Abstract
The loss of Ca2+ homeostasis during cerebral ischemia is a hallmark of impending neuronal demise. Accordingly, considerable cellular resources are expended in maintaining low resting cytosolic levels of Ca2+. These include contributions by a host of proteins involved in the sequestration and transport of Ca2+, many of which are expressed within intracellular organelles, including lysosomes, mitochondria as well as the endoplasmic reticulum (ER). Ca2+ sequestration by the ER contributes to cytosolic Ca2+ dynamics and homeostasis. Furthermore, within the ER Ca2+ plays a central role in regulating a host of physiological processes. Conversely, impaired ER Ca2+ homeostasis is an important trigger of pathological processes. Here we review a growing body of evidence suggesting that ER dysfunction is an important factor contributing to neuronal injury and loss post-ischemia. Specifically, the contribution of the ER to cytosolic Ca2+ elevations during ischemia will be considered, as will the signalling cascades recruited as a consequence of disrupting ER homeostasis and function.
Keywords: Ca2+ homeostasis, ischemia, ER stress, IP3R, RyR, SERCA, unfolded protein response(UPR), neuronal cell death
The endoplasmic reticulum (ER), an important organelle present in all eukaryotic cells, consists of a continuous network of tubules, cisterns and vesicles. The ER contributes to the synthesis of membrane lipids and proteins. It also contributes to the regulation of intracellular calcium dynamics. Nowhere is this more evident than in neurons where the ER has been proposed to function as a “neuron-within-a-neuron”1 due to its ability to rapidly integrate and respond to Ca2+ signals initiated at the plasma membrane (PM). Extending from dendritic spines, through the cell body, axon, and into presynaptic terminals, the ER contributes to all aspects of neuronal function including transmitter release, synaptic plasticity and gene transcription1,2.
The ability of the ER to integrate and contribute to rapid Ca2+ signalling is predicated upon its capacity to store, buffer and release Ca2+ to and from the cytosol. The intraluminal ER Ca2+ concentration ([Ca2+]ER) is primarily determined through the concerted activities of resident Ca2+ channels, transporters and Ca2+ binding proteins. An extensive review of all mechanisms contributing to the regulation of Ca2+ within the ER is beyond the scope of this review, but has been covered elsewhere1,3. Rather, in the following sections we will focus on ER-based mechanisms that have been proposed to contribute to neuronal cell injury and death in ischemic models of stroke.
Loss of ER Ca2+ homeostasis during ischemia
Obstructed blood flow during a stroke initiates a cascade of events that culminate in neuronal cell death. Deprived of oxygen and glucose, cellular energy stores (ie ATP levels) are rapidly depleted and ionic homeostasis is no longer possible; neurons begin to depolarize and release their transmitter stores. The resulting massive release of the excitatory transmitter glutamate provokes further depolarization due to activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPARs) and N-methyl-D-aspartate (NMDARs) glutamate receptor, as well as voltage-gated Ca2+ channels. Importantly, as NMDARs are permeable to Ca2+, neurons begin to accumulate toxic levels of this cation. The resulting pathological rise in Ca2+ triggers numerous downstream effectors and contributes to the generation of reactive oxygen and nitrogen species (ROS/RNS). As a result of the conditions prevailing during ischemia (ie hypoglycemia, elevated cytosolic Ca2+, ROS/RNS, etc) ER homeostasis is disrupted, resulting in Ca2+ store depletion4,5,6. Mechanistically, depletion of Ca2+ stores can be achieved through facilitated release and/or reduced re-uptake to and from the cytosol, respectively. Evidence suggests that Ca2+-store depletion during ischemia occurs as a result of both aberrant release, primarily achieved through ryanodine and IP3 receptor channels (RyRs and IP3Rs, respectively), as well as impaired re-uptake, mediated by the sarcoplasmic/endoplasmic reticulum ATPase (SERCA).
Ryanodine receptor channels (RyRs)
The RyR family consists of three members: RyR1, RyR2, and RyR3. While all of the isoforms are expressed within the CNS, RyR2 is considered the predominant neuronal isoform7. Within neurons, immunocytochemical evidence suggests a broad subcellular distribution of RyRs extending from the perikaryon to dendrites and even spines, where they have been shown to contribute to the generation of highly localized Ca2+ signals8 important for synaptic plasticity9 10 11 12. RyRs are predominantly gated by elevations in intracellular Ca2+, a process referred to as Ca2+-induced Ca2+ release (CICR)13. The activity of RyRs is further regulated by a number of intracellular modulators, including Mg2+, ATP, and cyclic ADP ribose (cADPR). In post-ischemic neurons, intracellular Ca2+ levels are elevated as a result of the excitotoxic activation of Ca2+ permeable NMDARs and voltage-gated Ca2+ channels. However, additional Ca2+ permeable channels, including TRP, ASIC, and pannexin channels14,15,16,17, are also likely to make important contributions in this respect. Consequently, elevated intracellular Ca2+ promotes RyR activation, resulting in Ca2+ release from the ER and a further rise in intracellular Ca2+ levels.
Aberrant RyR activation during ischemia is exacerbated through the post-translational modification of RyRs by ROS/RNS. This is proposed to occur through redox modification of cysteine residues, as first shown for RyRs expressed in cardiac muscle18. The relevance of such a mechanism to brain ischemia was demonstrated by Bull19 who demonstrated that S-glutathionylation of RyR2 augments CICR and proposed that such a mechanism might contribute to cortical neuronal death. Mechanistically, augmented RyR channel activity during ischemia may be due to an increased sensitivity of the oxidized channels to activation by Ca2+ and a reduced sensitivity to inhibition by Mg2+20. Moreover, maximal activation can be attained at lower concentrations of ATP for oxidized RyR channels21. Alternatively, Kakizawa22 recently showed that nitric oxide (NO) can induce RyR1 activation through S-nitrosylation at a specific cysteine residue (C3635) and evoke Ca2+ release from the ER. Using cultured neurons derived from RyR1−/− mice, in which NO-induced Ca2+ release is absent, they demonstrated that NO-induced neuronal cell death was reduced. Given the important contribution of NO to neuronal injury following ischemia23, the authors propose that NO-induced RyR1 activation could contribute to ischemic cell death. Regardless of the underlying mechanism, the contribution of RyR activation to cell death during ischemia is underscored by results demonstrating neuroprotection following treatment with dantrolene24,25,26, a blocker of this particular family of channels.
Inositol triphosphate receptor channels (IP3Rs)
In addition to RyRs, ER Ca2+ can also be released during ischemia through IP3R-dependent mechanisms. Like RyRs, three isoforms have been identified (IP3R1-3), with all isoforms being present within the CNS. While IP3R2 is strictly expressed within glial cells, both IP3R1 and IP3R3 are expressed neuronally. Each neuronal isoform is differentially expressed during development, IP3R1 being the predominant adult form27. As their names imply, the primary means of activating these receptors is through the intracellular production of IP3 downstream of phospholipase C (PLC) activation. Oxygen-glucose deprivation (OGD) followed by reoxygenation (REOX) has been shown to cause a dramatic increase in IP3 levels in cultured cortical neurons28 associated with IP3R-dependent Ca2+ release from the ER. Consistent with this, in a separate study, inhibition of PLC has been shown to protect cultured neurons from mild excitotoxic insult29. OGD/REOX-induced ER Ca2+ release was contingent on the PLC-coupled metabotropic glutamate receptor, mGluR1. Moreover, inhibition of mGluR1 receptors protected neurons from cell death, consistent with previous reports of neuroprotection by group I, mGluR antagonists30.
