Abstract
A diagnosis of small cell lung cancer (SCLC) today confers essentially the same terrible prognosis that it did 25 years ago, when common use of cisplatin-based chemotherapy began for this disease. In contrast to past decades of research on many other solid tumors, studies of combination chemotherapy using later generation cytotoxics and targeted kinase inhibitors have not had a significant impact on standard care for SCLC. The past few years have seen suggestions of incrementally improved outcomes using standard cytotoxics, including cisplatin-based combination studies of irinotecan and amrubicin by Japanese research consortia. Confirmatory phase III studies of these agents are ongoing in the United States. Antiangiogenic strategies are also of primary interest and are in late-phase testing. Several novel therapeutics, including high-potency small molecule inhibitors of Bcl-2 and the Hedgehog signaling pathway, and a recently discovered replication-competent picornavirus, have shown remarkable activity against SCLC in preclinical models and are currently in simultaneous phase I clinical development. Novel therapeutic approaches based on advances in understanding of the biology of SCLC have the potential to radically change the outlook for patients with this disease.
Keywords: Small cell lung cancer, novel therapeutics, Bcl-2, Hedgehog, SVV-001
Small Cell Lung Cancer: In Need of New Ideas
Small cell lung cancer (SCLC) is a common and nearly always fatal disease; it represents approximately 15% of all lung cancers, or approximately 26,000 cases annually in the United States.1 This disease is strongly associated with tobacco exposure and is expected to be an increasingly prevalent concern, particularly in Asia and the developing world, mirroring smoking trends worldwide.2
SCLC has a marked predilection for early, distant metastasis; approximately two thirds of SCLC cases are in advanced stage of disease at diagnosis. Patients with advanced-stage SCLC are considered incurable, and with current standard treatment have a median survival time of approximately 9 to 10 months from diagnosis;3 5-year survival is exceedingly rare. Limited-stage SCLC, functionally defined as disease confined to 1 hemithorax and which can be encompassed in a single radiation port, also carries a dismal prognosis, with median survival of approximately 18 months from diagnosis. However, in contrast to extensive-stage disease, limited-stage SCLC is potentially curable using current multimodality therapy, with long-term disease-free survival in approximately 20% to 25% of cases.4
Standard therapy for advanced- and limited-stage SCLC differs primarily in the addition of concomitant radiation for limited-stage disease. Typical first-line chemotherapy, regardless of stage, consists of a platinum agent (cisplatin or carboplatin) paired with a second agent, in the United States usually the topoisomerase II inhibitor etoposide. This essential treatment paradigm was initiated in the early 1980s and has undergone only minor subsequent modifications, primarily limited to improvements in supportive care medications and in the timing, focus, and intensity of radiation.5–7
Clinical Barriers to Progress in SCLC
The advent of platinum-based chemotherapy caused considerable optimism among lung cancer clinical researchers about the potential for further rapid progress against SCLC.8 SCLC, in contrast to non–small cell lung cancer (NSCLC), is a remarkably chemosensitive tumor, with objective response rates of 60% to 80% to standard chemotherapy. Several newer chemotherapeutic agents, including topotecan, paclitaxel, and gemcitabine, were found to have promising activity.9 However, throughout the 1980s and 1990s, none of the newer agents tested as single agents, as combinations, or added to cisplatin and etoposide seemed to offer any significant improvement over standard therapy. Despite initial chemosensitivity, extensive-stage SCLC recurs universally, and recurrent disease is relatively resistant to further therapy. Attempts at postinduction maintenance chemotherapy have been universally unsuccessful.10 These failures promoted a climate of nihilism about further clinical investigation in SCLC.
Solid tumor clinical research has focused predominantly on the development and testing of high-potency inhibitors of cell surface receptor tyrosine kinases over the past decade. SCLC, in contrast to NSCLC and several other common solid tumors, does not seem to depend on endothelial growth factor receptor (EGFR) family receptor tyrosine kinases for survival or proliferation.11 These key oncogenic factors are frequently undetectable in SCLC. Targeted inhibition of c-Kit, an alternative receptor tyrosine kinase that seems to be upregulated in a subset of SCLC, showed no clinical activity in SCLC.12 The oncogenic factors p53 and Rb, which are most strongly implicated in malignant transformation of SCLC, are not kinases and are notoriously difficult targets for selective pharmacologic intervention.13
Preclinical Barriers to Progress in SCLC
A final barrier to progress has been a relative absence of predictive preclinical models of human SCLC. Tobacco smoke–exposed mice do not typically develop lung tumors similar to SCLC. This issue was partially addressed by Meuwissen et al.14 in 2003 when they showed that targeted somatic inactivation of both p53 and Rb led to development of murine lung neuroendocrine tumors that were phenotypically similar to human SCLC.14 However, the usefulness of this 2-hit transgenic approach as a therapeutic model is unclear. Clinical SCLC, induced by tobacco carcinogens, is marked by a diversity of genetic alterations, is biologically heterogeneous, and most notably shows severe chromosomal instability.15 None of these features, which are key contributors to the emergence of therapeutic resistance, are reflected in extant transgenic models of SCLC.
