Abstract
The phosphoinositide-3-kinase (PI3-kinase)-Akt-mTOR pathway is a central signal transduction pathway that regulates many critical aspects of normal and cancer physiology, including cell proliferation, apoptosis, cell morphology and migration, protein synthesis, and integration of metabolism. In breast cancer, somatic mutations that activate the pathway occur in more than 50% of tumors, underscoring the potentially broad impact of targeting the pathway for therapy. A vast body of preclinical data demonstrates the efficacy of pathway inhibition on tumor growth, and evidence also shows that activation of the pathway occurs in models of acquired resistance to hormonal therapy. This preclinical work led to the investigation of allosteric mTOR inhibitors, everolimus and temsirolimus, in metastatic hormone receptor–positive breast cancer. The recent BOLERO-2 trial comparing everolimus plus exemestane versus placebo plus exemestane in women with resistance to nonsteroidal aromatase inhibitors demonstrated a 6-month improvement in progression-free survival and led to FDA approval of everolimus for this indication in the United States. This landmark trial is the first demonstration of significant clinical benefit using drugs targeting this pathway in breast cancer. Many questions remain about the role of everolimus and other pathway-targeting drugs in clinical development in breast cancer treatment. This article reviews the role of the PI3-kinase-Akt-mTOR pathway in breast cancer biology and the clinical trial evidence available to date.
NCCN: Continuing Education
Accreditation Statement
This activity has been designated to meet the educational needs of physicians and nurses involved in the management of patients with cancer. There is no fee for this article. No commercial support was received for this article. The National Comprehensive Cancer Network (NCCN) is accredited by the ACCME to provide continuing medical education for physicians.
NCCN designates this journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
NCCN is accredited as a provider of continuing nursing education by the American Nurses Credentialing Center`s Commission on Accreditation.
This activity is accredited for 1.0 contact hour. Accreditation as a provider refers to recognition of educational activities only; accredited status does not imply endorsement by NCCN or ANCC of any commercial products discussed/displayed in conjunction with the educational activity. Kristina M. Gregory, RN, MSN, OCN, is our nurse planner for this educational activity.
All clinicians completing this activity will be issued a certificate of participation. To participate in this journal CE activity: 1) review the learning objectives and author disclosures; 2) study the education content; 3) take the posttest with a 70% minimum passing score and complete the evaluation at http://education.nccn.org/ node/21665; and 4) view/print certificate.
Learning Objectives
Upon completion of this activity, participants will be able to:
Describe the role of the PI3-kinase-Akt-mTOR pathway in breast cancer treatment.
Outline the recent clinical trials for pathway-targeting drugs for the treatment of breast cancer.
PI3-Kinase-Akt-mTOR Pathway in Cancer Biology
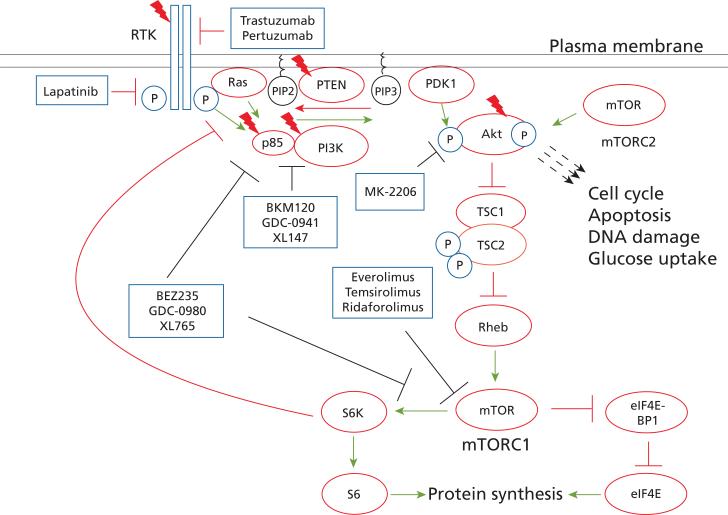
The phosphoinositide-3-kinase (PI3-kinase)-Akt-mTOR pathway is a major signaling pathway in normal and cancer physiology (Figure 1).1,2 The class I PI3-kinases consist of a catalytic subunit (p110) and a regulatory subunit (p85). PI3-kinase binds to phosphorylated tyrosines on a variety of receptor tyrosine kinases, including epidermal growth factor receptor (EGFR), insulin-like growth factor 1 receptor (IGF1R), insulin receptor, and HER2, leading to activation. PI3-kinase catalyzes the phosphorylation of the membrane lipid phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3). This reaction is reversed by the lipid phosphatases PTEN and INPP4B. PIP3 recruits pleckstrin homology domain– containing proteins to the plasma membrane, leading to their activation. Of particular importance are the phosphoinositide-dependent kinase Pdk1 and the Akt family of kinases, which includes 3 closely related serine/threonine kinases: Akt1, Akt2, and Akt3. Pdk1 phosphorylates threonine 308 and activates Akt.
Figure 1.
