INTRODUCTION
Following a penetrating injury to the central nervous system (CNS), transient unsuccessful regeneration is seen correlated with the production of a dense permanent scar. In the brain, basic fibroblast growth factor (basic FGF) is present in neurones, in the vascular basement membrane of blood vessels, and in the ependyma1 cells of the ventricles. In vitro studies suggest its potential as a neurotrophic factor and a growth factor promoting glial proliferation, angiogenesis, and fibrotic scar production after injury.1 In this study we have examined changes in basic FGF levels, distribution, and synthesis in the injured rat brain in order to further implicate it in the CNS wounding response.
MATERIALS AND METHODS
Groups of matched adult female rats had stereotactically defined lesions made unilaterally in the cerebrum. Between 4 hours and 14 days later they were terminated and processed. Some brains were frozen for later heparin-Sepharose basic FGF extraction and radioimmunoassay or RNA extraction and quantitative analysis by northern/dot blot hybridization. The amount of basic FGF mRNA in each sample was quantified by autoradiography of dot blots followed by densitometric scanning. Each sample was normalized to oligo dT and the results expressed as basic FGF mRNA as a percent of the 0 day control, which was taken as 100%. Standard errors of the mean (SEM) were calculated for each group (n = 4). Other animals were perfusion fixed and 20-μm cryostat sections were cut for in situ hybridization and immunocytochemistry. These analyses used established methods2,3 and a rabbit polyclonal antibody raised against the 1–24 synthetic fragment of bovine basic FGF(1–146), a cDNA containing the coding region [0.5 kilobase (kb)] of rat basic FGF cDNA clone RobFGF103 and a cRNA probe that was made from the XhoI-XhoI fragment of the RobFGF103 clone which was subcloned into pBluescript SK+ and linearized with NcoI. The cRNA probe encoding the antisense strand of coding sequence was transcribed using T7 RNA polymerase and [35S]UTP. An RNA probe encoding the sense strand of noncoding sequence was used on control tissue sections.
RESULTS
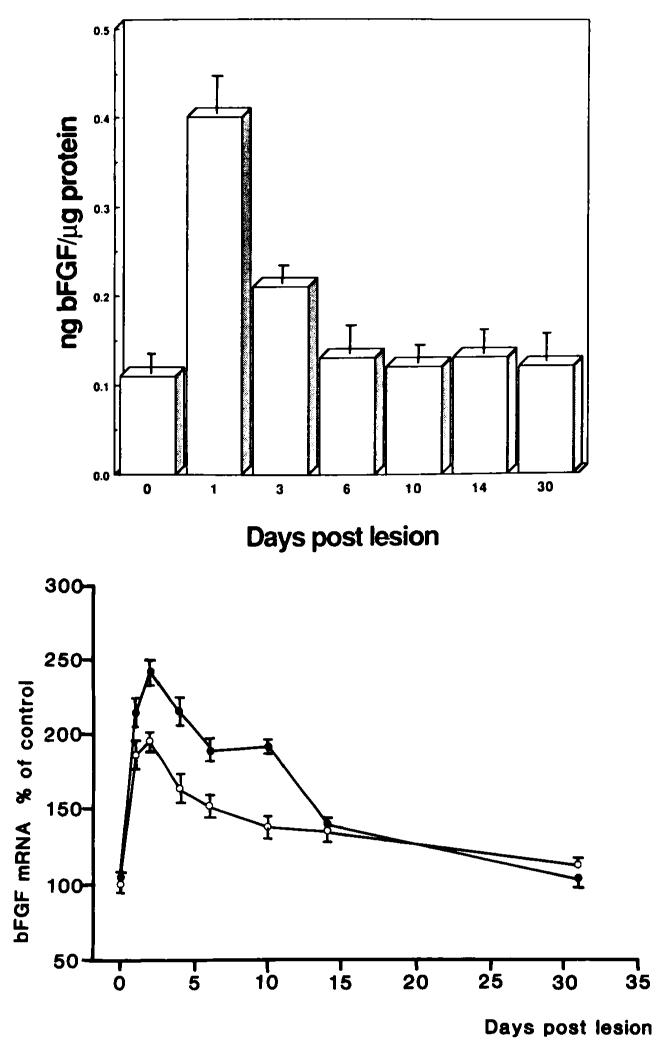
In the normal rat brain, multiple basic FGF mRNAs are seen by northern analysis, the major species being 1.7 and 5.9 kb. Following injury, basic FGF mRNA and protein levels rose rapidly and significantly in the lesioned cerebral hemisphere to peak between 1 and 2 days postlesion (see Figure 1). The increase in both had virtually disappeared 14 days after injury. At a cellular level both the intensity and the number of cells staining for basic FGF was increased focally around the wound when compared to controls as did the density of mRNA hybridization (see Figure 2). Moreover, there was a shift in the type of cells that contain basic FGF mRNA and protein. Early in the response (4 hours to 3 days) macrophagelike cells are clearly immunoreactive. By 7 days, basic FGF appears to be contained mostly in reactive astrocytes and this response subsided by 14 days.
FIGURE 1.
Top: Postinjury levels of immunoreactive basic FGF (expressed as ng basic FGF/kg protein) in the lesioned rat cerebral hemisphere. Bottom: Basic FGF mRNA levels in the lesioned (filled circles) and contralateral unlesioned (open circles) hemisphere of adult rats. The amount of specific basic FGF mRNA was quantified by autoradiography followed by densitometric scanning. Values represent means ± SEM expressed as a percentage of 0 day control animals.
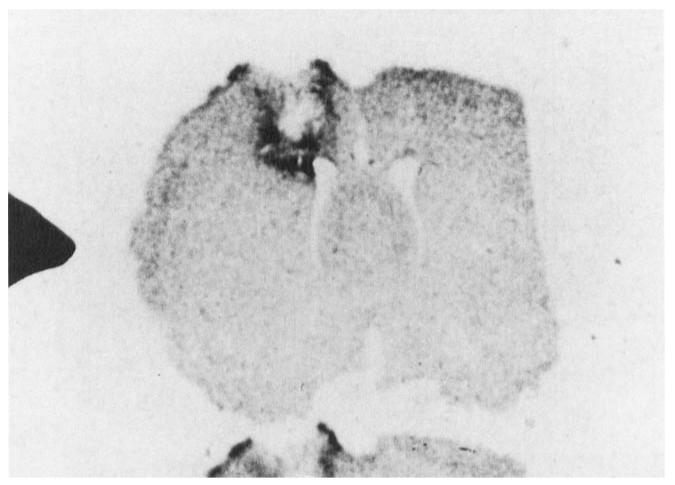
FIGURE 2.
In situ hybridization of basic FGF mRNA. Focal increases in basic FGF mRNA are seen in neural tissue around the lesion site at 3 days following injury.
CONCLUSIONS
We conclude that basic FGF is translocated and synthesized locally as a consequence of injury to the CNS. In vivo studies are under way to define the role of this growth factor in the injured brain.
REFERENCES
- 1.LOGAN A. Trends Endocrinol. Metab. 1990;1:149–154. doi: 10.1016/1043-2760(90)90027-z. [DOI] [PubMed] [Google Scholar]
- 2.GONZALEZ AM, BUSCAGLIA M, ONG M, BAIRD A. J. Cell Biol. 1990;110:753–765. doi: 10.1083/jcb.110.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.EMOTO N, GONZALEZ AM, WALICKE PA, WADA E, SIMMONS DM, SHIMASAKI S, BAIRD A. Growth Factors. 1989;2:21–29. doi: 10.3109/08977198909069078. [DOI] [PubMed] [Google Scholar]