The most common in vivo model for anticancer drug development is based on the use of human cancer cell lines implanted as tumor xenografts in recipient animals. Although it allows response analysis of actual human tumors, this model has clear limitations. Both genetic and epigenetic characteristics of cancer cells are influenced by culture conditions, and these changes may lead to misleading results in preclinical drug studies.16–18
Cancer cell line features that favor preferential selection ex vivo include rapid growth as a monolayer on plastic, growth in fetal calf serum and synthetic media, growth in high pO2 and glucose conditions, survival through trypsin passaging, and low mutagenicity resulting in a stable and consistent phenotype. Tumor features irrelevant to cell line derivation include angiogenic drive, metastatic potential, and survival in states of hypoxia, nutrient limitation, and high oncotic pressure. Implanted subcutaneously, most standard xenografts grow as localized, noninvasive, nonmetastatic nodules, in marked contrast to human cancers and particularly in contrast to the aggressive behavior of clinical SCLC.
Primary Xenografts: An Emerging Model
The authors’ laboratories have begun to explore an alternative approach to preclinical drug testing in SCLC, using primary xenografts or heterotransplants. This model depends on immediate transfer of human SCLC from patients into recipient mice, without intervening tissue culture or cell line derivation ex vivo. This technique avoids the selective pressures associated with ex vivo cell survival and proliferation through maintaining tumors in a biologically relevant context. Tumors passaged from animal to animal in this model maintain stable histologic and immunophenotypic characteristics. Recent data from several tumor models now suggest that primary xenografting, or xenografting from cells maintained in modified stem cell media, may better maintain the cellular morphology, growth characteristics, chromosome structure, gene copy number, and gene expression of the parental tumor.19–21 Taken together, these observations suggest that primary xenograft models may represent a better platform for preclinical therapeutic testing, one that may be more predictive of ultimate clinical efficacy.
Irinotecan and Amrubicin: Renewed Interest in Chemotherapeutics
The topoisomerase I inhibitor topotecan is the only FDA-approved agent for recurrent chemosensitive SCLC, defined as disease that remains stable for 3 months or longer after primary therapy is complete. In this context, topotecan has a response rate of approximately 25% to 30%.22,23 In chemorefractory relapse, defined as progressive disease during or within 3 months of completion of primary therapy, topotecan has a response rate of only 2% to 6%.22 No drugs for relapsed chemorefractory SCLC have been approved by the FDA.
Irinotecan, a topoisomerase I inhibitor closely related to topotecan, has shown promising activity in SCLC.24 Most notably, a phase III study conducted by the Japanese Clinical Oncology Group (JCOG) comparing cisplatin plus irinotecan with cisplatin plus etoposide in previously untreated advanced SCLC suggested that cisplatin plus irinotecan was a superior first-line regimen, associated with median survival of 12.8 months, compared with 9.4 months for cisplatin/etoposide (P = .002).25
Whether the efficacy of irinotecan in Japan will be reflected in similar activity in an American population is unclear. Irinotecan metabolism, tolerance, and efficacy have significant interindividual and interethnic differences, partly attributable to genomic polymorphisms affecting expression of UGT1A1.26,27 Two confirmatory phase III studies have been conducted in the United States. The one reported first used a slightly different schedule of cisplatin and irinotecan from that of the JCOG study, and failed to show any improvement in survival relative to cisplatin plus etoposide (P = .74).28 Survival data for the second study, which precisely mimicked the Japanese dose and schedule,29 has not yet been reported.
Amrubicin is a synthetic anthracycline, a class of chemotherapeutic agents that, among other mechanisms of action, are potent inhibitors of topoisomerase II. Recent studies from Japan have shown remarkable activity for amrubicin, both as a single agent and in combination with cisplatin.30,31 Most impressive has been its reported activity in recurrent, and even chemorefractory, SCLC. Two recent phase II studies reported amrubicin response rates in recurrent SCLC of more than 50%, with one of these studies showing major responses in 8 of 16 patients with chemorefractory SCLC.30 No phase III studies have been completed involving amrubicin. Nevertheless, these results are highly encouraging and separate confirmatory studies in both chemosensitive and chemorefractory recurrent SCLC are ongoing in the United States.32
Antiangiogenic Strategies in SCLC: Starving the Tumor
SCLC is a rapidly growing, highly metabolically active cancer that depends on a rapidly expansive tumor-associated vasculature. High microvessel density and high expression of a key angiogenic mediator, vascular endothelial growth factor (VEGF), are associated with poor outcome in SCLC.33 Several recently reported studies have tested angiogenic inhibitors in SCLC.