The phosphoinositide-3-kinase-Akt-mTOR pathway. Green arrows indicate activation or positive regulation, red bars indicate inhibition. Red lightning bolts indicate genes frequently mutated in human breast cancers. Blue rectangles depict drugs either approved or being evaluated in clinical trials for breast cancer, and the targets they inhibit (black bars). For simplicity, other targets of Akt are not shown. P, phosphorylation; RTK, receptor tyrosine kinase.
A second phosphorylation event on serine 473, mediated by the mTOR-containing TORC2 complex, is required for full Akt activation. Akt then phosphorylates several substrates, leading to pleiotropic effects on proliferation, apoptosis, differentiation, and cellular metabolism. One of the key downstream Akt targets is the mTOR protein kinase complex. mTOR, the mechanistic target of rapamycin, exists in 2 distinct multiprotein complexes: mTORC1 and mTORC2. Akt phosphorylates Tsc2 and PRAS40, which relieves inhibition of mTORC1, leading to increased mTORC1 kinase activity. mTORC1 regulates protein synthesis and cellular metabolism through 2 major substrates: p70 ribosomal protein S6-kinase (p70S6K) and eukaryotic initiation factor 4E binding protein 1 (EIF4EBP1). The mTORC2 complex functions upstream of Akt, phosphorylating Akt on the serine 473 residue critical for Akt activation.
Frequent Mutational Activation of the PI3-Kinase-Akt-mTOR Pathway in Breast Cancer
Although activation of the PI3-kinase-Akt-mTOR pathway has been observed in many different cancer types, the pathway plays an outsized role in breast cancer development, because breast cancers have the highest rate of mutational activation of the pathway.3,4 The pathway can be activated by genomic amplification or overexpression of receptor tyrosine kinases, such as HER2, EGFR, and IGF1R. Activating mutations in the catalytic PI3-kinase subunit PIK-3CA occur in 36% of breast cancers overall and are especially prevalent in luminal and HER2-amplified breast cancers (29%–45%).3–6 Activating mutations in the pleckstrin homology domain of AKT1 occur in another 3% of breast cancers, exclusively in estrogen receptor–positive (ER+) cases.7–9 Additional mutations occur in the PI3-kinase regulatory subunit PIK3R1 (3%), and other cancers have mutations in AKT2 or AKT3, or amplification of PIK3CA or AKT1–3. The tumor suppressor PTEN is mutated or deleted in 7% of breast cancers, and may also be silenced by promoter methylation. In contrast to PIK-3CA and AKT1 mutation, PTEN loss preferentially occurs in triple-negative breast cancers, and triple-negative cancers show evidence of strong PI3-kinase pathway activation.3 In most breast cancers, mutations in different genes in the PI3-kinase pathway are mutually exclusive.
Preclinical Data Supporting the PI3-Kinase-Akt-mTOR Pathway as a Breast Cancer Therapeutic Target
Multiple lines of evidence support PI3-kinase pathway activation as a driver of breast cancer development. Individuals with germline PTEN mutations (Cowden syndrome) have an elevated lifetime risk of breast cancer. Expression of mutant PIK3CA or constitutively active forms of Akt in human mammary epithelial cells causes growth factor independence and some hallmarks of cellular transformation, but is insufficient to make them fully tumorigenic.10–12 Mice with mam-mary gland–specific knock-in of activating PIK3CA kinase mutations develop mammary tumors.13,14
Although the PI3-kinase pathway can activate proliferation and resistance to apoptosis through a wide variety of mechanisms, evidence also indicates specific cross-talk between the estrogen receptor alpha (ERα) and the PI3-kinase pathway. Rapid membrane-initiated signaling in response to estrogen occurs in breast cancer cell lines to activate the PI3-kinase pathway.15,16 This signaling requires ERα and growth factor receptors, but does not require ERα transcriptional activity. ERα has been shown to bind to the p85 regulatory subunit of PI3-kinase.17 Akt and the mTOR target p70S6K can phosphory-late ERα serine 167, which increases both its ligand-dependent and ligand-independent transcriptional activity.18
Knockdown of PTEN in ER+ breast cancer cell lines leads to endocrine resistance, and forced expression of constitutively active forms of Akt or PI3-kinase can confer estrogen-independent growth in breast cancer cell lines.19–21 Long-term estrogen deprivation (LTED) culture of human ER+ breast cancer cell lines is a widely used model of estrogen independence/antiestrogen resistance.22 Typically, these LTED lines demonstrate upregulation of growth factor receptor tyrosine kinases and increased receptor tyrosine kinase signaling through the PI3-kinase and MAPK pathways.23,24 Pharmacologic inhibition of the PI3-kinase pathway can inhibit the estrogen-independent growth of LTED cell lines and resensitize these cells to hormonal therapies, including tamoxifen and aromatase inhibitors.20,23,25–28 In contrast, other studies have not found an increased sensitivity of LTED cells to dual PI3-kinase/mTOR inhibitors, and LTED cultures may not accurately model acquired tamoxifen resistance, which may occur through distinct mechanisms.29,30
Preclinical data show that PTEN loss or PIK-3CA mutation can confer trastuzumab resistance in HER2+ breast cancers, which can be overcome by combining trastuzumab with either PI3-kinase, Akt, or mTOR inhibitors.31–33 Conflicting results have been reported regarding whether PI3-kinase pathway activation predicts resistance to the dual HER2/ EGFR kinase inhibitor lapatinib.34–36 Retrospective studies have linked PI3-kinase pathway activation with shorter time to progression on trastuzumab in the metastatic setting, decreased pathologic complete response to neoadjuvant trastuzumab and chemotherapy, and decreased overall survival in patients treated with adjuvant trastuzumab and chemotherapy.31,37–39 Quantitatively measuring PTEN levels in tumors using immunohistochemistry is challenging, and methodologies differ among studies, which may affect the strength of the reported effects of PTEN expression. Ongoing clinical trials are exploring the use of PI3-kinase pathway inhibitors to overcome resistance to trastuzumab.40
Targeting the PI3-Kinase-Akt-mTOR Pathway
The PI3-kinase pathway can be inhibited proximally at the levels of growth factor receptors (trastuzumab, IGF1R inhibitors, EGFR inhibitors, lapatinib), PIK-3CA (nonselective and selective PI3K inhibitors), Akt (allosteric and kinase inhibitors of Akt), and mTOR (Figure 1). Drugs targeting mTOR include rapamycin and its analogues everolimus and temsirolimus, which allosterically inhibit mTORC1 only, and mTOR kinase inhibitors, which inhibit both mTORC1 and mTORC2.41 Because rapamycin's discovery and clinical use actually preceded the identification of its target, the clinical development of mTOR inhibitors has been more advanced than that of inhibitors at other levels of the pathway.