In addition to being gated by IP3, IP3R activation is regulated by Ca2+ thus allowing IP3Rs to contribute to CICR31,32,33,34. In this way, Ca2+ influx contributed by NMDARs, for example, can promote IP3R activation. The importance of such a mechanism to excitotoxic cell death was highlighted in a study by Ruiz29 in which they demonstrated that the inhibition of IP3Rs was especially effective in reducing Ca2+ overload and cell death during excitotoxicity. Interestingly, inhibition of IP3Rs may provide a neuroprotective effect by attenuating mitochondrial damage. Indeed, recent evidence has shown that IP3Rs are enriched at regions of close contact between the ER and mitochondria, called MAMs (mitochondria-associated membranes, for review see Decuypere35). Here IP3Rs play a critical role in initiating Ca2+ exchange between these two organelles under both physiological and pathological conditions. Ruiz et al demonstrated that the inhibition of IP3Rs prevented the loss of mitochondrial membrane potential induced by NMDA treatment of cultured neurons. Furthermore, inhibition of IP3Rs largely prevented NMDA-induced caspase-3 activation, whereas inhibition of RyRs was ineffective. This model may have important implications as recruitment of mitochondrial-mediated cell death pathways contribute to ischemic neuronal cell loss36.
Sarcoplasmic/endoplasmic Ca2+-ATPase (SERCA)
Ca2+ homeostasis within the ER, and indeed more broadly within the cytosol, is further compromised during ischemia as a result of the impairment of the SERCA. The primary transport mechanism responsible for the uptake of Ca2+ from the cytosol to the ER, SERCA pumps are encoded by a family of 3 highly homologous genes, with alternative splicing of SERCA2 generating further diversity (SERCA2a and SERCA2b). Of the two splice forms identified, SERCA2b is the dominant neuronal form37. Ischemia has been shown to cause inhibition of Ca2+ sequestration within the ER as a result of decreased SERCA activity38. As ATP is required for transport, inhibition of Ca2+ uptake by SERCA is likely a consequence of ischemia-induced ATP-depletion. However, recent evidence suggests that additional factors contribute to the associated inhibition of SERCA activity. Indeed, ATPase activity has been shown to be uncoupled from Ca2+ as a result of ischemia39. Mechanistically, inhibition of SERCA activity may be caused by the associated rise in ROS/RNS as several reports have shown reduced SERCA activity under conditions of oxidative/nitrosative stress40,41,42, including more specifically for SERCA2b43, the predominant neuronal isoform. Modifications of reactive tyrosine (protein nitration) and cysteine (S-glutathionylation) residues are thought to underlie the inhibition of pump activity. More specifically, hydroxyl radicals have been shown to disrupt the Ca2+-ATPase activity by attacking the ATP binding site, presumably through modification of cysteine residues localized within the active site of the enzyme44. Additionally, tyrosine nitration in response to peroxynitrate application has been proposed to affect SERCA activity45,46. The resulting protein modification targeted tyrosine residues in proximity to sites essential for Ca2+ translocation. Irrespective of the mechanistic basis, it is worth noting that inhibition of SERCA activity, by application of specific inhibitors (eg thapsigargin), is sufficient to disrupt ER function, leading to ER stress and the activation of downstream signalling cascades capable of initiating cell death.
ER response to ischemia
The evidence summarized in the preceding sections highlights mechanisms through which ER Ca2+ stores are depleted during ischemia. The release of Ca2+ from stores passively contributes to neuronal injury through the resulting rise of cytosolic Ca2+; however, the loss of ER Ca2+ homeostasis and resulting disruption of ER function may be equally meaningful in this respect. In addition to Ca2+ signalling, the ER contributes to the post-translational processing, folding and export of proteins47,48. This essential function of the ER is mediated by a complex multi-protein network of molecular chaperones and foldases, most commonly protein-disulfide-isomerase, binding immunoglobulin protein (BiP), calnexin and calreticulin. Critically, many of the proteins that assist with protein folding are reliant on [Ca2+]ER47,48. Moreover, in binding Ca2+ these same proteins contribute to ER Ca2+ homeostasis. For example, it is estimated that BiP, an Hsp70 family member, accounts for around 25% of Ca2+ storage within the ER49. Accordingly, protein folding and Ca2+ homeostasis within the ER are tightly coupled47,48,50. Consequently, disruption of luminal Ca2+ homeostasis leads to the accumulation of unfolded/misfolded proteins in the ER lumen, thereby causing ER stress. Interestingly, protein aggregates have been shown to accumulate following transient cerebral ischemia51,52,53. Severe protein aggregate formation was observed in vulnerable CA1 pyramidal neurons destined to die, but not in surviving neurons of the dentate gyrus, CA3 or cortex. Moreover, aggregate formation coincided with the time course of cell death. Further support for some intimate relation between protein aggregation and cell death comes from the finding that ischemic preconditioning, in which brief sublethal ischemic episodes confer resistance to subsequent ischemic insult, reduces protein aggregate formation and cell death in a model of transient ischemia54. Preconditioning is known to induce an array of stress response genes, including molecular chaperones, which are expected to counter the accumulation of misfolded proteins observed following ischemia.
The accumulation of misfolded proteins within the ER (ER stress) triggers a pro-survival adaptation, the unfolded protein response (UPR)55,56. Three ER resident proteins are responsible for initiating UPR; 1) PERK (double-stranded RNA-activated protein kinase-like ER kinase, 2) IRE1 (inositol requiring enzyme 1) and 3) ATF6 (activating transcription factor-6). Each of these single-pass transmembrane proteins functions as transducers relaying protein folding status within the ER lumen to the nucleus and cytosol through phosphorylation events as well as the generation and translocation of transcription factors.
The accumulation of misfolded proteins is thought to trigger the UPR by disrupting the association of PERK, IRE1, and ATF6 with BiP (binding immunoglobulin protein), a member of the HSP70 chaperone family and one of the most highly expressed proteins within the ER (Figure 1). A multifunctional protein, BiP possesses an N-terminal ATPase activity, but can also bind to the hydrophobic moiety of nascent unfolded proteins through its C-terminal peptide binding domain. Through its interaction with the luminal domains of UPR transducers, BiP is thought to constrain their signalling. The accumulation of unfolded proteins competitively displaces BiP, thereby initiating signalling downstream of three main arms of the UPR (however, note recent evidence suggesting that IRE1 may signal independently of BiP57). IRE1 is a serine/threonine protein kinase and endoribonuclease. Its activation is contingent on oligomerization and trans-autophosphorylation55,56. Signalling by IRE1 proceeds through a non-conventional splicing of mRNA transcripts for XBP1, a transcription factor. Like IRE1, PERK possesses kinase activity and is activated through self-assembly and autophosphorylation. Activated PERK primarily mediates translation attenuation through the phosphorylation of eIF2α (eukaryotic translation initiation factor 2α), an initiation factor required for protein translation. Lastly, ATF6 is a membrane-anchored transcription factor whose signalling is initiated following translocation to the Golgi, where it is subjected to regulated proteolysis. As a result of which, the cytoplasmic transcriptional domain (ATF6f) is released and translocates to the nucleus to affect changes in the expression of genes involved with chaperone activity and the degradation of misfolded proteins58. By reducing de novo protein synthesis and increasing the expression of ER resident chaperones, the UPR seeks to re-establish ER homeostasis by increasing misfolded protein handling capacity. However, if homeostasis cannot be restored, the main signalling pathways of the UPR are subverted from pro-survival to pro-apoptotic processes50,59. Accordingly, in their active form, UPR transducers serve as ER stress markers. Critically, cumulative evidence suggests that all three signalling arms of the UPR are activated following ischemia28,60,61,62,63,64,65,66,67,68,69,70,71.
