local and regional economies in their native and even more so in their invasive ranges; others provide multiple benefits to local communities. Management efforts are underway in only a small part of the invaded range. Countries where more research has been done are more likely to implement formal management than those where little published research is available. Management strategies differ among countries; developed nations use mainly mechanical and chemical control whereas developing nations tend to apply control through utilization approaches. A range of countries are also using biological control. Key gaps in knowledge and promising options for management are highlighted.
Introduction
The increased movement of humans around the world has facilitated transportation of many species to environments far from their native ranges. This has been done purposefully—to introduce new crops and horticultural and forestry species—and accidentally, for example as weed seed in grain shipments (Mack 2003). These introductions have led to the rise of biological invasions that cause substantial ecological, social and economic impacts, and they are one of the key drivers of global change (Vitousek et al. 1997; Pimentel et al. 2000). However, many alien species have been embraced by humans and are crucial for local livelihoods and national economies through the goods and services they provide (Shackleton et al. 2007; Kull et al. 2011; van Wilgen et al. 2011).
It is important to understand the dynamics of invasive species to reduce their negative impacts and maximize their benefits, but frameworks linking theory and management for biological invasions are lacking (Hulme 2003; Wilson et al. 2014). Management is inefficient in many areas due to lack of knowledge on key aspects of the invasive species. It is crucial to understand the reasons for introductions, uses (benefits), costs, ecology and scales of invasions and to elucidate perceptions and potential contentious issues when creating sustainable management plans (Kull et al. 2011; van Wilgen and Richardson 2014; Wilson et al. 2014). This is true for invasive species in the genus Prosopis.
Taxa of Prosopis (mesquite; Fabaceae) occur in most of the world's hot arid and semi-arid regions as native or introduced species (Pasiecznik et al. 2001). The genus Prosopis as described by Burkart (1976) consists of 44 species. They have been introduced globally and have become naturalized or invasive in many places (Rejmánek and Richardson 2013). Several Prosopis species are also ‘weedy’ in parts of their native ranges (Pasiecznik et al. 2001). In this paper we define native species as those whose presence in an area is not attributable to introduction by humans (this includes species that have spread into areas without assistance from humans by overcoming biogeographic barriers). Alien taxa are those whose presence in an area is attributable to introduction by humans. Naturalized taxa are alien taxa that are self-sustaining. Invasive taxa are naturalized taxa that have spread substantially from introduction sites (further details in Pyšek et al. 2004). We define ‘weedy’ taxa as native taxa that have increased in abundance and/or geographic range in their native ranges.
Numerous Prosopis taxa are recognized as major invaders across large parts of the world (Pasiecznik et al. 2001; Brown et al. 2004). ‘Prosopis’ is listed as one of the 20 weeds of national significance in Australia and taxa in the genus are declared as major invasive species in Ethiopia, India, Kenya and South Africa, and Sudan is advocating for its eradication (FAO 2006; Australian Weeds Committee 2012; Low 2012; van Wilgen et al. 2012). Factors that make many Prosopis species successful invaders include the production of large numbers of seeds that remain viable for decades, rapid growth rates, an ability to coppice after damage (Felker 1979; Shiferaw et al. 2004), root systems that allow them to efficiently utilize both surface and ground water (to depths of >50 m) (Nilsen et al. 1983; Dzikiti et al. 2013), and allelopathic and allelochemical effects on other plant species (Elfadl and Luukkanen 2006). Many Prosopis species can also withstand climatic extremes such as very high temperatures and low rainfall, and they are not limited by alkaline, saline or unfertile soils (Pasiecznik et al. 2001; Shiferaw et al. 2004). Interspecific hybridization also enhances invasiveness in many introduced regions (Zimmermann 1991).
Prosopis invasions generate environmental, social and economic benefits as well as harm (Chikuni et al. 2004; Geesing et al. 2004; Wise et al. 2012). This has led to contentious issues surrounding the genus (Richardson 1998b; van Wilgen and Richardson 2014). Some advocates promote it as a ‘wonder plant’ while others call for its eradication, or contrast its positive and negative aspects, e.g. ‘Boon or bane’ (Tiwari 1999), ‘Pest or providence, weed or wonder tree?’ (Pasiecznik 1999), ‘Invasive weed or valuable forest resource?’ (Pasiecznik 2002). Contrasting views, contradictory perceptions and unclear policies are limiting options for constructive dialogue between different parties. This is exacerbated by problems in identifying and differentiating morphologically similar species, and by a general lack of knowledge on the distribution, scale of invasion, benefits, impacts and effective management approaches. Furthermore, many different approaches for managing Prosopis have been tried in different situations, without a thorough evaluation of the relative effectiveness of the methods. The Food and Agricultural Organization has called for a sound, unbiased global overview of Prosopis to act as a prerequisite for the holistic management of the genus (FAO 2006). Such reviews have been useful for guiding and prioritizing management and improving knowledge in other groups of woody invasive plants (Richardson and Rejmánek 2004, 2011; Kull et al. 2011; Wilson et al. 2011).
The aims of this paper are thus to (i) contrast benefits and costs of invasive Prosopis, (ii) update knowledge on Prosopis occurrence and introductions globally and highlight the potential range expansion of Prosopis, (iii) elucidate ecological, economic and social factors that shape attempts at managing Prosopis, (iv) compare and contrast the effectiveness of different management approaches in different regions, and (v) identify priorities for research and policy development. We review the literature and collate data from many sources. Details on the approach for the literature review, approaches used for statistical analyses and climate matching are provided in Supporting Information.
Benefits and Costs
Benefits, costs and invasiveness of different species
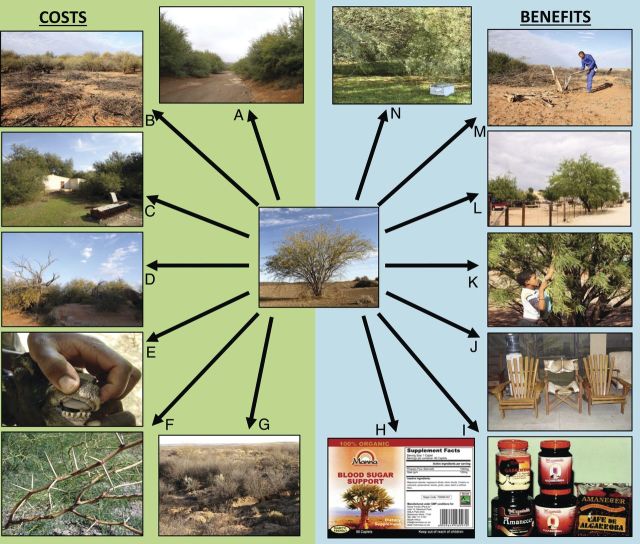
Prosopis provides benefits and generates costs which have led to contentious issues surrounding the genus (Fig. 1). The ‘usefulness’ of Prosopis has led to the large-scale introduction of five species in particular (P. chilensis, P. glandulosa, P. juliflora, P. pallida and P. velutina) and the subsequent naturalization and invasion of these taxa and their hybrids leading to the provision of benefits and costs in their new ranges [see Supporting Information]. Although P. pallida is invasive in many areas (Rejmánek and Richardson 2013), it appears to be less aggressive than some other species (Pasiecznik et al. 2006a, b).
Figure 1.
Costs and benefits of introduced Prosopis species: (A) invasive Prosopis stand altering hydrology in Loeriesfontein, South Africa; (B) cleared Prosopis in the foreground and uncleared in the background illustrating impenetrable thickets, loss of land, loss of grazing potential and the effort needed for its control in Kenhardt, South Africa; (C) loss of access to a barn and encroachment of fields in Calvinia, South Africa; (D) death of a native tree (Searsia lancea) due to competition from Prosopis in Kenhardt, South Africa; (E) effects of Prosopis pods on a goat's teeth in Kenya; (F) Prosopis thorns that cause tyre damage and injure humans and livestock; (G) Prosopis causing loss of topsoil and erosion in Prieska, South Africa; (H) ‘manna’—a blood sugar medicine made from Prosopis in South Africa (www.mannaplus.co.za); (I) food products made from Prosopis in Peru; (J) timber from Prosopis used to make furniture in Kenya; (K) a young boy collecting Prosopis pods to feed livestock in Askham, South Africa; (L) Prosopis used for shade and ornamentation in Askham, South Africa; (M) Prosopis used as a fuel in Kenhardt, South Africa; (N) a bee hive placed in an invasive Prosopis stand Calvinia, South Africa. Photos: S. Choge (J), G. Cruz (I), P. Manudu (E, F), R. Shackleton (A–D, G, K–N).
Several species are also weedy and thus provide both benefits and costs in their native ranges (P. affinis, P. caldenia, P. campestris, P. chilensis, P. cineraria, P. farcta, P. glandulosa, P. hassleri, P. humilis, P. juliflora, P. kuntzei, P. nigra, P. pubescens, P. ruscifolia, P. strombulifera, P. tamarugo, P. velutina) [see Supporting Information]. At least 19 (invasive and weedy) of the 44 species in the genus are known to generate benefits and costs, with the rest being only beneficial. The invasiveness and potential negative impacts of many Prosopis species are still unknown as only a handful have been introduced.
Uses/benefits
Prosopis species have been used for a variety of products for more than 5000 years in their native ranges (Pasiecznik et al. 2001). The numerous goods and services provided by Prosopis have led to global introductions and have made some species important for local communities. Prosopis is commonly used for fuel, fodder, windbreaks, shade, construction materials and soil stabilization through its invasive ranges in Africa and Asia (Pasiecznik et al. 2001; Wise et al. 2012). In some areas the benefits from Prosopis are, or were, regarded as a key income source for many households. In one village in Malawi, 44 % of people relied on Prosopis products as a primary or supplementary source of income (Chikuni et al. 2004). Communities in Kenya have benefited greatly from the sale of charcoal and Prosopis pods for fodder, boosting the local economy in some areas by US$1.5 million per year (Choge et al. 2012). In India, Prosopis provides up to 70 % of fuelwood needs for local households in some dry region villages (Pasiecznik et al. 2001).