Preclinical studies have shown that allosteric mTOR inhibitors, such as everolimus and temsirolimus, which predominantly inhibit the TORC1 complex, can lead to increased signaling through receptor tyrosine kinases, such as IGF1R and ERBB3.42–44 Relief of negative feedback mediated by TORC1 through S6K and IRS1 leads to a paradoxical increase in Akt and TORC2 activation (Figure 1). This mechanism has been hypothesized to underpin the limited efficacy of allosteric mTOR inhibitors in several clinical settings; however, a similar relief of feedback has been observed using Akt inhibitors.43,44 In some studies, dual PI3K/mTOR inhibition or combining PI3-kinase pathway and upstream receptor tyrosine kinase inhibition can overcome this paradoxical response to pathway inhibition. PIK3CA and KRAS mutations were shown to mediate sensitivity and resistance, respectively, to everolimus in isogenic mammary epithelial cell lines, and KRAS mutations have been shown to confer resistance to PI3-kinase inhibitors in other contexts.45,46 KRAS mutations are rare in human breast cancer, but other mechanisms that activate mitogen-activated protein kinase signaling may confer resistance to PI3-kinase pathway inhibition.
Identification of predictive biomarkers for mTOR pathway inhibition is a work in progress.40,47,48 Although several phosphoprotein markers correlate with activation of the pathway, including Akt S473 and T308, PRAS40, p70S6K, and S6, these markers have not been consistently predictive of response to pathway inhibitors in early clinical trials. Great interest has been shown in PI3K pathway mutations as biomarkers of inhibitor susceptibility. Several PI3-kinase and/or mTOR inhibitors show increased activity against breast cancer cell lines with mutations in PIK3CA or mutation/loss of PTEN.9,11,49–52 Importantly, mutations in the pathway may not all be functionally equivalent, and distinct mutations may behave differently in response to particular drugs.7,49–52 In addition, some tumors without these mutations are also sensitive to pathway inhibitors.53 For this reason, some investigators favor more systematic biomarker approaches, such as gene expression signatures or multiprotein markers of PI3-kinase pathway activation.52 Phase I studies of PI3-kinase–targeted therapies suggest an enrichment of responders in patients with activating PIK3CA mutations,54,55 whereas other studies have found no correlation between mutation and response, although most of these studies are underpowered.56 Recently, mutation of the TSC1 and TSC2 genes in bladder cancers was found to correlate with clinical benefit from everolimus.57
Clinical Trials Using mTOR Inhibitors in Breast Cancer
Studies of single-agent mTOR inhibitors in patients with pretreated metastatic breast cancer suggested an effect primarily on disease progression, predominantly in hormone receptor–positive and/or HER2+ patients. In a randomized phase II trial comparing weekly versus daily dosing schedules of everolimus for metastatic breast cancer, 4 of 33 patients in the daily dosing arm had complete or partial responses and 15 of 33 had stable disease.58 Weekly intravenous temsirolimus at 75 or 250 mg showed modest activity as a single agent in patients with heavily treated metastatic breast cancer (response rate, 9%; clinical benefit rate, 13.8%), whereas a smaller study using 25 mg showed no responses but a 9.7% stable disease rate.56,59
A randomized phase II trial comparing neoadjuvant letrozole versus everolimus plus letrozole in hormone receptor–positive breast cancer showed increased response rates with the combination.60 Biopsies performed on day 15 showed that everolimus decreased phosphorylation of ribosomal protein S6. An exploratory analysis showed a greater antiproliferative effect of everolimus in the subset of patients with PIK3CA helical domain mutations.