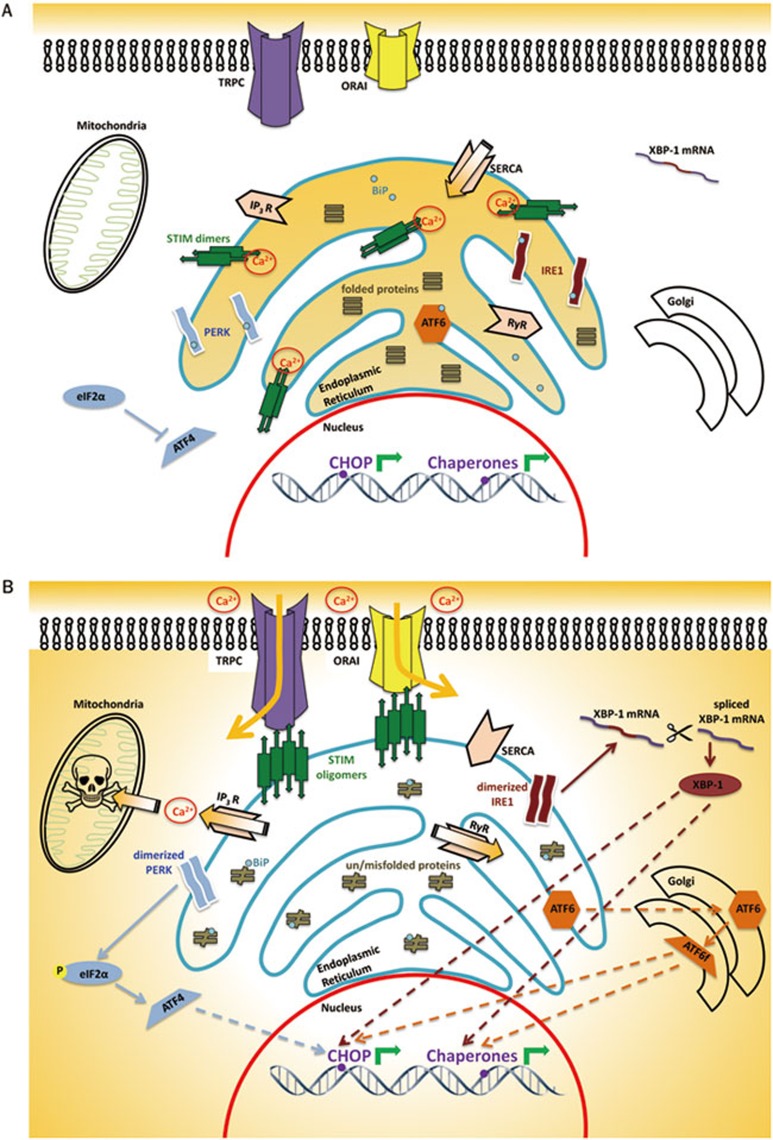
Figure 1.
(A) At physiological levels of luminal Ca2+, BiP remains bound to PERK, IRE1 and ATF6, suppressing their signalling activity. (B) Ischemia induced depletion of ER luminal calcium. ER protein folding capacity is exceeded causing competitive displacement of BiP from PERK, IRE1 and ATF6. Signalling pathways of the unfolded protein response are triggered when BiP dissociates from PERK, IRE1, and ATF6 allowing their dimerization and activation. In turn, transcription of CHOP and ER chaperones is upregulated. Release of ER Ca2+, particularly through IP3Rs, promotes uptake into mitochondria leading to mitochondrial injury and apoptosis. Store depletion further exacerbates the loss of cytosolic Ca2+ homeostasis through STIM-dependent signalling, possibly involving surface expressed Ca2+ permeable channels.
UPR activation during ischemia
Although the underlying mechanisms was not recognized at the time, one of the earliest evidence of UPR activation came from studies demonstrating a long lasting inhibition of protein synthesis following cerebral ischemia. We now know that increased eIF2α phosphorylation contributes to the observed suppression of protein synthesis. In this respect increased PERK activity has been well documented in several studies following ischemia72,73,74,75,76 as well as in cellular models of ischemia77. Increased PERK activity following ischemia can be inferred from its decreased association with BiP72, increased autophosphorylation as well as through increased phosphorylation of its target substrate eIF2α. Definitive evidence that PERK is responsible for eIF2α phosphorylation comes from a study by Owen76 in which they examined the consequence of ischemia/reperfusion in transgenic mice with targeted disruption of the PERK gene. They noted that in the absence of PERK expression, basal phosphorylation of eIF2α was reduced. More importantly, the increase in eIF2α phosphorylation following transient ischemia was completely prevented and a substantial rescue of protein synthesis was observed. An important consideration arising from these studies is whether protein synthesis inhibition is neuroprotective or cytotoxic. Circumstantially, increased eIF2α phosphorylation and transient inhibition of protein synthesis occurs in all post-ischemic neurons and while protein synthesis eventually recovers in regions resistant to ischemia-induced cell death, it remains depressed in vulnerable regions, suggesting that transient inhibition may serve as a protective mechanism. Consistent with this, a broad-based inactivation of eIF2α and protein synthesis inhibition is observed in neurons following ischemic preconditioning78. Moreover, salubrinal, an inhibitor of GADD34/PP1X, the phosphatase responsible for eIF2α dephosphorylation, has similarly been shown to be neuroprotective following acute ischemia29. Results from these and other studies suggest that transient inhibition of protein synthesis per se is not cytotoxic; in stark contrast, prolonged protein inhibition may serve as an indicator of impending neuronal demise78. It is however important to point out that mechanisms distinct from those involving PERK/eIF2α have been proposed to underlie long lasting inhibition of protein synthesis. Accordingly, eIF2α may be appropriate as a marker of ER stress, just not as a marker of impending neuronal demise. As will be discussed below, ER stress markers activated on a more protracted time course are more appropriate in this respect.
One of the most consistently reported markers of ER stress following ischemia is the up-regulation of BiP28,60,61,62,63,64,65,66,67,68,69,70,71. As outlined previously, BiP functions as the master regulator of the UPR. Yet BiP is also subject to transcriptional regulation downstream of UPR activation. Although the precise mechanisms contributing to BiP upregulation following ischemia have yet to be examined, previous work has proposed that BiP expression can be upregulated by ATF6 as well as by IRE1/XBP158. Recent evidence suggests that ATF6 signalling is not engaged following ischemia74,75, suggesting IRE1/XBP1 pathways may predominate in inducing BiP expression during ischemia-induced ER stress79. Note however, that ATF6 activation has been observed in cultured neurons following treatment with kainic acid (excitotoxic model of neuronal cell death)68, suggesting that ATF6 signalling may participate under some circumstances. BiP upregulation serves to protect the ER through several distinct mechanisms; 1) re-establishment of Ca2+ homeostasis, 2) protein folding and 3) suppression of ER stress signalling. The importance of BiP is underscored by several studies examining the effects of altered BiP expression on cell fate. Downregulation of BiP exacerbates cell death in response to excitotoxic insult71, conversely, its upregulation has been shown to have a neuroprotective effect62,63,71,80. With this knowlege in mind, Kudo et al62 used high throughput screening and identified Bix (BiP inducer X), a compound capable of inducing BiP expression. They demonstrated that Bix treatment could induce a 3-fold increase in BiP expression in a neuroblastoma cell line. More importantly, they went on to show that Bix treatment reduced infarct area, brain swelling and apoptosis in the ischemic penumbral regions following focal cerebral ischemia. Critically, the neuroprotective effect of Bix is preserved even if treatment is delayed for up to 3 h81, suggesting Bix may have potential therapeutic applications in stroke.