The most extensively studied antiangiogenic agent is the inhibitory VEGF antibody bevacizumab. Preliminary analysis of a phase II study of bevacizumab with first-line cisplatin plus etoposide suggests a response rate of 69% and 6-month survival of 33%.34 Similar data have been presented as interim reports for bevacizumab with first-line cisplatin plus irinotecan and carboplatin plus irinotecan.35,36 Encouragingly, and in contrast to reports in NSCLC, all 3 studies report no grade 3 or higher hemorrhage. Final outcome data from these phase II studies are awaited. A phase III study of the addition of bevacizumab to cisplatin plus etoposide has been initiated.
In addition to antibody-mediated inhibition of the ligand VEGF, several inhibitors of the VEGF receptor (VEGFR) and other angiogenic mediators are in active development. Vandetanib (ZD6474) is an orally bioavailable small molecule tyrosine kinase inhibitor suppressing activity of the key VEGF receptor, VEGFR2. In recent report of a randomized phase II study of vandetanib versus placebo in patients with SCLC after response to primary therapy, the National Cancer Institute of Canada found that vandetanib did not improve overall survival.37 However, trends were seen toward improved outcome in patients with limited-stage disease but worse outcome in those with extensive-stage disease.
Significant interest has been shown in thalidomide as an antiangiogenic agent that affects multiple vascular proliferative signaling pathways, including suppression of tumor-associated VEGF production.38 A French consortium recently completed a phase III study of thalidomide versus placebo as maintenance for patients with extensive-stage SCLC after response to first-line chemotherapy.39 No statistically significant difference was seen in overall survival. Benefit seemed to be limited to patients with a lesser performance status (PS 1 or 2), but this is a retrospective exploratory analysis of uncertain significance.
SCLC and Apoptotic Sensitivity: Bcl-2 as a Target
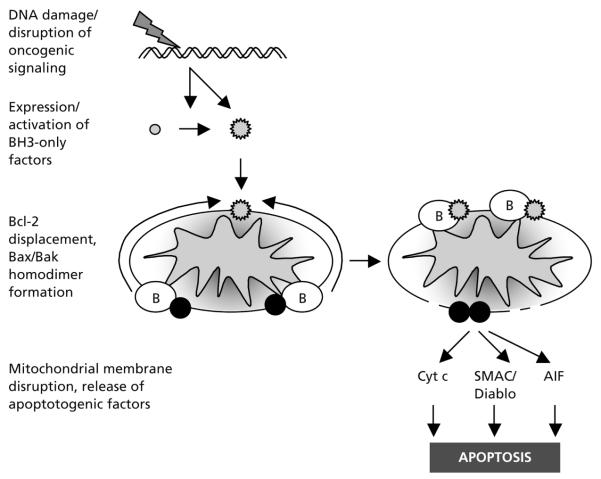
Dysregulation of apoptotic signaling pathways is one of the fundamental hallmarks of cancer.40 Two features of SCLC have made targeting apoptosis in this tumor type a particularly attractive strategy. First, in contrast with most other solid tumors, SCLC shows remarkable sensitivity to multiple apoptotic triggers.41 The apoptotic threshold of SCLC cell lines to standard cytotoxics is similar to that of many hematologic malignancies; this sensitivity may in part explain the exceptional clinical response rates of SCLC. Second, Bcl-2, a central contributor to the apoptotic dysregulation in SCLC seems to be a well-characterized factor amenable to therapeutic targeting. Bcl-2 is a central apoptotic regulator implicated in cell survival, tumorigenesis, and chemotherapeutic resistance (Figure 1).42 Bcl-2 has been reported to be upregulated in 73% to 90% of human SCLC tumors.43–45 Suppression of Bcl-2 levels using an antisense oligonucleotide targeting the bcl-2 mRNA increases the sensitivity of SCLC cell lines and xenografts to standard cytotoxics, including cisplatin and etoposide.46 Finally, and most notably, targeted inhibition using high-potency, high-specificity, small-molecule inhibitors of Bcl-2 has dramatic single-agent and combinatorial activity against SCLC cell lines treated in vitro and in xenograft models47,48 (Christine L. Hann, MD, Personal communication).
Figure 1.