The randomized phase III BOLERO-2 trial tested everolimus plus the steroidal aromatase inhibitor (AI) exemestane versus placebo plus exemestane in women with metastatic ER+/HER2– breast cancer that had progressed on prior therapy with nonsteroidal AIs.61 The trial was stopped early after a planned interim data analysis showed that the study had met its prespecified end point of progression-free survival. The everolimus arm showed an increase in response rate (7% vs 0.4%) and a significant increase in progression-free survival (10.6 vs 4.1 months by central review). A nonsignificant increase was seen in overall survival in the everolimus arm, but overall survival data are immature. The benefit of everolimus was consistent across subgroups divided by age, prior therapy, and sensitivity to previous hormonal therapy. Toxicity was significant, however, and consistent with toxicity profiles observed in other clinical trials of everolimus. Serious adverse events were more than twice as common in the everolimus arm and more likely to be treatment-related. Leading side effects were stomatitis (56% all grades; 8% grade 3), fatigue (33% all grades; 3% grade 3), diarrhea (30% all grades; 2% grade 3), and rash (36% all grades; 1% grade 3). This trial led to FDA approval of everolimus in combination with exemestane for metastatic ER+ breast cancer previously treated with nonsteroidal AIs.
In contrast, in the recently reported randomized phase III HORIZON trial, the combination of oral temsirolimus and letrozole did not improve response rates or progression-free survival when compared with letrozole plus placebo as first-line treatment for women with locally advanced or metastatic ER+ breast cancer.62 A key difference between the trial populations is that none of the patients in HORIZON had any prior AI exposure, although approximately 40% had received adjuvant tamoxifen, whereas BOLERO-2 participants essentially all experienced disease progression on prior nonsteroidal AI therapy. This difference is reflected in the response rates seen in the AI-plus-placebo arms: 27% in HORIZON and 0.4% in BOLERO-2. The median progression-free survival of both arms in HORIZON was 9 months, more than double that of the placebo arm in BOLERO-2, which reflects the benefit of active hormonal therapy in this AI-naïve group. Another difference between the trials is the choice of mTOR inhibitor and the use of oral rather than intravenous temsirolimus. Although differences between everolimus and temsirolimus could account for the disparate results, the response rates in the everolimus arm of BOLERO-2 are not very different from those seen with temsirolimus as a single agent, although this study used intravenous temsirolimus.59 Toxicities in the temsirolimus arm of HORIZON were similar to those reported in the everolimus arm of BOLERO-2 and in studies of intravenous temsirolimus, suggesting that orally dosed temsirolimus was likely hitting its targets.
The TAMRAD trial was a phase II trial of 111 patients that compared tamoxifen versus tamoxifen plus everolimus in patients with metastatic ER+/ HER2– breast cancer who had undergone prior AI therapy.63 The everolimus combination arm had a 6-month clinical benefit rate (the primary end point) of 61% versus 42% for the tamoxifen-only arm. Death occurred in 16 (30%) patients in the everolimus arm versus 31 (54%) in the tamoxifen-only arm, a 55% reduction in the risk of death, although this analysis was exploratory. An exploratory subgroup analysis suggested that the benefit of everolimus accrued mainly to patients with secondary hormone resistance, defined as those whose disease either relapsed more than 6 months after stopping adjuvant AIs or responded for at least 6 months to AIs in the metastatic setting. Although the patients were stratified according to these subgroups, the number in each group was small, and these results also can only be viewed as hypothesis-generating. In the everolimus arm, 35% of patients dose-reduced or discontinued therapy because of side effects.
BOLERO-1 is an ongoing phase III, randomized, double-blind, placebo-controlled trial comparing paclitaxel and trastuzumab with or without everolimus as first-line therapy for metastatic or locally advanced HER2+ breast cancer, and BOLERO-3 is comparing vinorelbine and trastuzumab with or without everolimus in the same population. Several phase II trials accruing in the United States are testing everolimus in combination with chemotherapy or fulvestrant, trastuzumab, or lapatinib in varied patient populations. (Clinical trial identifiers for currently open trials discussed in this article are available at ClinicalTrials.gov.) The planned Southwest Oncology Group/National Surgical Adjuvant Breast and Bowel Project S1207 phase III trial will evaluate the addition of 1 year of everolimus to standard adjuvant hormonal therapy in high-risk ER+/HER2– breast cancers.64 Other trials, mostly in phase I or II, are investigating other PI3-kinase pathway–targeted drugs alone or in combination with hormonal therapies or HER2–targeted therapies (Table 1). A phase III trial is randomizing AI-refractory patients to fulvestrant plus either placebo or BKM120, an oral pan-class I PI3-kinase inhibitor. An allosteric Akt inhibitor MK-2206 is being tested in combination with either chemotherapy or hormonal therapy in several phase I and II trials. The mTOR kinase inhibitor AZD2014, which inhibits both TORC1 and TORC2, unlike everolimus and other allosteric mTOR inhibitors, is in phase I testing in combination with fulvestrant. Some of these trials are screening tumors for PI3-kinase pathway mutations.
Table 1.