ER-initiated cell death cascades
The evidence summarized so far highlights early ER signalling events (eg increased phosphorylation of eIF2α by PERK, induction of BiP expression)82,83 that are triggered following ischemia and play a protective role attempting to restore cellular homeostasis. However, if balance cannot be restored, then elements of these same signalling pathways trigger pro-apoptotic processes. One of the best characterized cascades in this respect is through the induction of the transcription factor CHOP (C/EBP homologous protein, also known as GADD153). Indeed, in numerous cell types, overexpression of CHOP has been shown to induce apoptosis and, conversely, knockout of CHOP renders cells more resistant to cell death69,84,85,86. The promoter region for CHOP contains binding sites for ATF6 as well XBP1, allowing activation by each of these transcription factors. In addition, CHOP can be activated by activating transcription factor 4 (ATF4), itself regulated downstream of PERK/eIF2α. Accordingly, CHOP expression can be promoted through all 3 main arms of the UPR87. Increased neuronal CHOP expression has been demonstrated in response to transient forebrain ischemia61,63,69,88,89,90,91,92 as well as kainic acid treatment of cultured hippocampal neurons91. Increased CHOP expression is also observed in astrocytes OGD60. More recently, clarification of the mechanisms through which CHOP mediates cell death has emerged. CHOP has been reported to sensitize cells to ER stress through downregulation of the antiapoptotic protein Bcl-293. Moreover, expression of the pro-apoptotic mediator Bim is augmented by CHOP94. Of direct relevance to mechanisms contributing to neuronal apoptosis, CHOP was recently shown to bind to the promoter region of Puma (p53 upregulated modulator of apoptosis) and induce its expression84. CHOP-induced expression of Puma was shown to be critical for ER stress-mediated neuronal cell death. Accordingly, through transcriptional regulation, CHOP has been proposed to facilitate cell death by altering the balance between pro- and anti-apoptotic Bcl-2 family members95. The importance of CHOP as a contributor to ischemic neuronal cell death was demonstrated through the use of reverse genetic approaches69,96.
Apoptotic cell death induced by ER stress is ultimately effected through recruitment of caspases that contribute to the morphological and biochemical changes that are characteristic of this form of cell death97. Caspase-12 localizes to the ER and a number of studies have shown that it is subject to proteolytic processing as a consequence of extended ER stress98. Caspase-12 is proposed to function as an inducer caspase initiating the sequential recruitment of effector caspases-9 and -399. That being said, the importance of caspase-12 has been questioned by reports suggesting it is not always strictly necessary for ER stress-induced apoptosis100. Nevertheless, caspase-12 has been implicated in cell death associated with Alzheimer's disease101, prion disease102 as well as cerebral ischemia28,66,67,68,77,89,90,103,28,66,67,68,77,89,90,103. Several pathways leading to the recruitment of caspase-12 have been proposed. Like other caspase family members, caspase-12 is synthesized as a proenzyme and requires proteolytic processing to become active. In contrast to apoptotic pathways involving mitochondria, activation of caspase-12 is not reliant on cytochrome c. ER stress-induced mechanisms responsible for activation include proteolytic processing following translocation of cytosolic caspase-7104 to the ER surface as well as by Ca2+-dependent recruitment of calpain105. In addition, caspase-12 is capable of autolytic processing following its homodimerization, an activity promoted downstream of IRE1 activation106,107. Recent evidence has shown that proteolytic activation of procaspase-12 following OGD of cultured cortical neurons can be blocked by inhibitors of calpains, but not caspases, suggesting Ca2+-dependent calpain processing may predominate under these conditions77.
Store-operated Ca2+ entry, stromal-interacting molecules and ischemic neuronal cell death
The preceding section highlights an important and growing body of evidence implicating ER stress pathways as important contributors to the cascades of signalling events underlying neuronal injury and cell death following ischemia. A recurring theme in many of these studies is that depletion of ER Ca2+ stores, contributed through activation of RyRs and IP3Rs as well as impaired SERCA function, is an important trigger for ER stress. Consistent with this, depletion of ER Ca2+ stores through inhibition of SERCA activity is sufficient to initiate ER stress and cell death in a variety of cell types108. Typically, ER stress is operationally defined on the basis of UPR induction. However, canonical UPR signalling is unlikely to represent the earliest response of the ER to cellular stressors, which may include depletion of ATP, loss of Ca2+ homeostasis and ROS/RNS. Interestingly, emerging evidence suggests that additional non-canonical stress sensors may also contribute to neuronal cell death during ischemia. Specifically, STIM (stromal-interacting molecules) proteins, which function as ER resident Ca2+ sensors, have recently been identified as important contributors to neuronal cell death post-ischemia.
In the mid-1980s, it was proposed that when ER stores are depleted of Ca2+, a refilling process via Ca2+ influx involving ER proteins and plasma membrane channels is utilized109. This process has been termed store-operated Ca2+ entry (SOCE), sometimes referred to as capacitive calcium entry (CCE). The Ca2+ dependence of ER chaperones and foldases makes correct protein folding in the ER contingent on luminal Ca2+ levels being maintained by steady refilling processes thus avoiding activation of ER stress pathways. Recently identified ER resident proteins, stromal interaction molecules 1 and 2 (STIM1 and STIM2), act as Ca2+ sensors and relay messages of alterations in luminal Ca2+ to the plasma membrane110 where they interact directly with Ca2+ influx channels111. This interaction is a constituent of the aforementioned SOCE.
The single-pass transmembrane proteins STIM1 and STIM2, originally named GOK and proposed to be involved in tumor suppression and modifications of cell morphology112, detect decreases in ER-luminal Ca2+ through their N-terminal Ca2+ binding EF-hand domains. Also at the N-terminal are dense clusters of sterile α motif (SAM) domains that function to stabilize STIM in a dimeric form when EF-hands are Ca2+ bound113. In the case of depleted Ca2+ stores, as seen in ischemia induced ER stress, Ca2+ dissociates from the EF-hand domains of STIM, unfolding and destabilizing the EF-SAM clusters. This promotes activation wherein STIM dimers aggregate into oligomeric STIM complexes114. Effectively, STIM behaves as a Ca2+ sensor and is responsible for relaying the status of ER Ca2+ stores to the plasma membrane. Translocation of STIM along the ER membrane to ER-PM junctions enables STIM interaction with Ca2+ influx channels expressed at the cell surface to facilitate SOCE. So far, members of the Orai, TRPC and L-type voltage-gated Ca2+ channel families have been identified as coupling targets for activated STIM111,115,116,117. Aside from sensing decreases in ER Ca2+ levels, STIM has recently been regarded as a general cellular stress sensor because it is also activated by hypoxia-induced acidosis118, ER stress in dopamine neurons119, oxidative stress120 and transient temperature changes121. Hypoxia itself can cause STIM1 activation likely as a result of lowering ATP levels, reducing the activity of SERCA pump and depletion of Ca2+ stores. In response to supraphysiological ROS levels, S-glutathionylation of Cys56 on STIM, adjacent to its EF-SAM domains, causes a dissociation of Ca2+ from STIM and subsequent SOCE without ER Ca2+ depletion120. Additionally, STIM1 has been shown to oligomerize and translocate to ER-PM junctions when cells are heated from 37–40 oC. Subsequent cooling back to 37 oC triggers Ca2+ influx, independent of ER Ca2+ levels, thus implicating STIM as a sensor to transient temperature change as well122.