Although utilization is most common in rural settings to sustain local livelihoods, Prosopis products are also exploited on a large scale by private companies. In South Africa, pods are collected to produce organic medicines (‘manna’) that are said to have properties that stabilize blood sugar levels in humans. This company is making profits of US$100 000 per annum and has the potential to increase profits 10-fold if the product is marketed internationally (Wise et al. 2012). A company in Brazil, Riocon, has an annual turnover of US$6 million a year from the sale of Prosopis pod flour for animal feeds (A. Davi, Ricocon, pers. comm.).
Negative impacts/costs
Prosopis invasions also have a variety of negative social, ecological and economic impacts (Figs 1 and 2). They alter ecosystem services such as water supply, hydrological functioning, grazing potential and soil quality (DeLoach 1984; Bedunah and Sosebee 1986; Archer 1989; Le Maitre et al. 2000; van Klinken et al. 2006; Ndhlovu et al. 2011; Nie et al. 2012; Dzikiti et al. 2013). Native biodiversity in many parts of the world has also been negatively impacted by invasive Prosopis species (Steenkamp and Chown 1996; Dean et al. 2002; El-Keblawy and Al-Rawai 2007; Belton 2008; Kaur et al. 2012).
Figure 2.
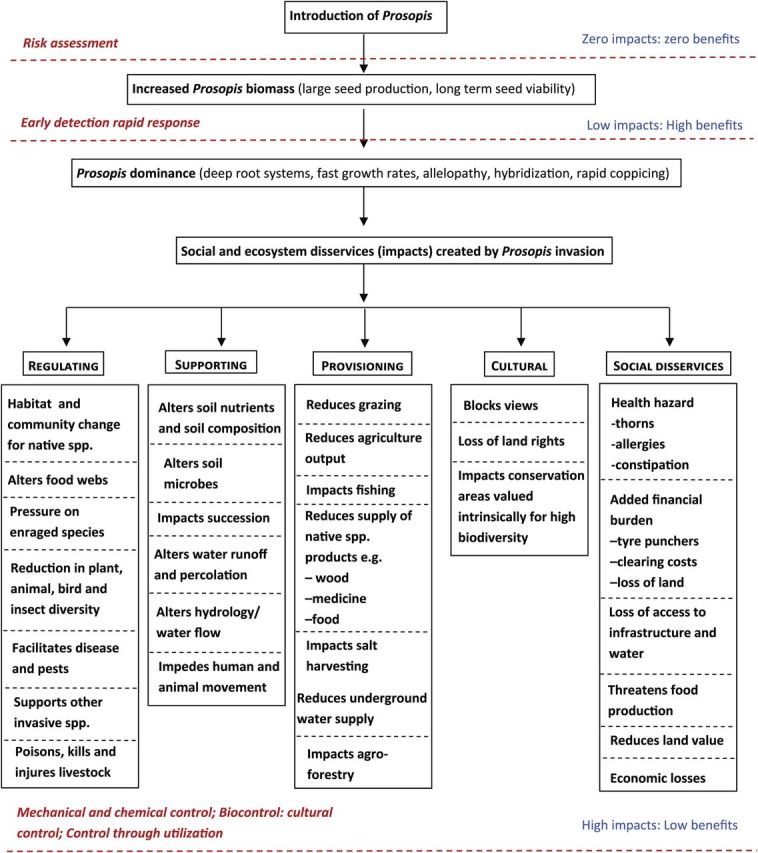
Cause-and-effect network diagram showing the negative effects of Prosopis invasions and management options that can be used to target each stage of invasion.
Local communities in Kenya, Sudan, Eritrea, Malawi and Pakistan noted a range of negative consequences arising from invasive Prosopis (Choge et al. 2002; Chikuni et al. 2004; Mwangi and Swallow 2005; Laxén 2007; Bokrezion 2008; Kazmi et al. 2009). These included effects on livestock health, Prosopis thorns causing tyre punctures and flesh wounds, dense thickets reducing access to water points, roads, infrastructure and agricultural and range lands, drying up of water sources, reducing natural forest cover and the services from these forests, as well as providing refuge for thieves.
In many parts of Africa Prosopis invasions are a leading cause of detrimental impacts on local community structure and functioning, leading to an increase in their vulnerability. This includes the potential loss of land rights for local livestock herders in Mali and violent conflict over limited natural resources between neighbouring communities in Ethiopia and Kenya (Centre for Sustainable Development Initiatives 2009; Djoudi et al. 2011; Stark et al. 2011). One Kenyan community has even taken the Food and Agricultural Organization (FAO) and the Kenyan government to court over the harm created by the introduction of Prosopis (Pasiecznik et al. 2006a).
Native weedy Prosopis taxa are also estimated to cause a loss of US$200–500 million per annum to the livestock industry in the USA (DeLoach 1984). In South Africa costs of managing Prosopis invasions are substantial, averaging $35.5 million per annum (van Wilgen et al. 2012).
Benefits vs. costs and the dimensions of contentious issues
Perceptions on the benefits and costs of invasive alien species are strongly influenced by invasion abundance (Binggeli 2001; Shackleton et al. 2007). As abundance increases, associated costs rise and benefits fall due to issues such as resource accessibility (Wise et al. 2012). In India, Prosopis was initially seen as beneficial, but over time the negative consequences became more apparent, leading to increasingly negative perceptions of the plant from some quarters (Pasiecznik et al. 2001). A similar situation arose in Kenya where, as Prosopis became invasive, it was described as a ‘bad omen’ by some local people (Choge and Chikamai 2004) and more than 65 % of people in three villages mentioned that their lives would have been better off if Prosopis was never introduced (Maundu et al. 2009). In Sudan, over 90 % of livestock farmers viewed Prosopis as a problem as it became more widespread (Elsidig et al. 1998).
In many areas, invasive Prosopis trees do not sustain their full use potential due to intraspecific competition in dense stands which, generally, form over time. In such cases relatively few pods are produced for fodder and human consumption and dense invasive stands become impenetrable for humans and livestock making utilization of resources difficult (Chikuni et al. 2004; Mwangi and Swallow 2005). Wise et al. (2012) show that net economic benefits decrease as invasion densities increase in South Africa. They predict that the net cost of having Prosopis in the country will become negative in 4–20 years depending on future rates of spread. A framework by Shackleton et al. (2007) also shows that useful invasive aliens initially have high benefits, but as invasion densities increase, costs rise which lead to an increase in human vulnerability. This raises questions about the introduction of ‘miracle’ species in the past such as Acacia, Leucaena and Prosopis because the adverse impacts tend to exceed the benefits as the invasions progress, if left unmanaged (de Wit et al. 2001; Pasiecznik 2004; Wise et al. 2012; Low 2012), as well as the continued promotion of invasive alien species like Prosopis for biofuels today (Witt 2010; Naseeruddin et al. 2013).
The fact that the detrimental effects emerge only after invasions have reached unmanageable levels exacerbates contentious issues surrounding invasive species and may delay management decisions, in many cases restricting the implementation of effective management. There have also been conflicts of interest regarding which form of management to implement—how best to preserve, exploit and even enhance benefits while reducing negative impacts of Prosopis invasions (Zimmermann 1991).
Introductions, Current and Potential Distribution of Prosopis
Introductions
Dates and sources of introduction
Intercontinental introductions of Prosopis species have occurred over several centuries (Fig. 3). The first reports were of the introduction of Prosopis species from the Americas to Senegal in 1822, and to Australia, Hawaii, India, Philippines, South Africa, Sri Lanka and Sudan in the late 1800s and early 1900s (Pasiecznik et al. 2001). However, most of the widespread introductions were made into Africa and Asia between the 1970s and 1990s (Fig. 3) as part of reforestation programmes after major droughts in the Sahel. Many areas, notably India, South Africa and Sudan, have had multiple introductions over many decades. There is no evidence of new introductions post 1990, with the last recorded introductions being in Malawi and Burkina Faso in 1986 (Ræbild et al. 2003; Chikuni et al. 2004). There have, however, been recent calls for the introduction of known invasive Prosopis species to new locations. Hasan and Alam (2006) recommend that the planting of Prosopis would be beneficial to combat degradation in Bangladesh. Parvaresh (2011) proposed using Prosopis to stabilize dunes to protect important biologically diverse wetlands and mangrove forests in Iran. The promotion of biofuels could also lead to the spread of invasive woody species such as Prosopis (Witt 2010). There has also been extensive natural spread (commonly by means of flood water) and human-assisted spread (livestock trade) into new areas within countries where it is already naturalized and invasive (Van den Berg 2010).
Figure 3.
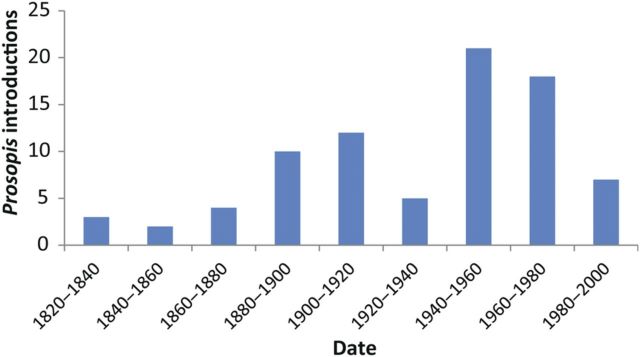
Time scale of all Prosopis introductions globally (n = 82 known species–country introduction dates).