Drugs Targetting the P13-AKT-m TOR Pathway Currently in Clinical Trials for Breast Cancer
| Drug | Class | Study Population(s) | Common Toxicities |
|---|---|---|---|
| Everolimus (RAD-001) | Allosteric mTOR inhibitor | Adjuvant HR+; locally advanced/metastatic, HER2+; advanced HER2-; neoadjuvant | Fatigue, stomatitis, diarrhea, rash61 |
| Temsirolimus | Allosteric mTOR inhibitor | HER2+ or TN | Fatigue, stomatitis, diarrhea, rash62 |
| Ridaforolimus (MK-8669) | Allosteric mTOR inhibitor | Advanced/metastatic HR+/HER2- | Fatigue, stomatitis, anorexia, diarrhea, nausea65 |
| AZD2014 | mTOR (TORC1/2) kinase inhibitor | Advanced/metastatic HR+ | Fatigue, stomatitis, anorexia, diarrhea, nausea66 |
| MK-2206 | Allosteric Akt inhibitor | HR+ neoadjuvant and advanced; preoperative biomarker, all subtypes; advanced HER2+ | Rash, nausea, pruritus, hyperglycemia, diarrhea67 |
| AZD5363 | Akt kinase inhibitor | Advanced/metastatic, all subtypes | Not reported |
| Triciribine | Akt inhibitor | Neoadjuvant; advanced HER2- | Hyperlipidemia, hyperglycemia, fatigue68 |
| GDC-0941 | PI3-kinase inhibitor | Advanced/metastatic HR+, HER2+, TN | Fatigue, nausea, diarrhea, rash, transient hyperglycemia69 |
| BKM120 | PI3-kinase inhibitor | Advanced/metastatic HR+ or TN; neoadjuvant HER2+; preoperative biomarker; advanced HER2+ resistant to trastuzumab | Fatigue, rash, nausea, mood alteration, hyperglycemia70 |
| BAY80-6946 | PI3-kinase inhibitor | Advanced/metastatic | Not reported |
| XL147 | PI3-kinase inhibitor | Advanced/metastatic HR+; advanced/metastatic HER2+ progressing on trastuzumab | Rash, hyperglycemia69 |
| BYL719 | PI3-kinase/PIK3CA- specific inhibitor | Advanced/metastatic HR+ | Hyperglycemia, nausea, vomiting, diarrhea, anorexia71 |
| XL765 | Dual PI3-kinase/ mTOR inhibitor | Advanced/metastatic HR+ | Nausea, diarrhea, anorexia, rash, elevated LFTs69 |
| BEZ235 | Dual PI3-kinase/ mTOR inhibitor | HER2+; preoperative biomarker; advanced/ metastatic HER2- | Nausea, vomiting, diarrhea, fatigue, anemia69 |
| GDC-0980 | Dual PI3-kinase/ mTOR inhibitor | Advanced/metastatic HR+ | Nausea, fatigue, diarrhea69 |
Abbreviations: HR, hormone receptor; LFTs, liver function tests; TN, triple-negative. Only trials that are currently open are listed (identifiers are available at ClinicalTrials.gov). Drugs being evaluated in phase I for multiple solid tumor types may not be listed. With the exception of the mTOR inhibitors, most toxicity data is from phase I studies.
Despite the large progression-free survival benefit in BOLERO-2, questions remain about the role of mTOR inhibitor therapy in clinical practice. First, although it is hypothesized that everolimus reverses endocrine resistance in these patients, this has not been proven, because BOLERO-2 and all other studies of AI plus everolimus lacked an everolimus-only arm. One cannot directly compare the activity of everolimus in BOLERO-2 with the activity of single-agent temsirolimus or everolimus in smaller phase II trials because of differences in levels of pretreatment and patient demographics. Second, these targeted therapies are toxic and certainly represent a signifi-cant decrement in quality of life for patients compared with endocrine therapy, as reflected in the greater discontinuation rates in the mTOR inhibitor arms of all of the trials discussed herein. This raises the question of whether adding an mTOR inhibitor is comparable to using traditional cytotoxic chemotherapy after the development of endocrine resistance. Furthermore, women who experience disease progression after AI therapy have other endocrine therapy options, such as fulvestrant and potentially tamoxifen.
Many questions remain to be answered by ongoing and future clinical trials and further basic and translational research. Is the role of mTOR inhibition limited to acquired hormonal therapy resistance? Despite frequent baseline mutational activation of the PI3-kinase pathway in ER+ tumors, initial endocrine therapy may select for increased dependence on PI3-kinase signaling, which could explain the more favorable results seen in BOLERO-2 and TAMRAD versus HORIZON. Reliable biomarkers of pathway dependence are needed. Will targeting mTOR or the PI3-kinase pathway in general prevent or reverse trastuzumab resistance in patients with HER2+ breast cancer? Will PI3-kinase inhibitors, dual PI3-kinase/mTOR inhibitors, mTOR kinase inhibitors, or Akt inhibitors show similar activity in ER+ breast cancers resistant to AIs or other hormonal therapies? Will inhibition of this pathway produce any benefit in triple-negative breast cancers? Can patients most likely to benefit from everolimus and other PI3-kinase pathway–targeting drugs be selected using mutational or phosphoprotein biomarkers? How should everolimus be sequenced with other available therapeutic options to optimize survival and quality of life? These questions present current and future challenges for researchers to address over the next few years as progress continues toward molecularly targeted breast cancer therapies.