The two isoforms, STIM1 and STIM2, possess homologous functional domains with the only differences between the two isoforms being slight variations in the amino acid sequences of their N and C termini. Functional consequences of these variations are in their affinities for Ca2+ binding, thus affecting each isoform's sensitivity of ER Ca2+ detection as well as activation kinetics and contribution to SOCE signalling123. The higher affinity isoform, STIM1, is rapidly activated under ER Ca2+ depletion making STIM1 the principal modulator of SOCE124. Contrastingly, the lower affinity STIM2 isoform, despite being more sensitive to ER Ca2+ depletion, demonstrates slower activation kinetics and is considered to be responsible for the maintenance of basal cytosolic and ER Ca2+ concentrations within tight limits125. This view has recently been called into question by Berna-erro et al126 as they implicated SOCE in ischemic neuronal cell death and label STIM2, rather than STIM1, as the critical mediator of SOCE. In their study, which asserted STIM2 as the predominant isoform in the brain, calcium-imaging experiments showed reduced SOCE in STIM2−/− but not in STIM1−/− or Orai1−/− mice. Furthermore, neurons from their STIM2−/− mice showed increased survival under hypoxic conditions and in vivo STIM2−/− mice under the middle-cerebral artery occlusion model of ischemic stroke did not demonstrate neurological damage. From this evidence, it was proposed that STIM2−/− mice are protected from ischemic stroke. In contrast to the findings of Berna-Erro, several other groups have detected neuronal STIM1 expression127,128,129. However, the precise contribution of STIM1 to neuronal function remains to be elucidated. In addition, a major unresolved question remains as to the identity of the plasma membrane Ca2+ channel responsible for STIM2-initiated Ca2+ influx during ischemia. Candidate channels include TRPC as well as Orai channel family members (Figure 1). This should represent an important focus for future studies.
Conclusions
Mounting evidence implicates the loss of ER homeostasis and function to the pathology associated with cerebral ischemia. ER function can be perturbed under numerous circumstances including, but not limited to, oxidative stress, Ca2+ dysregulation and the accumulation of misfolded proteins130,131. If sustained, these conditions lead to ER stress, increased Ca2+ influx, increased membrane permeability, and, eventually, cell death by apoptosis50,132. Neuroprotection has been demonstrated for several agents targeting various elements involved in regulating ER-dependent Ca2+ signalling (eg IP3R and RyR antagonists) and protein synthesis and folding (eg eIF2α phosphatase inhibitors, BiP inducers). However, numerous additional candidates targets have been identified that could be exploited in the development of new neuroprotective agents. Given recent evidence that STIM2 is a critical mediator of ischemic neuronal cell death, future studies elucidating the mechanisms through which STIM2 couples to cell death may identify novel therapeutic avenues.
Acknowledgments
This work was supported through funding from the Heart and Stroke Foundation of Canada to Dr Michael F JACKSON.
References
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Petersen OH. The endoplasmic reticulum as an integrating signalling organelle: from neuronal signalling to neuronal death. Eur J Pharmacol. 2002;447:141–54. doi: 10.1016/s0014-2999(02)01838-1. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–79. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- Kohno K, Higuchi T, Ohta S, Kohno K, Kumon Y, Sakaki S. Neuroprotective nitric oxide synthase inhibitor reduces intracellular calcium accumulation following transient global ischemia in the gerbil. Neurosci Lett. 1997;224:17–20. doi: 10.1016/s0304-3940(97)13459-0. [DOI] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- Xing H, zimi-Zonooz A, Shuttleworth CW, Connor JA. Caffeine releasable stores of Ca2+ show depletion prior to the final steps in delayed CA1 neuronal death. J Neurophysiol. 2004;92:2960–7. doi: 10.1152/jn.00015.2004. [DOI] [PubMed] [Google Scholar]
- Lanner JT. Ryanodine receptor physiology and its role in disease. Adv Exp Med Biol. 2012;740:217–34. doi: 10.1007/978-94-007-2888-2_9. [DOI] [PubMed] [Google Scholar]
- Emptage N, Bliss TV, Fine A. Single synaptic events evoke NMDA receptor-mediated release of calcium from internal stores in hippocampal dendritic spines. Neuron. 1999;22:115–24. doi: 10.1016/s0896-6273(00)80683-2. [DOI] [PubMed] [Google Scholar]
- Adasme T, Haeger P, Paula-Lima AC, Espinoza I, Casas-Alarcon MM, Carrasco MA, et al. Involvement of ryanodine receptors in neurotrophin-induced hippocampal synaptic plasticity and spatial memory formation. Proc Natl Acad Sci U S A. 2011;108:3029–34. doi: 10.1073/pnas.1013580108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkotian E, Segal M. Release of calcium from stores alters the morphology of dendritic spines in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 1999;96:12068–72. doi: 10.1073/pnas.96.21.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YF, Hawkins RD. Ryanodine receptors contribute to cGMP-induced late-phase LTP and CREB phosphorylation in the hippocampus. J Neurophysiol. 2002;88:1270–8. doi: 10.1152/jn.2002.88.3.1270. [DOI] [PubMed] [Google Scholar]
- Goussakov I, Chakroborty S, Stutzmann GE. Generation of dendritic Ca2+ oscillations as a consequence of altered ryanodine receptor function in AD neurons. Channels (Austin) 2011;5:9–13. doi: 10.4161/chan.5.1.14124. [DOI] [PubMed] [Google Scholar]
- Bardo S, Cavazzini MG, Emptage N. The role of the endoplasmic reticulum Ca2+ store in the plasticity of central neurons. Trends Pharmacol Sci. 2006;27:78–84. doi: 10.1016/j.tips.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, et al. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–77. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, et al. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–98. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312:924–7. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Xiong ZG, Jackson MF. Paradox of Ca(2+) signaling, cell death and stroke. Trends Neurosci. 2006;29:75–81. doi: 10.1016/j.tins.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–7. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- Bull R, Finkelstein JP, Galvez J, Sanchez G, Donoso P, Behrens MI, et al. Ischemia enhances activation by Ca2+ and redox modification of ryanodine receptor channels from rat brain cortex. J Neurosci. 2008;28:9463–72. doi: 10.1523/JNEUROSCI.2286-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull R, Finkelstein JP, Humeres A, Behrens MI, Hidalgo C. Effects of ATP, Mg2+, and redox agents on the Ca2+ dependence of RyR channels from rat brain cortex. Am J Physiol Cell Physiol. 2007;293:C162–71. doi: 10.1152/ajpcell.00518.2006. [DOI] [PubMed] [Google Scholar]
- Bull R, Marengo JJ, Finkelstein JP, Behrens MI, Alvarez O. SH oxidation coordinates subunits of rat brain ryanodine receptor channels activated by calcium and ATP. Am J Physiol Cell Physiol. 2003;285:C119–28. doi: 10.1152/ajpcell.00296.2002. [DOI] [PubMed] [Google Scholar]