Seed introductions have come both from native populations and from naturalized and invasive populations in countries where Prosopis was introduced previously. However, the original sources of seed and dates for introductions to many countries are poorly documented. Seed introduced to Hawaii came from a tree in France with a speculated provenance in Brazil (Pasiecznik et al. 2001) and P. pallida introduced to Australia came from Hawaii (Pasiecznik et al. 2001). South Africa had multiple introductions of many species and seed was most likely introduced from native ranges in Chile, Honduras, Mexico and USA (Zimmermann 1991). Seed from naturalized populations in South Africa was introduced into Egypt and seed introduced into Sudan came from South Africa and Egypt (Pasiecznik et al. 2001). The provenance of early Prosopis introductions to India is uncertain (likely Mexico or Jamaica); later introductions came from Argentina, Australia, Mexico, Peru and Uruguay (Pasiecznik et al. 2001).
Reasons for introduction
Most introductions of Prosopis were intentional, although there have been accidental cross-border introductions between neighbouring counties. Prosopis was introduced for many reasons: to provide fodder and shade in the arid areas of South Africa and Australia; for dune stabilization, afforestation and fuel wood supply in Sudan; for live fencing in Malawi; initially to rehabilitate old quarries and later for afforestation and the provision of fuelwood and fodder in Kenya; for fuelwood production and rehabilitating degraded soil in India; for local greening, ornamental cultivation and soil stabilization in many Middle Eastern countries; and for vegetation trials in Spain (Zimmermann 1991; Ghazanfar 1996; Pasiecznik et al. 2001; Choge et al. 2002; Chikuni et al. 2004; Elfadl and Luukkanen 2006; van Klinken et al. 2006; Laxén 2007; N. Pasiecznik and E. Peñalvo López, unpubl. res.). Prosopis was possibly first introduced unintentionally into Botswana, Nigeria and Yemen through livestock trading with neighbouring countries (Pasiecznik et al. 2001; Geesing et al. 2004).
Fate of introductions
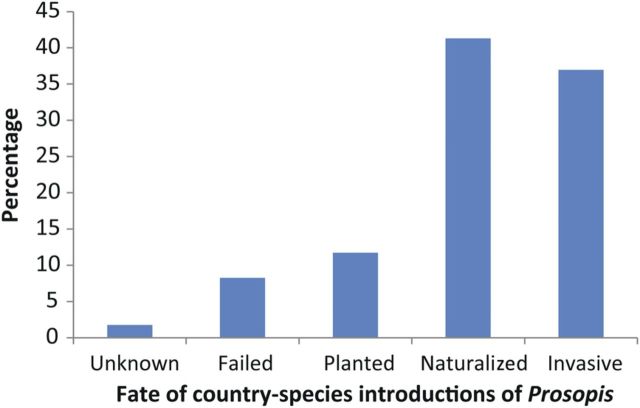
Of all the introductions of Prosopis species reviewed here, 79 % have led to naturalization, of which 38 % have become invasive (Fig. 4). No information on naturalization is available for 8 % of records, and 2 % of introductions are known to have failed (i.e. did not survive planting). Currently 12 % of introductions are only recorded as ‘planted’.
Figure 4.
Classification of all records of introduced Prosopis species (236 introductions in 103 countries); classification of ‘naturalized’ and ‘invasive’ follows the criteria of Pyšek et al. (2004).
Distribution
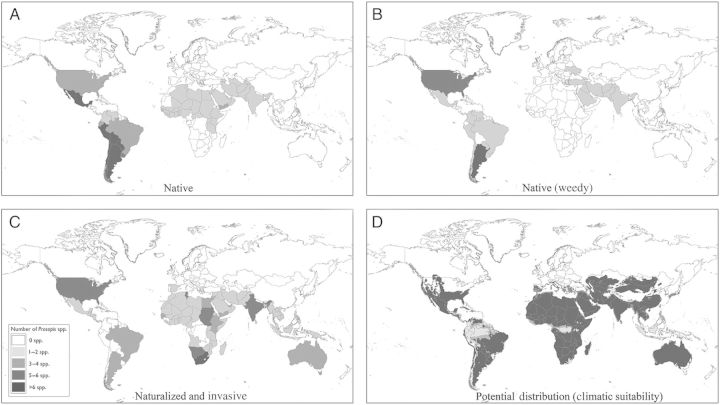
Prosopis currently occurs naturally or as an introduced species in at least 129 mainland and island countries and territories (Fig. 5; see Supporting Information). This includes the Caribbean islands (18) and mainland counties (19) in the Americas (excluding Canada, Suriname and Guyana), 40 countries in Africa, 26 in Asia, 4 in Europe, 24 island/atoll countries in the Pacific, Atlantic and Indian Oceans and Australia.
Figure 5.
Global distribution of Prosopis species: (A) species diversity in countries with native taxa; (B) species diversity of taxa recognized as being weedy within their native ranges; (C) species richness of introduced Prosopis taxa that have either naturalized or become invasive (following the criteria of Pyšek et al. 2004); and (D) potential Prosopis species richness based on climatic suitability.
The last comprehensive global review of Prosopis distribution listed the presence of taxa in 93 mainland and island/attol countries (Pasiecznik et al. 2001). It is unlikely that Prosopis has been introduced into more places in the 13 years since that review was undertaken, but rather that data availability has increased in the intervening period or that there has been unintentional spread e.g. into Tanzania. Of the 129 countries, 26 have only native species, 64 have only introduced Prosopis species, and 39 have both native and introduced species. Prosopis is weedy in 38 % of countries where it occurs naturally and 38 % of species in the genus are currently categorized as weedy in their native ranges. The distribution and scale of invasions in countries with invasive Prosopis are not well known, with only 13 % of countries having detailed distribution or percentage cover data and not just records of occurrence.
Potential distribution
Climate matching was used to assess areas of potential naturalization and invasion (Peel et al. 2007). We identified many regions that are climatically suitable for Prosopis where there are currently no records of any taxa (Fig. 5D).This includes countries in Europe (Greece, Italy, Portugal, Romania, etc.), South America (Guyana and Suriname), Asia (China, Japan, Nepal, South Korea, etc.) and numerous island/atoll countries and overseas territories (Comoros, Malta, Solomon Islands, Timor-Leste, etc.) (Fig. 5D; Supporting Information). All countries where at least one Prosopis species has been introduced and has established have the potential for the naturalization of additional Prosopis species. For example, there are currently seven naturalized and invasive Prosopis species recorded in South Africa, but the country is climatically suitable for many more species [see Supporting Information]. Maundu et al. (2009) also illustrated a high climatic suitability for Prosopis in southern and eastern Africa and showed that there are many areas that could have invasions but currently do not.
Management of Prosopis
Naturalized and/or weedy Prosopis are reported in 112 countries. Currently 23 countries with weedy or invasive Prosopis (21 %) implement some form of formal management. No countries rely exclusively on biological control, 6 (26 %) use only mechanical or chemical control, 5 (22 %) use control through utilization and 11 (48 %) apply an integrated approach (three or more methods, including biological control, mechanical control, chemical control, control through utilization and cultural control) (Table 2).
Table 2.
A comparison of techniques for managing Prosopis and their advantages and disadvantages.
| Control type | Advantages | Disadvantages |
|---|---|---|
| Biological control | • Relatively inexpensive once implemented • Works over large areas, including areas that are inaccessible for mechanical control • Minimal associated costs after biocontrol agent is released (monitoring is required) |
• Biocontrol agents have not yet had substantial impacts on reducing stand density or extent of invasions and rates of spread in some areas such as South Africa but have been more successful in places like Australia • Initial research is expensive • Potential to spread across borders unintentionally • Inapplicable in areas where native Prosopis is weedy • Conflicts of interest around the use of biological control in areas where Prosopis invasion is seen as beneficial (e.g. South Africa, Kenya) |
| Mechanical control | • Efficient at removing Prosopis over large areas | • Labour and capital intensive |
| Chemical control | • Efficient at removing Prosopis over large areas | • Labour and capital intensive |
| Utilization | • Maximizes on benefits to be had from biological invasions • Promotes rural social–economical development • Reduces overexploitation of native spp. • Profits counteract management costs |
• Encouraging utilization may create dependency on the species, thereby exacerbating conflicts of interest • Some areas have lower-value Prosopis spp. (more thorny, bitter pods, shrubby forms) making utilization more difficult • Many Prosopis invasions are in remote areas making large-scale utilization difficult |
| Cultural control/other control (e.g. fire, grazing and livestock transport management) | • Low costs • Can also prevent other types of degradation |
• Requires people to change perceptions • Large-scale education programmers are needed • Does not always work for all Prosopis spp.—e.g. fire-tolerant hybrids • Not applicable in all areas, e.g. places with low biomass and fire-tolerant hybrids |
Countries that use only chemical and mechanical control are mainly found in the Middle East and have small isolated invasions and are usually wealthier nations, whereas control through utilization is applied in poorer countries such as Kenya and Ethiopia. Biological control is driven by Australia and South Africa; however, there are also areas where ‘biological control agents’ are present but were not deliberately introduced, for example, Egypt (seed-feeding beetles—Coleoptera and Burchidae), Sudan and Yemen (Algarobis prosopis) (Delobel and Fediere 2002; Al-Shurai and Labrada 2006; Babiker 2006). In Yemen there is no evidence that the non-native A. prosopis feeds on the native Prosopis cineraria (Al-Shurai and Labrada 2006). There are concerns, however, that introduced insects could affect less invasive P. pallida populations in these areas that are utilized by local communities (Pasiecznik et al. 2006a, b). Another view is that any effect of such insects could improve the usefulness of less invasive taxa by reducing seed production and therefore potential invasiveness and could lead to less dense stands with larger trees and greater pod production (Zachariades et al. 2011).