Footnotes
The authors have disclosed that they have no financial interests, arrangements, affiliations, or commercial interests with the manufacturers of any products discussed in this article or their competitors. Dr. Lauring receives support from the Susan G. Komen for the Cure Career Catalyst Grant funded by Charlotte R. Nelson, the Flight Attendant Medical Research Institute, the Avon Foundation, and the Department of Defense Breast Cancer Research Program. Dr. Park receives support from the Breast Cancer Research Foundation, the Flight Attendant Medical Research Institute, the Avon Foundation, and the National Institutes of Health. Dr. Wolff receives support from a Susan G. Komen for the Cure Scholars Grant and from the Breast Cancer Research Foundation. This work is also supported in part by the NCI Cancer Center Support Grant P30 CA006973.
EDITOR
Kerrin M. Green, MA, Assistant Managing Editor, JNCCN—Journal of the National Comprehensive Cancer Network
Ms. Green has disclosed that she has no relevant financial relationships.
CE AUTHORS Nicole B. Harrold, BS, Manager, Continuing Education and Grants
Ms. Harrold has disclosed that she has no relevant financial relationships.
Kristina M. Gregory, RN, MSN, OCN, Vice President, Clinical Information Operations
Ms. Gregory has disclosed that she has no relevant financial relationships.
References
- 1.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 2.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stephens PJ, Tarpey PS, Davies H, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486:400–404. doi: 10.1038/nature11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachman KE, Argani P, Samuels Y, et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther. 2004;3:772–775. doi: 10.4161/cbt.3.8.994. [DOI] [PubMed] [Google Scholar]
- 6.Saal LH, Holm K, Maurer M, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2554–2559. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- 7.Lauring J, Cosgrove DP, Fontana S, et al. Knock in of the AKT1 E17K mutation in human breast epithelial cells does not recapitulate oncogenic PIK3CA mutations. Oncogene. 2010;29:2337–2345. doi: 10.1038/onc.2009.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpten JD, Faber AL, Horn C, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 9.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isakoff SJ, Engelman JA, Irie HY, et al. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res. 2005;65:10992–11000. doi: 10.1158/0008-5472.CAN-05-2612. [DOI] [PubMed] [Google Scholar]
- 11.Gustin JP, Karakas B, Weiss MB, et al. Knockin of mutant PIK3CA activates multiple oncogenic pathways. Proc Natl Acad Sci U S A. 2009;106:2835–2840. doi: 10.1073/pnas.0813351106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao JJ, Liu Z, Wang L, et al. The oncogenic properties of mutant p110alpha and p110beta phosphatidylinositol 3-kinases in human mammary epithelial cells. Proc Natl Acad Sci U S A. 2005;102:18443–18448. doi: 10.1073/pnas.0508988102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu P, Cheng H, Santiago S, et al. Oncogenic PIK3CA-driven mammary tumors frequently recur via PI3K pathway-dependent and PI3K pathway-independent mechanisms. Nat Med. 2011;17:1116–1120. doi: 10.1038/nm.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer DS, Brinkhaus H, Muller U, et al. Luminal expression of PIK3CA mutant H1047R in the mammary gland induces heterogeneous tumors. Cancer Res. 2011;71:4344–4351. doi: 10.1158/0008-5472.CAN-10-3827. [DOI] [PubMed] [Google Scholar]
- 15.Stoica GE, Franke TF, Moroni M, et al. Effect of estradiol on estrogen receptor-alpha gene expression and activity can be modulated by the ErbB2/PI 3-K/Akt pathway. Oncogene. 2003;22:7998–8011. doi: 10.1038/sj.onc.1206769. [DOI] [PubMed] [Google Scholar]
- 16.Stoica GE, Franke TF, Wellstein A, et al. Estradiol rapidly activates Akt via the ErbB2 signaling pathway. Mol Endocrinol. 2003;17:818–830. doi: 10.1210/me.2002-0330. [DOI] [PubMed] [Google Scholar]
- 17.Simoncini T, Hafezi-Moghadam A, Brazil DP, et al. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell RA, Bhat-Nakshatri P, Patel NM, et al. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001;276:9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 19.Miller TW, Perez-Torres M, Narasanna A, et al. Loss of Phosphatase and Tensin homologue deleted on chromosome 10 engages ErbB3 and insulin-like growth factor-I receptor signaling to promote antiestrogen resistance in breast cancer. Cancer Res. 2009;69:4192–4201. doi: 10.1158/0008-5472.CAN-09-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beeram M, Tan QT, Tekmal RR, et al. Akt-induced endocrine therapy resistance is reversed by inhibition of mTOR signaling. Ann Oncol. 2007;18:1323–1328. doi: 10.1093/annonc/mdm170. [DOI] [PubMed] [Google Scholar]
- 21.Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol Cancer Ther. 2002;1:707–717. [PubMed] [Google Scholar]