Apoptotic induction by cytotoxics and the role of Bcl-2 in therapeutic resistance. Many cytotoxic agents induce DNA damage or disrupt critical oncogenic signaling pathways. These alterations, through activation of p53, suppression of Akt, and other pathways, lead to activation of small proapoptotic Bcl-2 family members (grey circles), termed BH3-only factors, either by transcriptional induction (Puma, Noxa, and others) or posttranscriptional modification (Bid, Bim, Bad, and others). Activated BH3-only factors (grey stars) translocate to the mitochondrial outer membrane, where they bind with high affinity to Bcl-2 and related antiapoptotic family members (white circles with B). This displaces Bcl-2 from interaction with the critical proapoptotic family members Bak and Bax (black circles), which can then homodimerize, leading to disruption of mitochondrial membrane integrity and release of key activators of the downstream apoptotic cascade, including cytochrome c (Cyt c), SMAC/Diablo, and apoptosis-inducing factor (AIF). Tumors with excess expression of Bcl-2 have increased capacity to absorb activated BH3-only factors while still preventing Bax/Bak activation. Targeted inhibition of Bcl-2 leads to increased sensitivity to cytotoxic therapy and can directly promote death of tumors dependent on high levels of Bcl-2 for survival.
Initial clinical translation of these observations, through intravenous administration of a Bcl-2 antisense oligonucleotide, has been disappointing. Oblimersen is a phosphorothioate 18-mer oligonucleotide complementary to the first 6 codons of the bcl-2 mRNA.49 Two phase I studies and a randomized phase II study have been reported of oblimersen with cytotoxic chemotherapy in patients with SCLC.50–53
The first phase I study combined oblimersen with paclitaxel in patients with chemorefractory relapsed SCLC, based on observations that Bcl-2 and related family members contribute to paclitaxel resistance. Two patients among this cohort with very poor prognosis experienced stable disease, with 1 showing no progression for more than 6 months, but no responses were objective.50 The second study evaluated oblimersen, carboplatin, and etoposide in patients with newly diagnosed extensive-stage SCLC.51 This small phase I study reported a promising response rate of 83% (10 of 12 evaluable patients) for the combination, and led directly to a randomized phase II study in the Cancer and Leukemia Group B consortium comparing carboplatin and etoposide with or without oblimersen. Oblimersen did not improve response rate or survival.52,53
One explanation for the lack of evident enhancement of chemotherapeutic efficacy with oblimersen may have been inadequate intracellular delivery of the oligonucleotide. The authors’ correlative analyses of peripheral blood mononuclear cells of patients receiving oblimersen suggested no consistent effect on Bcl-2 protein level in vivo.52 Recent-generation small molecule inhibitors of Bcl-2, in contrast to Bcl-2 antisense oligonucleotides, show a high level of single-agent activity in SCLC xenograft models. These observations caused research in this area to shift from oligonucleotide vectors to small molecule inhibitors.
Clinical studies of at least 3 small molecule inhibitors of Bcl-2 and related Bcl-2 family members in patients with SCLC are either ongoing or will initiate enrollment in the next several months. ABT-263 is an orally bioavailable high-potency Bcl-2/Bcl-xL inhibitor currently being evaluated in a multicenter phase I-II study in patents with recurrent SCLC. Obatoclax and AT-101 are Bcl-2 inhibitors with lower affinity but differing and possibly complementary patterns of selective inhibition across the Bcl-2 family. A 2-institution National Cancer Institute (NCI)–supported phase I-II study is ongoing of obatoclax with topotecan in recurrent, chemosensitive SCLC. An NCI-supported phase II study involving the Mayo and California consortiums will evaluate efficacy of AT-101 as a single agent in patients with recurrent SCLC. The outcome of these studies is eagerly awaited.
SCLC and Embryonic (Stem Cell) Differentiation Pathways: Hedgehog as a Targe
The hedgehog pathway is an essential embryonic signaling cascade that regulates of stem/progenitor cell differentiation pathways. It is a critical factor in organismal morphogenesis, including mammalian lung development.54,55 Smoothened (Smo) is a transmembrane protein that is absolutely required for activating the downstream hedgehog signaling pathway. Patched, an inhibitory cell surface receptor, constitutively suppresses hedgehog pathway activation through inhibition of Smo. Any of 3 patched ligands (sonic, Indian, and desert hedgehog) can bind to and inactivate patched, derepressing Smo, and promoting pathway activation. Remarkably, evolution from flies to man has not resulted in the development of smoothened paralogs. In contrast, perhaps as many as 7 frizzled receptors exist, which are close relatives of Smo in the Wnt pathway. The discovery that the plant-derived alkaloid cyclopamine is a potent Smo antagonist has propelled efforts to develop high-potency and -specificity hedgehog inhibitors and make Smo one of the most attractive pharmacologic targets for cancer drug discovery.56
The hedgehog pathway has been implicated as a key oncogenic factor for multiple tumor types, which fall into at least 2 fundamentally different classes. One class, including medulloblastoma and basal cell carcinomas, show inactivating patched mutations, leading to constitutive ligand-independent pathway activation. Preclinical models of tumors with this phenotype are exquisitely sensitive to treatment with Smo inhibitors. A second class, typified by SCLC,57 seems to retain pathway integrity but shows ligand-dependent pathway upregulation. Tumors of this class are also inhibited by Smo inhibitors in multiple preclinical models. A high proportion of SCLCs show constitutive ligand-dependent activation of the hedgehog pathway, express high levels of the sonic hedgehog ligand, and are growth-inhibited by Smo inhibitors.57,58
Recent data from several laboratories suggest an unexpectedly close connection in the roles of Hedgehog signaling in regulating normal stem cell differentiation and tumorigenesis.59,60 It is becoming increasingly apparent that tumors, in direct analogy with other complex multicellular functional units, comprise large numbers of relatively differentiated cells with limited replicative potential and a very small population of pleuripotent progenitors, or cancer stem cells.61 In multiple tumor types, the hedgehog pathway seems to be differentially activated within the stem cell compartment. If confirmed, this concept has direct implications for clinical applications of hedgehog pathway inhibitors (Figure 2).