Logistic regressions were run to determine which factors underpin whether a country has formal management of Prosopis taking place or not. The degree of understanding of Prosopis invasion impacts and ecology (besides residence time—the time since introduction) is a better determinant of whether or not a country will manage Prosopis than the socioeconomic conditions of the country (Table 1). The stepwise regression revealed that the level of impacts and overall knowledge on Prosopis invasions are key determinants of the presence of management within a country or not. Having knowledge on invasion potential/risk allows countries either to act timeously or to develop protocols to guide management based on an overall understanding of impacts, ecology, uses and special scales. Having a good understanding surrounding Prosopis invasions also helps to highlight the need for management, and subsequent management also stimulates the accumulation of further knowledge on invasions. Residence time might not be a significant predictor, because in wetter areas invasions tend to establish much faster than in drier areas (Table 1). Also, all countries have had Prosopis long enough to have naturalized and invasive populations (Zimmermann et al. 2006).
Table 1.
Logistic regression highlighting the importance of different ecological, economical and social factors in determining management of Prosopis within a country.
| Explanatory variable | Nagelkerke R2 | Predictions—% correct | Wald stat | P value |
|---|---|---|---|---|
| No. of introduced Prosopis spp. | 0.540 | 84.3 | 13.04 | 0.000 |
| Source of introduction known | 0.234 | 70.0 | 4.815 | 0.999 |
| Time since introduction | 0.009 | 47.1 | 0.275 | 0.626 |
| Use level | 0.103 | 67.1 | 4.19 | 0.242 |
| Distribution and extent of Prosopis cover known | 0.616 | 81.4 | 7.087 | 0.069 |
| Level of Prosopis impacts | 0.685 | 87.1 | 19.638 | 0.000 |
| No. of publications relating to Prosopis | 0.960 | 88.6 | 20.765 | 0.000 |
| Overall knowledge of Prosopis invasions | 0.686 | 92.9 | 16.993 | 0.005 |
| GDP per capita | 0.013 | 65.7 | 0.680 | 0.410 |
| Human development index | 0.041 | 68.6 | 0.324 | 0.569 |
Simple socioeconomic variables are poor predictors of the existence of management strategies as there is evidence of management in countries at all levels of development (Table 1). Many of the poorer countries receive foreign aid to implement and run management programmes, at least at the outset.
The findings of this review contradict previous publications that have argued that less developed countries have conducted less research and management of invasive alien species (McNeely et al. 2005; Pyšek et al. 2008; Nuñez and Pauchard 2009; McGeoch et al. 2010). Some developing countries are at the forefront of Prosopis research and management such as Kenya (control through utilization, social impacts) and South Africa (biological control), along with developed countries such as Australia and the USA. Witt (2010) noted that the prominence and severity of the impacts of Prosopis in developing countries has motivated this investment in research and understanding. However, there may be a lack of research for less prominent invasive alien species in poorer regions of the world.
The classification and regression model highlights the factors that underpin which management approaches counties are likely to adopt (Fig. 6). Similar to the regression output, the overall level of knowledge of Prosopis is an important factor when predicting which management approach or technique a country will adopt (Fig. 6). Countries with a good understanding of Prosopis based on the number of publications and the diversity of published materials have a higher chance of having some form of management, and in general this takes the form of integrated management. The level of development of a county, indicated by gross domestic product per capita, also influences the type of management approach a country is likely to adopt. Wealthier countries are more likely to implement mechanical and chemical control methods, which are the most costly but also currently the most effective options. Middle-income countries most commonly implement integrated management, whereas poor countries predominantly adopt control through utilization for managing Prosopis.
Figure 6.
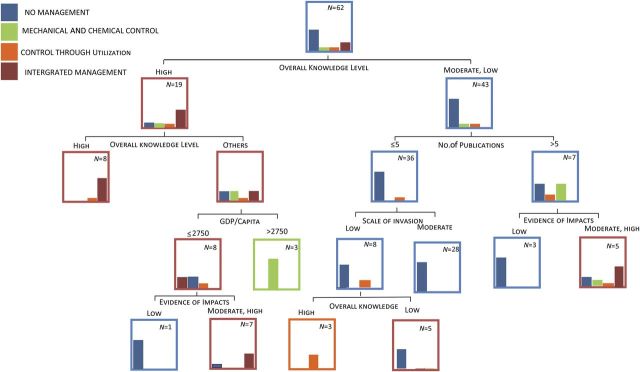
A classification and regression tree model using social, ecological and economic variables to explore the drivers of different types of Prosopis management globally.
The advantages and disadvantages of these approaches differ (Table 2), and are closely linked to the costs of the control method. For example, countries with limited invasions are more likely to use mechanical and chemical control, whereas those with large-scale invasions are more likely to adopt an integrated approach, as purely mechanical and chemical control becomes too costly (van Klinken et al. 2006). Control through utilization aims to aid local development while simultaneously controlling Prosopis impacts and is therefore promoted in poorer parts of the world.
Contentious issues surrounding invasive Prosopis taxa and their management
The benefits and impacts and choice of different management approaches of Prosopis have led to contentious issues regarding management. Control through utilization is advocated by some as a management technique that enables benefit of invasive Prosopis to be utilized while simultaneously reducing the negative impacts of invasions and promoting local development (Choge and Chikamai 2004). However, many believe that this approach is inefficient at reducing invasions and leads to other problems such as dependencies (Table 2) (van Wilgen et al. 2011) and that other approaches such as chemical and mechanical clearing should be prioritized, although they are costly (Witt 2010). To date, there is no evidence of the success of control through utilization as a management technique (Table 2). The control through utilization approach is motivated around local development (which is needed) more so than managing invasions at large spatial and temporal scales.
There are conflicting views on best management approaches (eradication vs. control through utilization) in different villages in Kenya (Mwangi and Swallow 2005; Njoroge et al. 2012). Similar cases of contentious issues and conflicts of interest have been seen for other management approaches such as biological control. In South Africa only seed-feeding beetles were introduced so that neither the Prosopis trees themselves nor the production of pods would be harmed (Richardson 1998a)—even though better biological control agents have been identified that would harm trees and be more effective in reducing invasions (Zachariades et al. 2011).
Case studies comparing different management approaches
Despite the growing body of research on management options for weedy and invasive Prosopis stands (van Klinken et al. 2006), there is an ongoing debate on how to effectively manage large-scale invasions. Different approaches are currently being used to manage Prosopis, each with their own set of advantages and disadvantages (Table 2). The following case studies were selected as being representative of different management strategies and also encompass the approaches most commonly employed in countries with different levels of socioeconomic development (developed—Australia; emerging economies—South Africa; developing—Kenya). The case studies are also characteristic of management strategies driven and implemented by different stakeholders, e.g. government driven with mainly private implementation (Australia), mainly government driven and implemented (South Africa) and government driven with some non-government organization (NGO) and international support (Kenya).
Australia
Prosopis has invaded over one million hectares and could potentially spread over 70 % of Australia's land area (Osmond 2003). Prosopis taxa are considered as one of the 20 worst invasives in Australia, and in accordance with the Weeds Management Act 2001, a strategic plan has been developed to guide management (Australian Weeds Committee 2012). Prosopis is a declared weed in all the mainland states and one territory in Australia and has been categorized in accordance with the threats it poses and the corresponding management responses that need to be implemented (van Klinken and Campbell 2009). This includes preventing introductions, trade, sale or movements of Prosopis taxa and the eradication of small populations and control of large populations (Australian Weeds Committee 2012). In general, most landowners use mechanical and chemical control measures to manage Prosopis. Although control and eradication programmes are primarily funded by the state, many private landowners also fund management operations. For example, in Queensland $A4 million was allocated for Prosopis management by the government, which was supplemented further by over $A600 000 by landholders between 1995 and 1999 and over $A2 million was spent on clearing between 2001 and 2005 (Martin and van Klinken 2006).
Control of Prosopis first started in 1954 at Mardie Station, Western Australia, and by 1962 a major reduction in Prosopis density had been achieved. Populations increased again when funding diminished, but in the mid-1970s the allocation of government funding led to substantial progress with clearing (van Klinken and Campbell 2009). In other areas of Western Australia control was improving, but after funding lapsed many infestations returned in the 1990s with the exception of some areas such as Yeeda Station where control had been successful due to annual monitoring and clearing (van Klinken and Campbell 2009). In Queensland substantial funding was invested for clearing in the area around Comongin Station, and by 2005 over 4000 ha of dense Prosopis stands had been removed (van Klinken and Campbell 2009). In northern Queensland research concluded that eradication was feasible in the region and significant steps have been made towards this goal (van Klinken and Campbell 2009). New South Wales and South Australia have similar examples of good control efforts and others that have had limited success due to a lapse in control and monitoring (van Klinken and Campbell 2009).
Four biological control agents have been released in Australia: Algarobius bottimeri and A. prosopis (seed-feeding bruchids), Evippe species (a leaf-tying moth) and Prosopidopsylla flava (a sap sucker) (van Klinken et al. 2003; van Klinken 2012). Two have established widely (A. prosopis, Evippe species), and the latter has had noticeable impacts on Prosopis populations through reducing long-term growth rates (van Klinken 2012). Biological control in Australia has been more successful than in other places like South Africa and the benefit-to-cost ratios are positive (0.5), with expectations to increase in the future (Page and Lacey 2006). The release of more agents is recommended to further improve control (van Klinken et al. 2003; van Klinken 2012).