- 22.Santen RJ, Song RX, Zhang Z, et al. Long-term estradiol deprivation in breast cancer cells up-regulates growth factor signaling and enhances estrogen sensitivity. Endocr Relat Cancer. 2005;12(Suppl 1):S61–73. doi: 10.1677/erc.1.01018. [DOI] [PubMed] [Google Scholar]
- 23.Martin MB, Franke TF, Stoica GE, et al. A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology. 2000;141:4503–4511. doi: 10.1210/endo.141.12.7836. [DOI] [PubMed] [Google Scholar]
- 24.Cavazzoni A, Bonelli MA, Fumarola C, et al. Overcoming acquired resistance to letrozole by targeting the PI3K/AKT/mTOR pathway in breast cancer cell clones. Cancer Lett. 2012;323:77–87. doi: 10.1016/j.canlet.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 25.Ghayad SE, Bieche I, Vendrell JA, et al. mTOR inhibition reverses acquired endocrine therapy resistance of breast cancer cells at the cell proliferation and gene-expression levels. Cancer Sci. 2008;99:1992–2003. doi: 10.1111/j.1349-7006.2008.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boulay A, Rudloff J, Ye J, Zumstein-Mecker S, et al. Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer. Clin Cancer Res. 2005;11:5319–5328. doi: 10.1158/1078-0432.CCR-04-2402. [DOI] [PubMed] [Google Scholar]
- 27.deGraffenried LA, Friedrichs WE, Russell DH, et al. Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt Activity. Clin Cancer Res. 2004;10:8059–8067. doi: 10.1158/1078-0432.CCR-04-0035. [DOI] [PubMed] [Google Scholar]
- 28.Miller TW, Hennessy BT, Gonzalez-Angulo AM, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120:2406–2413. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung E, Kim JE, Rewcastle GW, et al. Comparison of the effects of the PI3K/mTOR inhibitors NVP-BEZ235 and GSK2126458 on tamoxifen-resistant breast cancer cells. Cancer Biol Ther. 2011;11:938–946. doi: 10.4161/cbt.11.11.15527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yue W, Fan P, Wang J, et al. Mechanisms of acquired resistance to endocrine therapy in hormone-dependent breast cancer cells. J Steroid Biochem Mol Biol. 2007;106:102–110. doi: 10.1016/j.jsbmb.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 32.Lu CH, Wyszomierski SL, Tseng LM, et al. Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin Cancer Res. 2007;13:5883–5888. doi: 10.1158/1078-0432.CCR-06-2837. [DOI] [PubMed] [Google Scholar]
- 33.Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 34.Xia W, Husain I, Liu L, et al. Lapatinib antitumor activity is not dependent upon phosphatase and tensin homologue deleted on chromosome 10 in ErbB2-overexpressing breast cancers. Cancer Res. 2007;67:1170–1175. doi: 10.1158/0008-5472.CAN-06-2101. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien NA, Browne BC, Chow L, et al. Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol Cancer Ther. 2010;9:1489–1502. doi: 10.1158/1535-7163.MCT-09-1171. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Zhang Q, Zhang J, et al. PI3K pathway activation results in low efficacy of both trastuzumab and lapatinib. BMC Cancer. 2011;11:248. doi: 10.1186/1471-2407-11-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen JD, Knoop A, Laenkholm AV, et al. PIK3CA mutations, PTEN, and pHER2 expression and impact on outcome in HER2-positive early-stage breast cancer patients treated with adjuvant chemotherapy and trastuzumab. Ann Oncol. 2012;23:2034–2042. doi: 10.1093/annonc/mdr546. [DOI] [PubMed] [Google Scholar]
- 38.Dave B, Migliaccio I, Gutierrez MC, et al. Loss of phosphatase and tensin homolog or phosphoinositol-3 kinase activation and response to trastuzumab or lapatinib in human epidermal growth factor receptor 2-overexpressing locally advanced breast cancers. J Clin Oncol. 2011;29:166–173. doi: 10.1200/JCO.2009.27.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Razis E, Bobos M, Kotoula V, et al. Evaluation of the association of PIK3CA mutations and PTEN loss with efficacy of trastuzumab therapy in metastatic breast cancer. Breast Cancer Res Treat. 2011;128:447–456. doi: 10.1007/s10549-011-1572-5. [DOI] [PubMed] [Google Scholar]
- 40.Morrow PK, Wulf GM, Ensor J, et al. Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy. J Clin Oncol. 2011;29:3126–3132. doi: 10.1200/JCO.2010.32.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chakrabarty A, Sanchez V, Kuba MG, et al. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc Natl Acad Sci U S A. 2012;109:2718–2723. doi: 10.1073/pnas.1018001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chandarlapaty S, Sawai A, Scaltriti M, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engelman JA, Chen L, Tan X, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Nicolantonio F, Arena S, Tabernero J, et al. Deregulation of the PI3K and KRAS signaling pathways in human cancer cells determines their response to everolimus. J Clin Invest. 2010;120:2858–2866. doi: 10.1172/JCI37539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macaskill EJ, Bartlett JM, Sabine VS, et al. The mammalian target of rapamycin inhibitor everolimus (RAD001) in early breast cancer: results of a pre-operative study. Breast Cancer Res Treat. 2011;128:725–734. doi: 10.1007/s10549-010-0967-z. [DOI] [PubMed] [Google Scholar]