- Kakizawa S, Yamazawa T, Chen Y, Ito A, Murayama T, Oyamada H, et al. Nitric oxide-induced calcium release via ryanodine receptors regulates neuronal function. EMBO J. 2012;31:417–28. doi: 10.1038/emboj.2011.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boys JA, Toledo AH, Naya-Prado R, Lopez-Neblina F, Toledo-Pereyra LH. Effects of dantrolene on ischemia-reperfusion injury in animal models: a review of outcomes in heart, brain, liver, and kidney. J Investig Med. 2010;58:875–82. doi: 10.231/JIM.0b013e3181e5d719. [DOI] [PubMed] [Google Scholar]
- Inan S, Wei H. The cytoprotective effects of dantrolene: a ryanodine receptor antagonist. Anesth Analg. 2010;111:1400–10. doi: 10.1213/ANE.0b013e3181f7181c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlschlegel S, Sims JR. Dantrolene: mechanisms of neuroprotection and possible clinical applications in the neurointensive care unit. Neurocrit Care. 2009;10:103–15. doi: 10.1007/s12028-008-9133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp AH, Nucifora FC, Jr, Blondel O, Sheppard CA, Zhang C, Snyder SH, et al. Differential cellular expression of isoforms of inositol 1,4,5-triphosphate receptors in neurons and glia in brain. J Comp Neurol. 1999;406:207–20. [PubMed] [Google Scholar]
- Chen X, Kintner DB, Luo J, Baba A, Matsuda T, Sun D. Endoplasmic reticulum Ca2+ dysregulation and endoplasmic reticulum stress following in vitro neuronal ischemia: role of Na+-K+-Cl-cotransporter. J Neurochem. 2008;106:1563–76. doi: 10.1111/j.1471-4159.2008.05501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Matute C, Alberdi E. Endoplasmic reticulum Ca(2+) release through ryanodine and IP(3) receptors contributes to neuronal excitotoxicity. Cell Calcium. 2009;46:273–81. doi: 10.1016/j.ceca.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Bruno V, Battaglia G, Copani A, D'Onofrio M, Di IP, De BA, et al. Metabotropic glutamate receptor subtypes as targets for neuroprotective drugs. J Cereb Blood Flow Metab. 2001;21:1013–33. doi: 10.1097/00004647-200109000-00001. [DOI] [PubMed] [Google Scholar]
- Hagar RE, Burgstahler AD, Nathanson MH, Ehrlich BE. Type III InsP3 receptor channel stays open in the presence of increased calcium. Nature. 1998;396:81–4. doi: 10.1038/23954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasri NN, Holmes AM, Bultynck G, Parys JB, Bootman MD, Rietdorf K, et al. Regulation of InsP3 receptor activity by neuronal Ca2+-binding proteins. EMBO J. 2004;23:312–21. doi: 10.1038/sj.emboj.7600037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, McBride S, Mak DO, Vardi N, Palczewski K, Haeseleer F, et al. Identification of a family of calcium sensors as protein ligands of inositol trisphosphate receptor Ca(2+) release channels. Proc Natl Acad Sci U S A. 2002;99:7711–6. doi: 10.1073/pnas.102006299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock JT, Sinkins WG, Schilling WP. Protein S-glutathionylation enhances Ca2+-induced Ca2+ release via the IP3 receptor in cultured aortic endothelial cells. J Physiol. 2012;590:3431–47. doi: 10.1113/jphysiol.2012.230656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decuypere JP, Monaco G, Missiaen L, De SH, Parys JB, Bultynck G. IP(3) receptors, mitochondria, and Ca signaling: implications for aging. J Aging Res. 2011;2011:920178. doi: 10.4061/2011/920178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham SH, Chen J. Programmed cell death in cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:99–109. doi: 10.1097/00004647-200102000-00001. [DOI] [PubMed] [Google Scholar]
- Baba-Aissa F, Raeymaekers L, Wuytack F, Dode L, Casteels R. Distribution and isoform diversity of the organellar Ca2+ pumps in the brain. Mol Chem Neuropathol. 1998;33:199–208. doi: 10.1007/BF02815182. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Churn SB, DeLorenzo RJ. Ischemia-induced inhibition of calcium uptake into rat brain microsomes mediated by Mg2+/Ca2+ ATPase. J Neurochem. 1997;68:1124–34. doi: 10.1046/j.1471-4159.1997.68031124.x. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Churn SB, DeLorenzo RJ. Global ischemia-induced inhibition of the coupling ratio of calcium uptake and ATP hydrolysis by rat whole brain microsomal Mg(2+)/Ca(2+) ATPase. Brain Res. 1999;834:32–41. doi: 10.1016/s0006-8993(99)01504-8. [DOI] [PubMed] [Google Scholar]
- Viner RI, Huhmer AF, Bigelow DJ, Schoneich C. The oxidative inactivation of sarcoplasmic reticulum Ca(2+)-ATPase by peroxynitrite. Free Radic Res. 1996;24:243–59. doi: 10.3109/10715769609088022. [DOI] [PubMed] [Google Scholar]
- Tang WH, Cheng WT, Kravtsov GM, Tong XY, Hou XY, Chung SK, et al. Cardiac contractile dysfunction during acute hyperglycemia due to impairment of SERCA by polyol pathway-mediated oxidative stress. Am J Physiol Cell Physiol. 2010;299:C643–53. doi: 10.1152/ajpcell.00137.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Martin Y, Martin-Romero FJ, Inesta-Vaquera FA, Gutierrez-Merino C, Henao F. Modulation of sarcoplasmic reticulum Ca(2+)-ATPase by chronic and acute exposure to peroxynitrite. Eur J Biochem. 2004;271:2647–57. doi: 10.1111/j.1432-1033.2004.04193.x. [DOI] [PubMed] [Google Scholar]
- Grover AK, Kwan CY, Samson SE. Effects of peroxynitrite on sarco/endoplasmic reticulum Ca2+ pump isoforms SERCA2b and SERCA3a. Am J Physiol Cell Physiol. 2003;285:C1537–43. doi: 10.1152/ajpcell.00299.2003. [DOI] [PubMed] [Google Scholar]
- Xu KY, Zweier JL, Becker LC. Hydroxyl radical inhibits sarcoplasmic reticulum Ca(2+)-ATPase function by direct attack on the ATP binding site. Circ Res. 1997;80:76–81. doi: 10.1161/01.res.80.1.76. [DOI] [PubMed] [Google Scholar]
- Viner RI, Ferrington DA, Williams TD, Bigelow DJ, Schoneich C. Protein modification during biological aging: selective tyrosine nitration of the SERCA2a isoform of the sarcoplasmic reticulum Ca2+-ATPase in skeletal muscle. Biochem J. 1999;340:657–69. [PMC free article] [PubMed] [Google Scholar]
- Knyushko TV, Sharov VS, Williams TD, Schoneich C, Bigelow DJ. 3-Nitrotyrosine modification of SERCA2a in the aging heart: a distinct signature of the cellular redox environment. Biochemistry. 2005;44:13071–81. doi: 10.1021/bi051226n. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Petersen OH, Verkhratsky A. Intraluminal calcium as a primary regulator of endoplasmic reticulum function. Cell Calcium. 2005;38:303–10. doi: 10.1016/j.ceca.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Michalak M, Robert Parker JM, Opas M. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium. 2002;32:269–78. doi: 10.1016/s0143416002001884. [DOI] [PubMed] [Google Scholar]
- Lievremont JP, Rizzuto R, Hendershot L, Meldolesi J. BiP, a major chaperone protein of the endoplasmic reticulum lumen, plays a direct and important role in the storage of the rapidly exchanging pool of Ca2+ J Biol Chem. 1997;272:30873–9. doi: 10.1074/jbc.272.49.30873. [DOI] [PubMed] [Google Scholar]
- Banhegyi G, Mandl J, Csala M. Redox-based endoplasmic reticulum dysfunction in neurological diseases. J Neurochem. 2008;107:20–34. doi: 10.1111/j.1471-4159.2008.05571.x. [DOI] [PubMed] [Google Scholar]