Experiments have shown that some species are highly fire tolerant (especially the hybrids), which reduces the potential for using fire as a control method in many areas (van Klinken et al. 2006). Grazing control has also been advised to help prevent establishment and further spread of Prosopis (Csurhes 1996), although this approach has had limited success in Argentina and the USA (Dussart et al. 1998; Brown and Archer 1989). There are also regulations on the transport of livestock in areas infested with Prosopis to prevent its spread and accidental introduction elsewhere in Australia (Australian Weeds Committee 2012). Management policy is backed up by good legislation; Australia is one of two countries with a national management strategy. The government has also published many easily accessible documents on Prosopis management methods to inform landowners on control measures, and the Prosopis strategic plan places a lot of emphasis on educating and making stakeholders aware of Prosopis invasions and how to manage them (Australian Weeds Committee 2012). There have been rewarding examples of control success (van Klinken and Campbell 2009); however, Prosopis populations continue to spread in many areas and further management is needed.
South Africa
Prosopis invasions in South Africa cover an estimated 1.8 million hectares, and are increasing at 8 % per annum (Versfeld et al. 1998; Van den Berg 2010). They have the potential to invade between 5 and 32 million hectares of South Africa based on climatic suitability—about a third of the area of the country (Rouget et al. 2004). Prosopis is declared as a category 2 invasive alien species because it provides benefits and causes harm; this status means that it is legal to grow Prosopis in demarcated areas once a permit has been issued. A combination of mechanical, chemical and biological control methods is used to control Prosopis, mainly by the government-managed Working for Water programme. Three seed-feeding beetles (A. prosopis, A. bottimeri and Neltumius arizonensis) were introduced as biological control agents to try and reduce spread while maintaining its benefits (Zimmermann 1991; Coetzer and Hoffmann 1997). Neltumius arizonensis failed to establish (Zachariades et al. 2011). Although biological control is considered the most cost-effective way of managing large-scale invasions of many species, there are many cases where the agents fail to make a significant impact and Prosopis is one of them (van Wilgen et al. 2012). The overall return on investment is low compared with biological control programmes for Opuntia species and Australian Acacia species in South Africa (van Wilgen et al. 2012). There is potential to release more agents, such as the Evippe species which is already successful in Australia (see above), should the contentious issues surrounding the benefits and costs of Prosopis be resolved (Zachariades et al. 2011). Prosopis cover increased by ∼35 % between 1996 and 2008, despite the expenditure of R435.5 million (US$42.7 million) on control over this period. Only 15 100 ha were cleared using mechanical and chemical control with this substantial budget (van Wilgen et al. 2012), which makes the cost/ha very expensive (US$2828). The limited success to date may be due to lack of a management strategy and of prioritization of management projects (Forsyth et al. 2012). There is a need for researchers, managers and policy-makers to agree on new strategies for prioritizing areas for interventions to curb the spread of Prosopis and to ensure that the limited resources are used effectively (Forsyth et al. 2012). There have been some attempts at controlling Prosopis through utilization, but they had no noticeable impacts on invasions, and these initiatives failed as input and transport costs were too high and financial returns were low (Zimmermann et al. 2006). South Africa also has many particularly aggressive hybrids that form dense shrub-dominated stands, which makes the utilization approach difficult (Zimmermann et al. 2006).
Kenya
Prosopis is estimated to have invaded one million hectares and has the potential to invade nearly half of Kenya's surface (Maundu et al. 2009; Witt 2010). It was declared a noxious weed in 2008 (Low 2012). Biological and mechanical control was initially proposed as the management approach to combat Prosopis invasions, but the government later opted for a control-by-utilization approach (FAO 2006; Pasiecznik and Felker 2006). The FAO, with support from several NGOs, initiated programmes to manage Prosopis through utilization. These efforts were continued by the government's forestry department and forestry research organization (KEFRI) following the end of these projects. Considerable time and effort was taken to build capacity, formulate good policies and educate communities to utilize the goods and services from Prosopis (Pasiecznik et al. 2006a). For example, small-scale utilization projects were established and a cookbook using Prosopis flour was created and supplied to communities to promote its use (Choge et al. 2006; Pasiecznik et al. 2006a). Although initial costs for training and purchasing appropriate small-scale processing machinery are high, they are considered to be lower than other control approaches (Pasiecznik et al. 2006a). In 2002, trade in Prosopis goods and services was worth US$2122 per household per year in some villages (Choge et al. 2002). Ten years later, trade in Prosopis products in four selected areas was estimated to exceed US$1.5 million (Choge et al. 2012). Each tonne of pods that are collected and milled into flour is estimated to remove approximately two million viable seeds (Pasiecznik et al. 2006a). Changes in legislation, and the promotion of Prosopis use, helped drive the substantial rise in use and led to 100 % of the locals in one village supporting control through utilization as the most preferred management method to adopt in Kenya (Njoroge et al. 2012). However, in other villages 85–90 % of people surveyed considered complete eradication of Prosopis to be the best option (Mwangi and Swallow 2005). There are still, however, contentious issues surrounding the benefits and costs of the species and management approaches in Kenya (Pasiecznik et al. 2006a). There are many publications on the profits that are being made through utilization, but there is no evidence that these utilization programmes have contained or reduced the extent of Prosopis invasions. There is, therefore, a need for further investigation of the successes and failure of control through utilization programmes (Geesing et al. 2004). A common problem with trying to promote Prosopis utilization is that it is seen as an inferior resource in many communities, with people preferring to use native species (Geesing et al. 2004). Recently, a new utilization approach to increase invasive Prosopis use has been adopted in Kenya—a power station (based on technology from India) is currently being built in the Kenyan Rift Valley which aims to produce electricity for the local area from burning Prosopis biomass (S. Choge, pers. comm.).
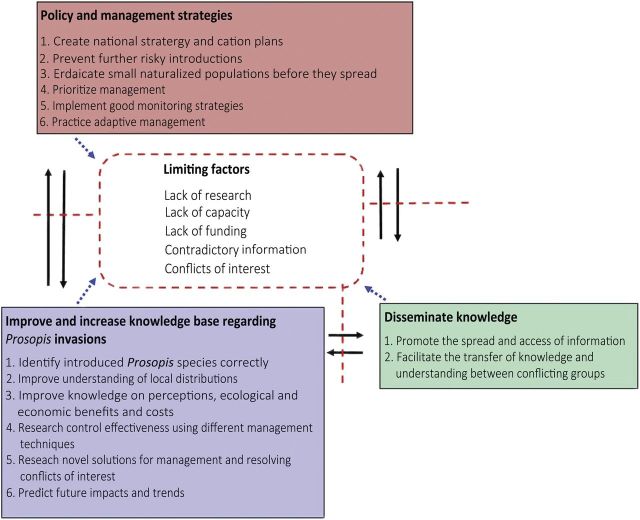
Research and management needs
This section highlights key management and research issues that need to be addressed to improve Prosopis control and the factors that currently constrain progress in these areas (Fig. 7). There is a great need for countries to develop national and even regional strategies, to provide guidelines for research and management in a targeted way, as each country has unique requirements and needs. Australia and Ascension Island are the only counties/territories to have strategic plans for Prosopis management and countries with long-standing Prosopis control programmes such as South Africa and Kenya still do not. Some broad-scale factors that need to be considered are suggested below.
Figure 7.
Requirements for research and management needs regarding Prosopis and factors limiting success.
Policy and management
National strategies and management/action plans need to be created and adopted to guide the coordinated control of Prosopis (Fig. 7). Such national strategies and plans are important to set up frameworks on how to guide Prosopis management and research. Numerous organizations and national governments globally have undertaken projects to control Prosopis, and planning and prioritization from the outset would ensure greater success. Country-specific strategic plans need to be created as there are large differences in invasion rates and scales and socio-economic situations within different areas of the world.
Introductions of known invasive Prosopis species to climatically suitable countries where it does not already exist should be undertaken such as in China, European countries along the Mediterranean and North East Asia, and spread of Prosopis into new areas within countries where it is invasive should be prevented. Risk assessments for purposeful introductions need to be conducted in the future. Pathways of accidental introductions between neighbouring countries and into new areas in countries with invasive Prosopis need to be managed. This could include regulations on livestock and fodder transport which is currently implemented in Australia (Australian Weeds Committee 2012). This is done by holding livestock in feed lots for a week before they are transported to ensure that all Prosopis seeds have excreted.
Countries need to eradicate small naturalized populations before they become invasive. Early detection and rapid response is a cost-effective way of preventing invasive species from getting out of hand and causing devastating, irreversible impacts in the future. For example, in Spain, Prosopis has started to show signs of naturalization at a single location where it was planted for experiments and eradication attempts now would be most cost effective in the long run (N. Pasiecznik and E. Peñalvo López, unpubl. res.).
There is also an urgent need for managers and researchers to monitor the effectiveness of control measures. Adaptive management needs to be promoted and applied for controlling Prosopis invasions where operational success is so far limited, so that the causes of the failures can be identified and addressed to improve overall control. Managers and researchers need to collaborate in research to design from the outset successful adaptive management strategies to be implemented.
Improve knowledge
There are many research questions regarding Prosopis invasions in many parts of the world that need to be answered to improve management (Fig. 7).
These include correctly identifying Prosopis species present and gaining consensus on the status introduced and weedy species (e.g. following the criteria proposed by Pyšek et al. 2013). There have been numerous misidentifications of introduced Prosopis species, especially in Africa. This has caused much taxonomic confusion and contradictions between different sources of information that are only starting to be clarified. There are also hybridized populations in many areas where Prosopis has been introduced, further hindering identification (Zimmermann 1991. It was recently recognized that P. pallida, which was seen as not being as invasive as other species, is more widespread than originally thought as it was misidentified as P. juliflora in Africa (Pasiecznik et al. 2006b). Most species introduced to Africa were described as P. chilensis, but this is not the case, and accurate species lists are not available for many African countries such as Angola. Molecular methods are useful for clarifying taxonomic issues, especially in areas where hybridization has taken place. It is important to know which taxa are present for management, e.g. when looking for biological control agents and understanding ecology and rates of spread (Pyšek et al. 2013).