- 48.Meric-Bernstam F, Akcakanat A, Chen H, et al. PIK3CA/PTEN mutations and Akt activation as markers of sensitivity to allosteric mTOR inhibitors. Clin Cancer Res. 2012;18:1777–1789. doi: 10.1158/1078-0432.CCR-11-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brachmann SM, Hofmann I, Schnell C, et al. Specific apoptosis induction by the dual PI3K/mTor inhibitor NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells. Proc Natl Acad Sci U S A. 2009;106:22299–22304. doi: 10.1073/pnas.0905152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serra V, Markman B, Scaltriti M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 51.Weigelt B, Warne PH, Downward J. PIK3CA mutation, but not PTEN loss of function, determines the sensitivity of breast cancer cells to mTOR inhibitory drugs. Oncogene. 2011;30:3222–3233. doi: 10.1038/onc.2011.42. [DOI] [PubMed] [Google Scholar]
- 52.O'Brien C, Wallin JJ, Sampath D, et al. Predictive biomarkers of sensitivity to the phosphatidylinositol 3’ kinase inhibitor GDC-0941 in breast cancer preclinical models. Clin Cancer Res. 2010;16:3670–3683. doi: 10.1158/1078-0432.CCR-09-2828. [DOI] [PubMed] [Google Scholar]
- 53.Dan S, Okamura M, Seki M, et al. Correlating phosphatidylinositol 3-kinase inhibitor efficacy with signaling pathway status: in silico and biological evaluations. Cancer Res. 2010;70:4982–4994. doi: 10.1158/0008-5472.CAN-09-4172. [DOI] [PubMed] [Google Scholar]
- 54.Janku F, Wheler JJ, Naing A, et al. PIK3CA mutation H1047R is associated with response to PI3K/AKT/mTOR signaling pathway inhibitors in early-phase clinical trials. Cancer Res. 2013;73:276–284. doi: 10.1158/0008-5472.CAN-12-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janku F, Wheler JJ, Westin SN, et al. PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations. J Clin Oncol. 2012;30:777–782. doi: 10.1200/JCO.2011.36.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fleming GF, Ma CX, Huo D, et al. Phase II trial of temsirolimus in patients with metastatic breast cancer. Breast Cancer Res Treat. 2012;136:355–363. doi: 10.1007/s10549-011-1910-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iyer G, Hanrahan AJ, Milowsky MI, et al. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338:221. doi: 10.1126/science.1226344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ellard SL, Clemons M, Gelmon KA, et al. Randomized phase II study comparing two schedules of everolimus in patients with recurrent/metastatic breast cancer: NCIC Clinical Trials Group IND.163. J Clin Oncol. 2009;27:4536–4541. doi: 10.1200/JCO.2008.21.3033. [DOI] [PubMed] [Google Scholar]
- 59.Chan S, Scheulen ME, Johnston S, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2005;23:5314–5322. doi: 10.1200/JCO.2005.66.130. [DOI] [PubMed] [Google Scholar]
- 60.Baselga J, Semiglazov V, van Dam P, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2630–2637. doi: 10.1200/JCO.2008.18.8391. [DOI] [PubMed] [Google Scholar]
- 61.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolff AC, Lazar AA, Bondarenko I, et al. Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol. 2013;31:195–202. doi: 10.1200/JCO.2011.38.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bachelot T, Bourgier C, Cropet C, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol. 2012;30:2718–2724. doi: 10.1200/JCO.2011.39.0708. [DOI] [PubMed] [Google Scholar]
- 64.Chavez-Mac Gregor M, Barlow WE, Gonzalez-Angulo AM, et al. A phase III randomized, placebo-controlled clinical trial evaluating the use of adjuvant endocrine therapy +/− one year of everolimus in patients with high-risk, hormone receptor-(HR) positive and Her2-negative breast cancer: SWOG/NSABP S1207. Cancer Res. 2012;72:42s. [Google Scholar]
- 65.Hartford CM, Desai AA, Janisch L, et al. A phase I trial to determine the safety, tolerability, and maximum tolerated dose of deforolimus in patients with advanced malignancies. Clin Cancer Res. 2009;15:1428–1434. doi: 10.1158/1078-0432.CCR-08-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banerji U, Dean EJ, Gonzalez M, et al. First-in-human phase I trial of the dual mTORC1 and mTORC2 inhibitor AZD2014 in solid tumors [abstract]. J Clin Oncol. 2012:30. Abstract 3004. [Google Scholar]
- 67.Yap TA, Yan L, Patnaik A, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 68.Hoffman K, Holmes FA, Fraschini G, et al. Phase I-II study: triciribine (tricyclic nucleoside phosphate) for metastatic breast cancer. Cancer Chemother Pharmacol. 1996;37:254–258. doi: 10.1007/BF00688325. [DOI] [PubMed] [Google Scholar]
- 69.Shuttleworth SJ, Silva FA, Cecil AR, et al. Progress in the preclinical discovery and clinical development of class I and dual class I/IV phosphoinositide 3-kinase (PI3K) inhibitors. Curr Med Chem. 2011;18:2686–2714. doi: 10.2174/092986711796011229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 71.Juric D, Rodon J, Gonzalez-Angulo AM, et al. BYL719, a next generation PI3K alpha specific inhibitor: preliminary safety, PK, and efficacy results for first-in-human study [abstract]. Cancer Res. 2012;72(8 Suppl) Abstract CT-01. [Google Scholar]