Figure 2.
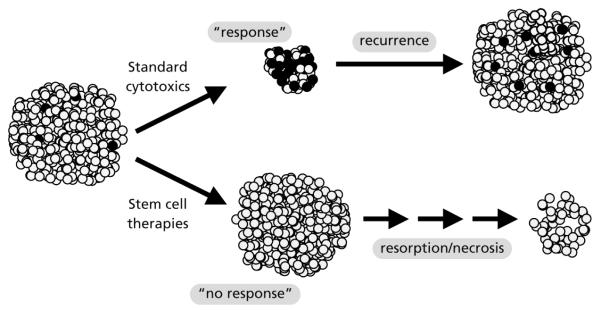
Differential capacities of standard cytotoxics and stem cell-directed therapeutics. Small cell lung cancer (SCLC) is highly chemosensitive, but responses to standard cytotoxics are almost never durable. This suggests SCLC contains a subset of cells with tumorigenic potential (black) that may be selectively chemoresistant. New combinations of standard cytotoxics targeting the same chemosensitive bulk population may show minor improvements in response rate but are unlikely to have durable anticancer activity. In contrast, novel therapeutics, such as hedgehog pathway inhibitors, that may primarily target tumor stem cell populations may show minimal objective response rates, but may have longer-term durable effects, especially if combined with standard cytotoxics.
Several pharmaceutical companies are pursuing development of synthetic hedgehog inhibitors. The first agent to be clinically tested is an orally bioavailable inhibitor, GDC-0449, which is currently in a phase I safety and dose-finding study in patients with solid tumors.
SCLC and a Virus Targeting Neuroendocrine Cancers: Fighting Biology with Biology
Over the past decade several attempts have been made to develop live, replication-competent anticancer viruses. Agents tested include retrovirus and adenoviral constructs. A novel, naturally derived picornavirus was discovered as a contaminant of a laboratory adenoviral preparation. This picornavirus, Seneca Valley Virus or SVV-001, has selective tropism for cancer cells of neuroendocrine differentiation and shows remarkable activity in SCLC xenografts.62 One pathognomonic feature of SCLC is expression of neuroendocrine markers. A phase I dose-escalation study of SVV-001 in patients with tumors having neuroendocrine features is ongoing. Intratumoral viral replication after intravenous administration was documented in the first patient receiving this agent.63 The essential biologic characteristics of this virus, including definition of its cellular receptor and mechanisms of selective tropism for neuroendocrine tumors, are areas of ongoing research in the authors’ laboratory.
Summary: A Time of Renewed Hope for SCLC
SCLC remains almost a universally fatal malignancy, marked by rapid disease recurrence and short survival times. Developments over the past several years suggest a reason for renewed hope in SCLC. Improved in vivo laboratory models of disease, based on transgenic modification of key oncogenic factors leading to murine SCLC and on primary xenografting of human SCLC, have generated new and possibly more predictive platforms for rapidly assessing therapeutic strategies. An improved understanding of the determinants of malignant transformation, clonogenic potential, tumor growth, and metastatic spread has led to the identification of novel targets for drug development in SCLC, several of which are currently being translated into the earliest phases of clinical development. These include concurrent first-in-human, first-in-class phase I studies of a novel anticancer virus with tropism for neuroendocrine tumors, a high-potency Hedgehog pathway inhibitor, and multiple selective Bcl-2 family inhibitors. In the next several years, other targeted therapeutics relevant to SCLC are likely to follow from emerging insights into the SCLC kinome, which is the set of somatically activated kinases characteristic of SCLC. Combinations of these novel therapeutics with the standard regimens, which have been remarkably effective against the bulk of SCLC tumor cells, have the potential to change the prognosis for patients with this disease.
Acknowledgment
This work is supported in part by the Burroughs Wellcome Fund and the Flight Attendant Medical Research Institute (CMR).