There is a need to improve the understanding of Prosopis distribution and population sizes in introduced ranges to guide management planning (Wilson et al. 2014). As indicated earlier, only 13 % of countries with naturalized and invasive Prosopis have maps or detailed records of occurrence and scale of invasion. No information is available on the scale of Prosopis invasions on any of the Pacific (besides Hawaii), Indian Ocean or Caribbean Islands. Only a few African countries have a good understanding of the scale of invasions and, in Asia, information on the distribution of invasive Prosopis is only available for India and Pakistan. Such knowledge is essential for planning and implementing management. Bioclimatic mapping at board local scales is useful for understanding potential spread and occurrence of invasive species. However, bioclimatic models can be of limited value at very local scales as other biotic and abiotic factors come into play (Robinson et al. 2011). On a global scale, bioclimatic modelling is useful for highlighting which countries and species need risk assessments for purposeful introductions, and where introduction pathways need to be monitored to prevent unintentional introductions, e.g. between India and China or Iran and Turkmenistan.
Further knowledge on the ecology, local perceptions, and the ecological, economic and social benefits and impacts of Prosopis is needed to guide management (Wilson et al. 2014). Our study has highlighted that knowledge on Prosopis invasions is essential for management (Table 1; Fig. 6). Most of the literature comes from a handful of countries (Australia, India, Kenya, South Africa, USA), and research in other areas is needed since each region has its own set of factors that drive invasions and complicate management. There is also a need for research to better predict trends such as future densities, extent and impacts which is particularly important when it comes down to developing strategic responses. Drivers of weediness in areas where it is native such as Argentina, Mexico, Middle East and the USA require further study to improve understanding of what drives native plants to become invasive and provide insight into how to manage them.
The issue of the lack of knowledge is also present for research on the effectiveness of controlling populations using different methods. Utilization as a control method is becoming popular in many areas such as Djibouti, Ethiopia and Kenya. However, despite many reports showing how much monetary benefit Prosopis has provided, there is no information on how successful this approach is for controlling Prosopis invasions. There are also conflicting ideas on the role and success of biological control in Australia and South Africa and further work is needed (Zachariades et al. 2011). There is scope for identifying and potentially releasing additional biological control agents to improve control success in areas where this has been limited until now, such as in South Africa (Zachariades et al. 2011). Research is needed to identify novel solutions to aid the dilemma of management and contentious issues regarding invasive Prosopis globally. These include methods that retain the benefits, but reduce the impacts substantially.
Risk assessments need to be run for Prosopis species that have not been introduced yet to determine whether they might be better candidates for introduction, by providing benefits with fewer costs associated with invasiveness.
Dissemination of knowledge
Organizations involved in addressing land degradation and invasions should promote the dissemination of knowledge and awareness of both the impacts and benefits of Prosopis to prevent unwise introductions and promote management (Fig. 7). Some people still advocate the introduction of Prosopis species long after the severe impacts caused by invasions of these species were widely publicized; this has been described as ‘dangerous aid’ (Low 2012). Having regular multidisciplinary international meetings or workshops on Prosopis invasions may help to spread knowledge and create dialogue between parties, which could help to reduce contentious issues surrounding many invasive Prosopis species. The creation of management strategies using transdisciplinary approaches would also help to provide solutions acceptable to all stakeholders in situations where conflicting goals exist.
Conclusions
Prosopis species are among the most widespread and damaging invasive woody plants in semi-arid and arid regions of the world and there is much potential for taxa to spread further. The detrimental effects on the environment and human livelihoods are escalating rapidly and there is an urgent need to devise more effective management approaches to drastically reduce adverse impacts and enhance benefits. However, there are still critical gaps in our knowledge of its ecology, impacts and how to retain benefits and reduce costs, and a lack of management capacity in many countries. Clearly focused research and strategic planning is needed to improve management, reduce costs and improve benefit flows.
Sources of Funding
The DST-NRF Centre of Excellence for Invasion Biology and Working for Water Programme through their collaborative research project on ‘Integrated management of invasive alien species in South Africa’ – National Research Foundation (grant 85417).
Contributions by the Authors
R.T.S. and D.M.R. conceived the idea. R.T.S., D.C.L. and N.M.P. collected the data. N.M.P. provided specialist taxonomic advice and management information. R.T.S. ran the statistics and R.T.S. and D.C.L. undertook the climate matching. R.T.S. led the writing with assistance from the others.
Conflicts of Interest Statement
None declared.
Supporting Information
The following Supporting Information is available in the online version of this article –
File 1. Methods for literature review, climate matching, regression analysis, classification and regression tree.
File 2. Global distribution of Prosopis species. Status codes (sensu Pyšek et al. 2004) are given in brackets: N, naturalized; I, invasive; NA, native; W, weedy; U, unknown. Countries partaking in management of Prosopis species are marked with an asterisk.
File 3. Climate matching output—list of climatically suitable countries and the associated species (excluding known native species).
File 4. Underlying information on Prosopis invasions worldwide.
Acknowledgements
We thank the many researchers and managers who were consulted about the occurrence and management of Prosopis.
Literature Cited
- Al-Shurai A, Labrada R FAO. Problems posed by the introduction of Prosopis in selected countries. Rome, Italy: FAO; 2006. Problems posed by Prosopis in Yemen; pp. 21–28. [Google Scholar]
- Archer S. Have southern Texas savannas been converted to woodlands in recent history? The American Naturalist. 1989;134:545–561. [Google Scholar]
- Australian Weeds Committee. Mesquite (Prosopis spp.) strategic plan 2012–17. Canberra: Weeds of National Significance, Australian Governmental Department of Agriculture, Fisheries and Forestry; 2012. [Google Scholar]
- Babiker AGT FAO. Problems posed by the introduction of Prosopis in selected countries. Rome, Italy: FAO; 2006. Mesquite (Prosopis spp.) in Sudan: history, distribution and control; pp. 11–20. [Google Scholar]
- Bedunah DJ, Sosebee RE. Influence of mesquite control on soil erosion on a depleted range site. Journal of Soil and Water Conservation. 1986;41:131–135. [Google Scholar]
- Belton T. Management strategy for Mexican thorn (Prosopis juliflora) on Ascension Island: an assessment of this species, and recommendations for management. Bedfordshire: RSPB; 2008. [Google Scholar]
- Binggeli P. The human dimensions of invasive woody plants. In: McNeely JA, editor. The great reshuffling: human dimensions of invasive alien species. Gland and Cambridge: IUCN; 2001. pp. 145–160. [Google Scholar]
- Bokrezion H. Mainz: 2008. The ecological and socio-economic role of Prosopis juliflora in Eritrea: an analytical assessment within the context of rural development in the Horn of Africa. PhD Thesis. [Google Scholar]
- Brown JR, Archer S. Woody plant invasion grassland: establishment of honey mesquite (Prosopis glandulosa var. glandulosa) on sites differing in herbaceous biomass and grazing history. Oecologia. 1989;80:19–26. doi: 10.1007/BF00789926. [DOI] [PubMed] [Google Scholar]
- Brown LS, Boudjelas S, De Poorter M. 100 of the world's worst invasive alien species: a selection from the global Invasive Species Database. Auckland, New Zealand: The Invasive Species Specialist Group (ISSG), Species Survival Commission (SSC) of the World Conservation Union (IUCN); 2004. [Google Scholar]
- Burkart A. A monograph of the genus Prosopis (Leguminosae subfam. Mimosoideae). Part 1 and 2. Catalogue of the recognised species of Prosopis. Journal of the Arnold Arboretum. 1976;57:219–249. 450–526. [Google Scholar]
- Centre for Sustainable Development Initiatives. Nairobi: Centre for Sustainable Development Initiatives; 2009. Working paper on Prosopis: the case for local-level initiatives in Prosopis management. [Google Scholar]