- Hu BR, Martone ME, Jones YZ, Liu CL. Protein aggregation after transient cerebral ischemia. J Neurosci. 2000;20:3191–9. doi: 10.1523/JNEUROSCI.20-09-03191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge P, Luo Y, Liu CL, Hu B. Protein aggregation and proteasome dysfunction after brain ischemia. Stroke. 2007;38:3230–6. doi: 10.1161/STROKEAHA.107.487108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGracia DJ, Rudolph J, Roberts GG, Rafols JA, Wang J. Convergence of stress granules and protein aggregates in hippocampal cornu ammonis 1 at later reperfusion following global brain ischemia. Neuroscience. 2007;146:562–72. doi: 10.1016/j.neuroscience.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Chen S, Kamme F, Hu BR. Ischemic preconditioning prevents protein aggregation after transient cerebral ischemia. Neuroscience. 2005;134:69–80. doi: 10.1016/j.neuroscience.2005.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- Gardner BM, Walter P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science. 2011;333:1891–4. doi: 10.1126/science.1209126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti A, Chen AW, Varner JD. A review of the mammalian unfolded protein response. Biotechnol Bioeng. 2011;108:2777–93. doi: 10.1002/bit.23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–30. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- Benavides A, Pastor D, Santos P, Tranque P, Calvo S. CHOP plays a pivotal role in the astrocyte death induced by oxygen and glucose deprivation. Glia. 2005;52:261–75. doi: 10.1002/glia.20242. [DOI] [PubMed] [Google Scholar]
- Hotokezaka Y, van LK, Lo EH, Beatrix B, Katayama I, Jin G, et al. alphaNAC depletion as an initiator of ER stress-induced apoptosis in hypoxia. Cell Death Differ. 2009;16:1505–14. doi: 10.1038/cdd.2009.90. [DOI] [PubMed] [Google Scholar]
- Kudo T, Kanemoto S, Hara H, Morimoto N, Morihara T, Kimura R, et al. A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ. 2008;15:364–75. doi: 10.1038/sj.cdd.4402276. [DOI] [PubMed] [Google Scholar]
- Oida Y, Shimazawa M, Imaizumi K, Hara H. Involvement of endoplasmic reticulum stress in the neuronal death induced by transient forebrain ischemia in gerbil. Neuroscience. 2008;151:111–9. doi: 10.1016/j.neuroscience.2007.10.047. [DOI] [PubMed] [Google Scholar]
- Oida Y, Izuta H, Oyagi A, Shimazawa M, Kudo T, Imaizumi K, et al. Induction of BiP, an ER-resident protein, prevents the neuronal death induced by transient forebrain ischemia in gerbil. Brain Res. 2008;1208:217–24. doi: 10.1016/j.brainres.2008.02.068. [DOI] [PubMed] [Google Scholar]
- Roberts GG, Di Loreto MJ, Marshall M, Wang J, DeGracia DJ. Hippocampal cellular stress responses after global brain ischemia and reperfusion. Antioxid Redox Signal. 2007;9:2265–75. doi: 10.1089/ars.2007.1786. [DOI] [PubMed] [Google Scholar]
- Shibata M, Hattori H, Sasaki T, Gotoh J, Hamada J, Fukuuchi Y. Activation of caspase-12 by endoplasmic reticulum stress induced by transient middle cerebral artery occlusion in mice. Neuroscience. 2003;118:491–9. doi: 10.1016/s0306-4522(02)00910-7. [DOI] [PubMed] [Google Scholar]
- Shimoke K, Matsuki Y, Fukunaga K, Matsumura Y, Fujita E, Sugihara K, et al. Appearance of nuclear-sorted caspase-12 fragments in cerebral cortical and hippocampal neurons in rats damaged by autologous blood clot embolic brain infarctions. Cell Mol Neurobiol. 2011;31:795–802. doi: 10.1007/s10571-011-9687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokka AL, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L, et al. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci. 2007;27:901–8. doi: 10.1523/JNEUROSCI.4289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajiri S, Oyadomari S, Yano S, Morioka M, Gotoh T, Hamada JI, et al. Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ. 2004;11:403–15. doi: 10.1038/sj.cdd.4401365. [DOI] [PubMed] [Google Scholar]
- Wang S, Longo FM, Chen J, Butman M, Graham SH, Haglid KG, et al. Induction of glucose regulated protein (grp78) and inducible heat shock protein (hsp70) mRNAs in rat brain after kainic acid seizures and focal ischemia. Neurochem Int. 1993;23:575–82. doi: 10.1016/0197-0186(93)90106-f. [DOI] [PubMed] [Google Scholar]
- Yu Z, Luo H, Fu W, Mattson MP. The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis. Exp Neurol. 1999;155:302–14. doi: 10.1006/exnr.1998.7002. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Saito A, Okuno S, Ferrand-Drake M, Dodd RL, Nishi T, et al. Oxidative damage to the endoplasmic reticulum is implicated in ischemic neuronal cell death. J Cereb Blood Flow Metab. 2003;23:1117–28. doi: 10.1097/01.WCB.0000089600.87125.AD. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Saito A, Okuno S, Ferrand-Drake M, Dodd RL, Chan PH. Oxidative injury to the endoplasmic reticulum in mouse brains after transient focal ischemia. Neurobiol Dis. 2004;15:229–39. doi: 10.1016/j.nbd.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Kumar R, Azam S, Sullivan JM, Owen C, Cavener DR, Zhang P, et al. Brain ischemia and reperfusion activates the eukaryotic initiation factor 2alpha kinase, PERK. J Neurochem. 2001;77:1418–21. doi: 10.1046/j.1471-4159.2001.00387.x. [DOI] [PubMed] [Google Scholar]
- Kumar R, Krause GS, Yoshida H, Mori K, DeGracia DJ. Dysfunction of the unfolded protein response during global brain ischemia and reperfusion. J Cereb Blood Flow Metab. 2003;23:462–71. doi: 10.1097/01.WCB.0000056064.25434.CA. [DOI] [PubMed] [Google Scholar]
- Owen CR, Kumar R, Zhang P, McGrath BC, Cavener DR, Krause GS. PERK is responsible for the increased phosphorylation of eIF2alpha and the severe inhibition of protein synthesis after transient global brain ischemia. J Neurochem. 2005;94:1235–42. doi: 10.1111/j.1471-4159.2005.03276.x. [DOI] [PubMed] [Google Scholar]
- Badiola N, Penas C, Minano-Molina A, Barneda-Zahonero B, Fado R, Sanchez-Opazo G, et al. Induction of ER stress in response to oxygen-glucose deprivation of cortical cultures involves the activation of the PERK and IRE-1 pathways and of caspase-12. Cell Death Dis. 2011;2:e149. doi: 10.1038/cddis.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGracia DJ, Hu BR. Irreversible translation arrest in the reperfused brain. J Cereb Blood Flow Metab. 2007;27:875–93. doi: 10.1038/sj.jcbfm.9600388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen W, Aufenberg C, Hotop S, Mengesdorf T. Transient cerebral ischemia activates processing of xbp1 messenger RNA indicative of endoplasmic reticulum stress. J Cereb Blood Flow Metab. 2003;23:449–61. doi: 10.1097/01.WCB.0000054216.21675.AC. [DOI] [PubMed] [Google Scholar]