Footnotes
The authors have no financial interest, arrangement, or affiliation with the manufacturers of any products discussed in the article or their competitors.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Alberg AJ, Brock MV, Samet JM. Epidemiology of lung cancer: looking to the future. J Clin Oncol. 2005;23:3175–3185. doi: 10.1200/JCO.2005.10.462. [DOI] [PubMed] [Google Scholar]
- 3.Murray N, Turrisi AT., III A review of first-line treatment for small-cell lung cancer. J Thorac Oncol. 2006;1:270–278. doi: 10.1016/s1556-0864(15)31579-3. [DOI] [PubMed] [Google Scholar]
- 4.Lee CB, Morris DE, Fried DB, Socinski MA. Current and evolving treatment options for limited stage small cell lung cancer. Curr Opin Oncol. 2006;18:162–172. doi: 10.1097/01.cco.0000208790.45312.25. [DOI] [PubMed] [Google Scholar]
- 5.Murray N, Coy P, Pater JL, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1993;11:336–344. doi: 10.1200/JCO.1993.11.2.336. [DOI] [PubMed] [Google Scholar]
- 6.Takada M, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol. 2002;20:3054–3060. doi: 10.1200/JCO.2002.12.071. [DOI] [PubMed] [Google Scholar]
- 7.Turrisi AT, III, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–271. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 8.Oldham RK, Greco FA. Small-cell lung cancer. A curable disease. Cancer Chemother Pharmacol. 1980;4:173–177. doi: 10.1007/BF00254014. [DOI] [PubMed] [Google Scholar]
- 9.Argiris A, Murren JR. Advances in chemotherapy for small cell lung cancer: single-agent activity of newer agents. Cancer J. 2001;7:228–235. [PubMed] [Google Scholar]
- 10.Schiller JH, Adak S, Cella D, et al. Topotecan versus observation after cisplatin plus etoposide in extensive-stage small-cell lung cancer: E7593—a phase III trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:2114–2122. doi: 10.1200/JCO.2001.19.8.2114. [DOI] [PubMed] [Google Scholar]
- 11.Fischer B, Marinov M, Arcaro A. Targeting receptor tyrosine kinase signalling in small cell lung cancer (SCLC): what have we learned so far? Cancer Treat Rev. 2007;33:391–406. doi: 10.1016/j.ctrv.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Johnson BE, Fischer T, Fischer B, et al. Phase II study of imatinib in patients with small cell lung cancer. Clin Cancer Res. 2003;9(16 Pt 1):5880–5887. [PubMed] [Google Scholar]
- 13.McNeish IA, Bell SJ, Lemoine NR. Gene therapy progress and prospects: cancer gene therapy using tumour suppressor genes. Gene Ther. 2004;11:497–503. doi: 10.1038/sj.gt.3302238. [DOI] [PubMed] [Google Scholar]
- 14.Meuwissen R, Linn SC, Linnoila RI, et al. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell. 2003;4:181–189. doi: 10.1016/s1535-6108(03)00220-4. [DOI] [PubMed] [Google Scholar]
- 15.Ninomiya H, Nomura K, Satoh Y, et al. Genetic instability in lung cancer: concurrent analysis of chromosomal, mini- and microsatellite instability and loss of heterozygosity. Br J Cancer. 2006;94:1485–1491. doi: 10.1038/sj.bjc.6603121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandita A, Aldape KD, Zadeh G, et al. Contrasting in vivo and in vitro fates of glioblastoma cell subpopulations with amplified EGFR. Genes Chromosomes Cancer. 2004;39:29–36. doi: 10.1002/gcc.10300. [DOI] [PubMed] [Google Scholar]
- 17.Peterson JK, Houghton PJ. Integrating pharmacology and in vivo cancer models in preclinical and clinical drug development. Eur J Cancer. 2004;40:837–844. doi: 10.1016/j.ejca.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Wales MM, Biel MA, el Deiry W, et al. p53 activates expression of HIC-1, a new candidate tumour suppressor gene on 17p13.3. Nat Med. 1995;1:570–577. doi: 10.1038/nm0695-570. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 20.Embuscado EE, Laheru D, Ricci F, et al. Immortalizing the complexity of cancer metastasis: genetic features of lethal metastatic pancreatic cancer obtained from rapid autopsy. Cancer Biol Ther. 2005;4:548–554. doi: 10.4161/cbt.4.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morton CL, Houghton PJ. Establishment of human tumor xenografts in immunodeficient mice. Nat Protoc. 2007;2:247–250. doi: 10.1038/nprot.2007.25. [DOI] [PubMed] [Google Scholar]
- 22.Ardizzoni A, Hansen H, Dombernowsky P, et al. Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative Group. J Clin Oncol. 1997;15:2090–2096. doi: 10.1200/JCO.1997.15.5.2090. [DOI] [PubMed] [Google Scholar]