- Chikuni MF, Dudley CO, Sambo EY. Prosopis glandulosa Torry (Leguminosae-Mimosoidae) at Swang'oma, Lake Chilwa plain: a blessing in disguise. Malawi Journal of Science and Technology. 2004;7:10–16. [Google Scholar]
- Choge SK, Chikamai BN. Experiences of Prosopis utilization and management from outside Kenya. Proceedings of the Workshop on Integrated Management of Prosopis Species in Kenya; Nairobi, Kenya: KEFRI; 2004. [Google Scholar]
- Choge SK, Ngujiri FD, Kuria MN, Busaka EA, Muthondeki JK. The status and impact of Prosopis spp. in Kenya. Nairobi: KEFRI; 2002. [Google Scholar]
- Choge SK, Harvey M, Chesang S, Pasiecznik NM. Cooking with Prosopis flour. Recipes tried and tested in Bango District, Kenya. Nairobi, Coventry: KEFRI and HDRA; 2006. [Google Scholar]
- Choge SK, Clement N, Gitonga M, Okuye J. Nairobi: KEFRI; 2012. Status report on commercialization of Prosopis tree resources in Kenya. Technical report for the KEFRI/KFS Technical Forest Management and Research Liaison Committee. [Google Scholar]
- Coetzer W, Hoffmann JH. Establishment of Neltumius arizonensis (Coleoptera: Burchidae) on Prosopis (Prosopis species: Mimosaceae) in South Africa. Biological Control. 1997;10:187–192. [Google Scholar]
- Csurhes S. Mesquite (Prosopis spp.) in Queensland. Australia: Department of Natural Resources and Mines; 1996. [Google Scholar]
- Dean WRJ, Anderson MD, Milton SJ, Anderson TA. Avian assemblages in native Acacia and alien Prosopis drainage line woodland in the Kalahari, South Africa. Journal of Arid Environments. 2002;51:1–19. [Google Scholar]
- DeLoach CJ. Conflicts of interest over beneficial and undesirable aspects of Prosopis (Prosopis spp.) in the United States as related to biological control. Sixth International Symposium on Biological Control; 19–25 August 1984; Vancouver, Canada. 1984. pp. 301–340. [Google Scholar]
- Delobel A, Fediere G. First report in Egypt of two seed-beetles (Coleoptera: Burchidae) noxious to Prosopis spp. Bulletin of the Faculty of Agriculture, Cairo University. 2002;53:129–140. [Google Scholar]
- de Wit MP, Crookes DJ, van Wilgen BW. Conflicts of interest in environmental management: estimating the costs and benefits of a tree invasion. Biological invasions. 2001;3:167–178. [Google Scholar]
- Djoudi H, Brockhaus M, Locatelli B. Once there was a lake: vulnerability to environmental changes in northern Mali. Regional Environmental Change. 2011;11:1–16. [Google Scholar]
- Dussart E, Lerner P, Peinetti R. Long-term dynamics of 2 populations of Prosopis caldenia Burkart. Journal of Rangeland Management. 1998;51:985–991. [Google Scholar]
- Dzikiti S, Schachtschneider K, Naiken V, Gush M, Moses G, Le Maitre DC. Water relations and the effects of clearing invasive Prosopis trees on groundwater in an arid environment in the Northern Cape, South Africa. Journal of Arid Environments. 2013;90:103–113. [Google Scholar]
- Elfadl MA, Luukkanen O. Field studies on ecological strategies of Prosopis juliflora in a dry land ecosystem. Journal of Arid Environments. 2006;66:1–15. [Google Scholar]
- El-Keblawy A, Al-Rawai A. Impacts of the invasive exotic Prosopis juliflora (Sw.) D.C. on the native flora and soils of the UAE. Plant Ecology. 2007;190:23–35. [Google Scholar]
- Elsidig NA, Abdelsalam AH, Abdelmagid TD. Socio-economic, environmental and management aspects of mesquite in Kassala State (Sudan) Sudan: Sudanese Social Forestry Society; 1998. [Google Scholar]
- FAO. Problems posed by the introduction of Prosopis spp. in selected countries. Rome: Plant Production and Protection Division, Food and Agricultural Organization of the United Nations; 2006. [Google Scholar]
- Felker P. Mesquite: an all-purpose leguminous arid land tree. In: Eitchie GA, editor. New agricultural crops, American Association for the Advancement of Science Symposium Proceedings. Vol. 38. Boulder: Westview Press; 1979. pp. 89–132. [Google Scholar]
- Forsyth GG, Le Maitre DC, O'Farrell PJ, van Wilgen BW. The prioritization of invasive alien plant control projects using multi-criteria decision model informed by stakeholder impute and special data. Journal of Environmental Management. 2012;103:51–57. doi: 10.1016/j.jenvman.2012.01.034. [DOI] [PubMed] [Google Scholar]
- Geesing D, Al-Khawlani M, Abba ML. Management of introduced Prosopis species: can economic exploitation control and invasive species? Unasylva. 2004;217:36–44. [Google Scholar]
- Ghazanfar SA. Invasive Prosopis in Sultanate of Oman. Aliens. 1996;3:10. [Google Scholar]
- Hasan MK, Alam AKMA. Land degradation situation in Bangladesh and the role of agroforestry. Journal of Agriculture and Rural Development. 2006;4:19–25. [Google Scholar]
- Hulme PE. Biological invasions: winning the science battles but losing the conservation war? Oryx. 2003;37:178–193. [Google Scholar]
- Kaur R, Gonzales WL, Llambi LD, Soriano PJ, Callaway RM, Rout ME, Gallaher JT, Inderjit Community impacts of Prosopis juliflora invasion: biogeographic and congeneric comparisons. PLoS One. 2012;7:e44966. doi: 10.1371/journal.pone.0044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmi SJH, Shaikh S, Zamir UB, Zafar H, Rasool A, Tariq F, Afzal A, Arif T. Ecological and socio-economic evaluation of the use of Prosopis juliflora for bio-char production in Pakistan. Pakistan: Drynet; 2009. [Google Scholar]
- Kull CA, Shackleton CM, Cunningham PJ, Ducatillon C, Dufour-Dror J, Esler KJ, Friday JB, Gouveia AC, Griffin AR, Marchante E, Midgley SJ, Pauchard A, Rangan H, Richardson DM, Rinaudo T, Tassin J, Urgenson LS, von Maltitz GP, Zenni RD, Zylstra MJ. Adoption, use and perception of Australian acacias around the world. Diversity and Distributions. 2011;17:822–836. [Google Scholar]
- Laxén J. Is Prosopis a curse or a blessing? An ecological and economic analysis of an invasive alien tree species in Sudan. In: Lukkanan O, editor. Tropical forestry reports. Finland: VITRI, University of Helsinki; 2007. pp. 1–199. [Google Scholar]
- Le Maitre DC, Versfeld DB, Chapman RA. The impact of invading alien plants on surface water resources in South Africa: a preliminary assessment. Water SA. 2000;26:397–408. [Google Scholar]
- Low T. In denial about dangerous aid. Biological Invasions. 2012;14:2235–2236. [Google Scholar]
- Mack RN. Global plant dispersal, naturalization, and invasion: pathways, modes and circumstances. In: Ruiz GM, Carlton JT, editors. Invasive species: vectors and management strategies. Washington, DC: Island Press; 2003. pp. 3–30. [Google Scholar]
- Martin T, van Klinken RD. Value for money? Investment in weed management in Australian rangelands. Rangeland Journal. 2006;28:63–75. [Google Scholar]
- Maundu P, Kibet S, Morimoto Y, Imbumi M, Adeka R. Impacts of Prosopis juliflora on Kenya's semi-arid and arid ecosystems and local livelihoods. Biodiversity. 2009;10:33–50. [Google Scholar]
- McGeoch MA, Butchart SHM, Spear D, Marais E, Kleynhans EJ, Symes A, Chanson J, Hoffmann M. Global indicators of biological invasion: species numbers, biodiversity impact and policy responses. Diversity and Distributions. 2010;16:95–108. [Google Scholar]
- McNeely JA, Mooney HA, Neville LE, Schei PJ, Waage JK. A global strategy on invasive alien species: synthesis and ten strategic elements. In: Mooney AH, Mack RN, McNeely AJ, Neville LE, Schei PJ, Waage JK, editors. Invasive alien species: a new synthesis. Washington, DC: Island Press; 2005. pp. 326–345. [Google Scholar]
- Mwangi M, Swallow B. Invasion of Prosopis juliflora and local livelihoods: case study from the Lake Baringo area of Kenya. Nairobi: World Agroforestry Centre; 2005. ICRAF Working Paper no. 3. [Google Scholar]
- Naseeruddin S, Yadav KS, Sateesh L, Manikyam A, Sesai S, Rao LV. Selection of the best chemical pretreatment for lignocellulosic substrate Prosopis juliflora. Bioresource Technology. 2013;136:542–549. doi: 10.1016/j.biortech.2013.03.053. [DOI] [PubMed] [Google Scholar]
- Ndhlovu T, Milton-Dean SJ, Esler KJ. Impact of Prosopis (mesquite) invasion and clearing on the grazing capacity of semiarid Nama Karoo rangeland, South Africa. African Journal of Range and Forage Science. 2011;28:129–137. [Google Scholar]
- Nie W, Yuan Y, Kepner W, Erickson C, Jackson M. Hydrological impacts of mesquite encroachment in the upper San Pedro watershed. Journal of Arid Environments. 2012;82:147–155. [Google Scholar]
- Nilsen ET, Sharifi MR, Rundel PW, Jarrell WM, Virginia RA. Diurnal and seasonal water relations of the desert phreatophyte Prosopis glandulosa (honey mesquite) in the Sonoran Desert of California. Ecology. 1983;64:1381–1393. [Google Scholar]
- Njoroge E, Sirmah P, Mburu F, Koech E, Mware M, Chepkwony J. Preference and adoption of farmer field school (FFS) Prosopis juliflora management practices: experiences in Baringo District, Kenya. Forestry Studies in China. 2012;14:283–290. [Google Scholar]
- Nuñez MA, Pauchard A. Biological invasions in developing and developed countries: does one model fit all? Biological Invasions. 2009;12:707–714. [Google Scholar]
- Osmond R. Best practice manual, mesquite: control and management options for mesquite (Prosopis spp.) in Australia. Queensland: National Weeds Programme and Queensland Department of Natural Resources and Mines; 2003. [Google Scholar]