- 23.von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17:658–667. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- 24.Saijo N. Progress in treatment of small-cell lung cancer: role of CPT-11. Br J Cancer. 2003;89:2178–2183. doi: 10.1038/sj.bjc.6601456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 26.Kim TW, Innocenti F. Insights, challenges, and future directions in irinogenetics. Ther Drug Monit. 2007;29:265–270. doi: 10.1097/FTD.0b013e318068623b. [DOI] [PubMed] [Google Scholar]
- 27.Innocenti F, Undevia SD, Iyer L, et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol. 2004;22:1382–1388. doi: 10.1200/JCO.2004.07.173. [DOI] [PubMed] [Google Scholar]
- 28.Hanna N, Bunn PA, Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24:2038–2043. doi: 10.1200/JCO.2005.04.8595. [DOI] [PubMed] [Google Scholar]
- 29.Lara PN, Jr, Gandara DR, Natale RB. Randomized phase III trial of cisplatin/irinotecan versus cisplatin/etoposide in patients with extensive-stage small-cell lung cancer. Clin Lung Cancer. 2006;7:353–356. doi: 10.3816/CLC.2006.n.019. [DOI] [PubMed] [Google Scholar]
- 30.Onoda S, Masuda N, Seto T, et al. Phase II trial of amrubicin for treatment of refractory or relapsed small-cell lung cancer: Thoracic Oncology Research Group Study 0301. J Clin Oncol. 2006;24:5448–5453. doi: 10.1200/JCO.2006.08.4145. [DOI] [PubMed] [Google Scholar]
- 31.Yana T, Negoro S, Takada M, et al. Phase II study of amrubicin in previously untreated patients with extensive-disease small cell lung cancer: West Japan Thoracic Oncology Group (WJTOG) study. Invest New Drugs. 2007;25:253–258. doi: 10.1007/s10637-006-9012-9. [DOI] [PubMed] [Google Scholar]
- 32.Ettinger DS. Amrubicin for the treatment of small cell lung cancer: does effectiveness cross the Pacific? J Thorac Oncol. 2007;2:160–165. doi: 10.1097/jto.0b013e31802f1cd9. [DOI] [PubMed] [Google Scholar]
- 33.Lucchi M, Mussi A, Fontanini G, et al. Small cell lung carcinoma (SCLC): the angiogenic phenomenon. Eur J Cardiothorac Surg. 2002;21:1105–1110. doi: 10.1016/s1010-7940(02)00112-4. [DOI] [PubMed] [Google Scholar]
- 34.Sandler A, Szwaric S, Dowlati A, et al. A phase II study of cisplatin plus etoposide plus bevacizumab for previously untreated extensive stage small cell lung cancer (E3501): a trial of the Eastern Cooperative Oncology Group [abstract] J Clin Oncol. 2007;25(Suppl 1):400s. doi: 10.1200/JCO.2009.23.7545. Abstract 7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ready N, Dudek AZ, Wang XF, et al. CALGB 30306: a phase II study of cisplatin, irinotecan and bevacizumab for untreated extensive stage small cell lung cancer [abstract] J Clin Oncol. 2007;25(Suppl 1):400s. doi: 10.1200/JCO.2011.35.6923. Abstract 7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spiegel DR, Hainsworth JD, Yardley DA, et al. Phase II trial of irinotecan, carboplatin, and bevacizumab in patients with extensive-stage small cell lung cancer [abstract] J Clin Oncol. 2007;25(Suppl 1):694s. doi: 10.1097/JTO.0b013e3181bbc540. Abstract 18130. [DOI] [PubMed] [Google Scholar]
- 37.Arnold AM, Seymour L, Smylie M, et al. Phase II study of vandetanib or placebo in small-cell lung cancer patients after complete or partial response to induction chemotherapy with or without radiation therapy: National Cancer Institute of Canada Clinical Trials Group Study BR.20. J Clin Oncol. 2007;25:4278–4284. doi: 10.1200/JCO.2007.12.3083. [DOI] [PubMed] [Google Scholar]
- 38.D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pujol JL, Breton JL, Gervais R, et al. Phase III double-blind, placebo-controlled study of thalidomide in extensive-disease small-cell lung cancer after response to chemotherapy: an intergroup study FNCLCC cleo04 IFCT 00-01. J Clin Oncol. 2007;25:3945–3951. doi: 10.1200/JCO.2007.11.8109. [DOI] [PubMed] [Google Scholar]
- 40.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 41.Shivapurkar N, Reddy J, Chaudhary PM, Gazdar AF. Apoptosis and lung cancer: a review. J Cell Biochem. 2003;88:885–898. doi: 10.1002/jcb.10440. [DOI] [PubMed] [Google Scholar]
- 42.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ikegaki N, Katsumata M, Minna J, Tsujimoto Y. Expression of bcl-2 in small cell lung carcinoma cells. Cancer Res. 1994;54:6–8. [PubMed] [Google Scholar]