- Page AR, Lacey KL. Economic impact assessment of Australia weed biological control. Australia: CRC for Australian Weed Management; 2006. [Google Scholar]
- Parvaresh H. 2011. Identification of threats on mangrove forests in Gabrik International Wetland for sustainable management. 2011 International Conference on Biology, Environment and Chemistry IPCBEE, Vol. 24. Singapore: IACSIT Press. [Google Scholar]
- Pasiecznik NM. Prosopis—pest or providence, weed or wonder tree? ETFRN News. 1999;28:12–14. [Google Scholar]
- Pasiecznik NM. Prosopis (mesquite, algarrobo): invasive weed or valuable forest resource? Coventry, UK: HDRA; 2002. Policy brief. [Google Scholar]
- Pasiecznik NM. Cinderella species and what happens after midnight? Aliens. 2004;19/20:20. [Google Scholar]
- Pasiecznik NM, Felker P. Safeguarding valued old world native Prosopis species from biocontrol introductions. Biocontrol News and Information. 2006;27:1–26. [Google Scholar]
- Pasiecznik NM, Felker P, Harris PJC, Harsh LN, Cruz G, Tewari JC, Cadoret K, Maldonado LJ. The Prosopis juliflora–Prosopis pallida complex: a monograph. Coventry, UK: HDRA; 2001. [Google Scholar]
- Pasiecznik NM, Choge SK, Muthike GM, Chesang S, Fehr C, Bakewell-Stone P, Wright J, Harris PJC. Putting knowledge on Prosopis into use in Kenya. Pioneering advances in 2006. Nairobi and Coventry, UK: KEFRI and HDRA; 2006a. [Google Scholar]
- Pasiecznik NM, Harris PJC, Trenchard EJ, Smith SJ. Implications of uncertain Prosopis taxonomy for biocontrol. Biocontrol News and Information. 2006b;27:1–26. [Google Scholar]
- Peel MC, Finlayson BL, McMahon TA. Updated world map of the Köppen-Geiger climate classification. Hydrology and Earth System Science. 2007;11:1633–1644. [Google Scholar]
- Pimentel D, McNair S, Janecka J, Wightman J, Simmonds C, O'Connell C, Wong E, Russel L, Zern J, Aquino T, Tsomondo T. Economics and environmental threats of alien plant, animal and microbe invasions. Agriculture, Ecosystems and Environment. 2000;84:1–20. [Google Scholar]
- Pyšek P, Richardson DM, Rejmánek M, Webster GL, Williamson M, Kirschner J. Alien plants in checklists and floras: towards better communication between taxonomists and ecologists. Taxon. 2004;53:131–143. [Google Scholar]
- Pyšek P, Richardson DM, Pergl J, Jarošík V, Sixtova Z, Weber E. Geographical and taxonomic biases in invasion ecology. Trends in Ecology and Evolution. 2008;23:237–244. doi: 10.1016/j.tree.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Pyšek P, Hulme PE, Meyerson LA, Smith GF, Boatwright JS, Crouch NR, Figueiredo E, Foxcroft LC, Jarošík V, Richardson DM, Suda J, Wilson JRU. Hitting the right target: taxonomic challenges for, and of, plant invasions. AoB PLANTS. 2013;5:plt042. doi:10.1093/aobpla/plt042. [Google Scholar]
- Ræbild A, Diallo BO, Graudal L, Dao M, Sanou J. Evaluation of a species and provenance trial of Prosopis at Gonsé, Burkina Faso. Humlebaek, Denmark: Danida Forest Seed Centre; 2003. Trial no. 14 in the arid zone series. Results and Documentation No. 11. [Google Scholar]
- Rejmánek M, Richardson DM. Trees and shrubs as invasive alien species—2013 update of the global database. Diversity and Distributions. 2013;19:1093–1094. [Google Scholar]
- Richardson DM. Commercial forestry and agroforestry as sources of invasive alien trees and shrubs. In: Sandlund OT, Schei PJ, Viken A, editors. Invasive species and biodiversity management. Dordrecht: Kluwer Academic Publishers; 1998a. pp. 237–257. [Google Scholar]
- Richardson DM. Forestry trees as invasive aliens. Conservation Biology. 1998b;12:18–26. [Google Scholar]
- Richardson DM, Rejmánek M. Conifers as invasive aliens: a global survey and predictive framework. Diversity and Distributions. 2004;10:321–331. [Google Scholar]
- Richardson DM, Rejmánek M. Trees and shrubs as invasive alien species—a global review. Diversity and Distributions. 2011;17:788–809. [Google Scholar]
- Robinson TP, van Klinken RD, Metternicht G. Comparison of alternative strategies for invasive species distribution modelling. Ecological Modelling. 2011;221:2261–2269. [Google Scholar]
- Rouget M, Richardson DM, Nel J, Le Maitre DC, Egoh B, Mgidi T. Mapping potential ranges of major plant invaders in South Africa, Lesotho and Swaziland using climatic suitability. Diversity and Distributions. 2004;10:475–484. [Google Scholar]
- Shackleton CM, McGarry D, Fourie S, Gambiza J, Shackleton SE, Fabricius C. Assessing the effects of invasive alien species on rural livelihoods: case examples and a framework from South Africa. Human Ecology. 2007;35:113–127. [Google Scholar]
- Shiferaw H, Teketay D, Nemomissa S, Assefa F. Some biological characteristics that foster the invasion of Prosopis juliflora (Sw.) DC. at Middle Awash Rift Valley Area, north-eastern Ethiopia. Journal of Arid Environments. 2004;58:135–154. [Google Scholar]
- Stark J, Terasawa K, Ejigu M. Climate change and conflict in pastoralist regions of Ethiopia: mounting challenges, emerging responses. United States Agency for International Development; 2011. CMM Discussion Paper No. 4. Washington, DC. [Google Scholar]
- Steenkamp HE, Chown SL. Influence of dense stands of an exotic tree Prosopis glandulosa Benson, on a savanna dung beetle (Coleoptera: Scarabeidae) assemblage in southern Africa. Biological Conservation. 1996;78:305–311. [Google Scholar]
- Tiwari JWK. Exotic weed Prosopis juliflora in Gujarat and Rajasthan, India—boon or bane? Tigerpaper. 1999;26:21–25. [Google Scholar]
- Van den Berg EC. Potchefstroom: 2010. Detection, quantification and monitoring Prosopis spp. in the Northern Cape Province of South Africa using Remote Sensing and GIS. MSc Thesis. [Google Scholar]
- van Klinken R. Prosopis spp.—mesquite. In: Julien M, McFadyen R, Cullen J, editors. Biological control of weeds in Australia. Melbourne, Australia: CSIRO; 2012. pp. 477–485. [Google Scholar]
- van Klinken RD, Campbell S. Australian weeds series: Prosopis species. In: Panetta FD, editor. The biology of Australian weed. Vol. 3. Melbourne: R.G. and F.J. Richardson; 2009. pp. 238–273. [Google Scholar]
- van Klinken RD, Fichera G, Cordo H. Targeting biological control across diverse landscapes: the release, establishment and early success of two insects on mesquite (Prosopis spp.) insects in Australian rangelands. Biological Control. 2003;26:8–20. [Google Scholar]
- van Klinken RD, Graham J, Flack LK. Population ecology of hybrid mesquite (Prosopis species) in Western Australia: how does it differ from native range invasions and what are the implications for impacts and management? Biological Invasions. 2006;8:727–741. [Google Scholar]
- van Wilgen BW, Richardson DM. Challenges and trade-offs in the management of invasive alien trees. Biological Invasions. 2014;16:721–734. [Google Scholar]
- van Wilgen BW, Dyer C, Hoffmann JH, Ivey P, Le Maitre DC, Moore JL, Richardson DM, Roget M, Wannenburgh A, Wilson JRU. National-scale strategic approaches for managing introduced plants: insights from Australian acacias in South Africa. Diversity and Distributions. 2011;17:1060–1075. [Google Scholar]
- van Wilgen BW, Forsyth GG, Le Maitre DC, Wannenburgh A, Kotze DF, van den Berg E, Henderson L. An assessment of the effectiveness of a large, national-scale invasive alien plant control strategy in South Africa. Biological Conservation. 2012;148:28–38. [Google Scholar]
- Versfeld DB, Le Maitre DC, Chapman RA. Pretoria: Water Research Commission; 1998. Alien invading plants and water resources in South Africa. Report no. TT 99/98. [Google Scholar]
- Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human domination of Earth's ecosystems. Science. 1997;277:494–499. [Google Scholar]
- Wilson JRU, Gairifo C, Gibson MR, Arianoutsou M, Baker BB, Baret SB, Celesti-Grapow L, DiTomaso JM, Dufour-Dror J, Kueffer C, Kull CA, Hoffmann JH, Impson FAC, Loope LL, Marchant E, Marchante H, Moore JL, Murphy DJ, Tassin J, Witt A, Zenni RD, Richardson DM. Risk assessment, eradication, and biological control: global efforts to limit Australian acacia invasions. Diversity and Distributions. 2011;17:1030–1046. [Google Scholar]
- Wilson JRU, Caplat P, Dickie IA, Hui C, Maxwell BD, Nuñez MA, Pauchard A, Rejmánek M, Richardson DM, Robertson MP, Spear D, Webber BL, van Wilgen BW, Zenni RD. A standardized set of metrics to assess and monitor tree invasions. Biological Invasions. 2014;16:535–551. [Google Scholar]
- Wise RM, van Wilgen BW, Le Maitre DC. Costs, benefits and management options for an invasive alien tree species: the case of mesquite in the Northern Cape, South Africa. Journal of Arid Environments. 2012;84:80–90. [Google Scholar]
- Witt ABR. Biofuels and invasive species from an African perspective—a review. GCB Bioenergy. 2010;2:321–329. [Google Scholar]
- Zachariades C, Hoffmann JH, Roberts A. Biological control of mesquite (Prosopis species) (Fabaceae) in South Africa. African Entomology. 2011;19:402–415. [Google Scholar]
- Zimmermann HG. Biological control of Prosopis, Prosopis spp. (Fabaceae), in South Africa. Agriculture, Ecosystems and Environment. 1991;37:175–186. [Google Scholar]
- Zimmermann HG, Hoffmann JH, Witt ABR. A South African perspective on Prosopis. Biocontrol News and Information. 2006;27:1–26. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.