- 44.Jiang SX, Sato Y, Kuwao S, Kameya T. Expression of bcl-2 oncogene protein is prevalent in small cell lung carcinomas. J Pathol. 1995;177:135–138. doi: 10.1002/path.1711770206. [DOI] [PubMed] [Google Scholar]
- 45.Stefanaki K, Rontogiannis D, Vamvouka C, et al. Immunohistochemical detection of bcl2, p53, mdm2 and p21/waf1 proteins in small-cell lung carcinomas. Anticancer Res. 1998;18(2A):1167–1173. [PubMed] [Google Scholar]
- 46.Zangemeister-Wittke U, Schenker T, Luedke GH, Stahel RA. Synergistic cytotoxicity of bcl-2 antisense oligodeoxynucleotides and etoposide, doxorubicin and cisplatin on small-cell lung cancer cell lines. Br J Cancer. 1998;78:1035–1042. doi: 10.1038/bjc.1998.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 48.Tahir SK, Yang X, Anderson MG, et al. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 2007;67:1176–1183. doi: 10.1158/0008-5472.CAN-06-2203. [DOI] [PubMed] [Google Scholar]
- 49.Reed JC, Stein C, Subasinghe C, et al. Antisense-mediated inhibition of BCL2 protooncogene expression and leukemic cell growth and survival: comparisons of phosphodiester and phosphorothioate oligodeoxynucleotides. Cancer Res. 1990;50:6565–6570. [PubMed] [Google Scholar]
- 50.Rudin CM, Otterson GA, Mauer AM, et al. A pilot trial of G3139, a bcl-2 antisense oligonucleotide, and paclitaxel in patients with chemorefractory small-cell lung cancer. Ann Oncol. 2002;13:539–545. doi: 10.1093/annonc/mdf124. [DOI] [PubMed] [Google Scholar]
- 51.Rudin CM, Kozloff M, Hoffman PC, et al. Phase I study of G3139, a bcl-2 antisense oligonucleotide, combined with carboplatin and etoposide in patients with small-cell lung cancer. J Clin Oncol. 2004;22:1110–1117. doi: 10.1200/JCO.2004.10.148. [DOI] [PubMed] [Google Scholar]
- 52.Rudin CM, Salgia R, Wang XF, et al. CALGB 30103: a randomized phase II study of carboplatin and etoposide with or without G3139 in patients with extensive stage small cell lung cancer [abstract] J Clin Oncol. 2005;23(Suppl 1):662s. doi: 10.1200/JCO.2007.14.3461. Abstract 7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rudin CM, Salgia R, Wang X, et al. A randomized phase II study of carboplatin and etoposide with or without oblimersen, antisense bcl-2, for extensive stage small cell lung cancer: CALGB 30103. J Clin Oncol. 2008;26:870–876. doi: 10.1200/JCO.2007.14.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watkins DN, Peacock CD. Hedgehog signalling in foregut malignancy. Biochem Pharmacol. 2004;68:1055–1060. doi: 10.1016/j.bcp.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 55.Velcheti V, Govindan R. Hedgehog signaling pathway and lung cancer. J Thorac Oncol. 2007;2:7–10. doi: 10.1097/JTO.0b013e31802c0276. [DOI] [PubMed] [Google Scholar]
- 56.Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026–1033. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- 57.Watkins DN, Berman DM, Burkholder SG, et al. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–317. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- 58.Watkins DN, Berman DM, Baylin SB. Hedgehog signaling: progenitor phenotype in small-cell lung cancer. Cell Cycle. 2003;2:196–198. [PubMed] [Google Scholar]
- 59.Lou H, Dean M. Targeted therapy for cancer stem cells: the patched pathway and ABC transporters. Oncogene. 2007;26:1357–1360. doi: 10.1038/sj.onc.1210200. [DOI] [PubMed] [Google Scholar]
- 60.Peacock CD, Wang Q, Gesell GS, et al. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc Natl Acad Sci U S A. 2007;104:4048–4053. doi: 10.1073/pnas.0611682104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Al-Hajj M, Becker MW, Wicha M, et al. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev. 2004;14:43–47. doi: 10.1016/j.gde.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 62.Reddy PS, Burroughs KD, Hales LM, et al. Seneca Valley virus, a systemically deliverable oncolytic picornavirus, and the treatment of neuroendocrine cancers. J Natl Cancer Inst. 2007;99:1623–1633. doi: 10.1093/jnci/djm198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rudin CM, Lansey D, Burroughs KD, et al. Phase I trial of Seneca Valley Virus, a newly discovered systemically deliverable oncolytic picornavirus, in patients with solid tumors with neuroendocrine features [abstract] J Clin Oncol. 2007;25(Suppl 1):685s. Abstract 18014. [Google Scholar]