- Lee J, Bruce-Keller AJ, Kruman Y, Chan SL, Mattson MP. 2-Deoxy-D-glucose protects hippocampal neurons against excitotoxic and oxidative injury: evidence for the involvement of stress proteins. J Neurosci Res. 1999;57:48–61. doi: 10.1002/(SICI)1097-4547(19990701)57:1<48::AID-JNR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Oida Y, Hamanaka J, Hyakkoku K, Shimazawa M, Kudo T, Imaizumi K, et al. Post-treatment of a BiP inducer prevents cell death after middle cerebral artery occlusion in mice. Neurosci Lett. 2010;484:43–6. doi: 10.1016/j.neulet.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–9. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- Lee dY, Lee KS, Lee HJ, Kim DH, Noh YH, Yu K, et al. Activation of PERK signaling attenuates Abeta-mediated ER stress. PLoS One. 2010;5:e10489. doi: 10.1371/journal.pone.0010489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galehdar Z, Swan P, Fuerth B, Callaghan SM, Park DS, Cregan SP. Neuronal apoptosis induced by endoplasmic reticulum stress is regulated by ATF4-CHOP-mediated induction of the Bcl-2 homology 3-only member PUMA. J Neurosci. 2010;30:16938–48. doi: 10.1523/JNEUROSCI.1598-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525–32. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–95. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–64. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Saito A, Okuno S, Ferrand-Drake M, Dodd RL, Chan PH. Damage to the endoplasmic reticulum and activation of apoptotic machinery by oxidative stress in ischemic neurons. J Cereb Blood Flow Metab. 2005;25:41–53. doi: 10.1038/sj.jcbfm.9600005. [DOI] [PubMed] [Google Scholar]
- Nakka VP, Gusain A, Raghubir R. Endoplasmic reticulum stress plays critical role in brain damage after cerebral ischemia/reperfusion in rats. Neurotox Res. 2010;17:189–202. doi: 10.1007/s12640-009-9110-5. [DOI] [PubMed] [Google Scholar]
- Osada N, Kosuge Y, Kihara T, Ishige K, Ito Y. Apolipoprotein E-deficient mice are more vulnerable to ER stress after transient forebrain ischemia. Neurochem Int. 2009;54:403–9. doi: 10.1016/j.neuint.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Osada N, Kosuge Y, Ishige K, Ito Y. Characterization of neuronal and astroglial responses to ER stress in the hippocampal CA1 area in mice following transient forebrain ischemia. Neurochem Int. 2010;57:1–7. doi: 10.1016/j.neuint.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Paschen W, Gissel C, Linden T, Althausen S, Doutheil J. Activation of gadd153 expression through transient cerebral ischemia: evidence that ischemia causes endoplasmic reticulum dysfunction. Brain Res Mol Brain Res. 1998;60:115–22. doi: 10.1016/s0169-328x(98)00180-6. [DOI] [PubMed] [Google Scholar]
- McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–59. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–49. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Szegezdi E, Macdonald DC, Ni CT, Gupta S, Samali A. Bcl-2 family on guard at the ER. Am J Physiol Cell Physiol. 2009;296:C941–53. doi: 10.1152/ajpcell.00612.2008. [DOI] [PubMed] [Google Scholar]
- He Z, Ostrowski RP, Sun X, Ma Q, Huang B, Zhan Y, et al. CHOP silencing reduces acute brain injury in the rat model of subarachnoid hemorrhage. Stroke. 2012;43:484–90. doi: 10.1161/STROKEAHA.111.626432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RV, Castro-Obregon S, Frankowski H, Schuler M, Stoka V, Del RG, et al. Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J Biol Chem. 2002;277:21836–42. doi: 10.1074/jbc.M202726200. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Kalai M, Vandenabeele P. Caspase-12: an overview. Cell Death Differ. 2004;11:365–8. doi: 10.1038/sj.cdd.4401364. [DOI] [PubMed] [Google Scholar]
- Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem. 2002;277:34287–94. doi: 10.1074/jbc.M204973200. [DOI] [PubMed] [Google Scholar]
- Obeng EA, Boise LH. Caspase-12 and caspase-4 are not required for caspase-dependent endoplasmic reticulum stress-induced apoptosis. J Biol Chem. 2005;280:29578–87. doi: 10.1074/jbc.M502685200. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Hetz C, Russelakis-Carneiro M, Maundrell K, Castilla J, Soto C. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 2003;22:5435–45. doi: 10.1093/emboj/cdg537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouw G, Zechel JL, Gamboa J, Lust WD, Selman WR, Ratcheson RA. Activation of caspase-12, an endoplasmic reticulum resident caspase, after permanent focal ischemia in rat. Neuroreport. 2003;14:183–6. doi: 10.1097/00001756-200302100-00004. [DOI] [PubMed] [Google Scholar]
- Rao RV, Hermel E, Castro-Obregon S, Del RG, Ellerby LM, Ellerby HM, et al. Coupling endoplasmic reticulum stress to the cell death program. Mechanism of caspase activation. J Biol Chem. 2001;276:33869–74. doi: 10.1074/jbc.M102225200. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–94. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita E, Kouroku Y, Jimbo A, Isoai A, Maruyama K, Momoi T. Caspase-12 processing and fragment translocation into nuclei of tunicamycin-treated cells. Cell Death Differ. 2002;9:1108–14. doi: 10.1038/sj.cdd.4401080. [DOI] [PubMed] [Google Scholar]
- Roy S, Sharom JR, Houde C, Loisel TP, Vaillancourt JP, Shao W, et al. Confinement of caspase-12 proteolytic activity to autoprocessing. Proc Natl Acad Sci U S A. 2008;105:4133–8. doi: 10.1073/pnas.0706658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann GE, Mattson MP. Endoplasmic reticulum Ca(2+) handling in excitable cells in health and disease. Pharmacol Rev. 2011;63:700–27. doi: 10.1124/pr.110.003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, et al. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–10. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- Sabbioni S, Barbanti-Brodano G, Croce CM, Negrini M. GOK: a gene at 11p15 involved in rhabdomyosarcoma and rhabdoid tumor development. Cancer Res. 1997;57:4493–7. [PubMed] [Google Scholar]
- Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. J Biol Chem. 2006;281:35855–62. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–22. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–9. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, Shcheglovitov A, Dolmetsch R. The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science. 2010;330:101–5. doi: 10.1126/science.1191027. [DOI] [PubMed] [Google Scholar]
- Wang Y, Deng X, Mancarella S, Hendron E, Eguchi S, Soboloff J, et al. The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science. 2010;330:105–9. doi: 10.1126/science.1191086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancarella S, Wang Y, Deng X, Landesberg G, Scalia R, Panettieri RA, et al. Hypoxia-induced acidosis uncouples the STIM-Orai calcium signaling complex. J Biol Chem. 2011;286:44788–98. doi: 10.1074/jbc.M111.303081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S, Sun Y, Watt JA, Wang S, Lei S, Birnbaumer L, et al. Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling. J Clin Invest. 2012;122:1354–67. doi: 10.1172/JCI61332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BJ, Irrinki KM, Mallilankaraman K, Lien YC, Wang Y, Bhanumathy CD, et al. S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J Cell Biol. 2010;190:391–405. doi: 10.1083/jcb.201004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboloff J, Madesh M, Gill DL. Sensing cellular stress through STIM proteins. Nat Chem Biol. 2011;7:488–92. doi: 10.1038/nchembio.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Coste B, Mathur J, Patapoutian A. Temperature-dependent STIM1 activation induces Ca(2)+ influx and modulates gene expression. Nat Chem Biol. 2011;7:351–8. doi: 10.1038/nchembio.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Mancarella S, Wang Y, Yue C, Ritchie M, Gill DL, et al. The short N-terminal domains of STIM1 and STIM2 control the activation kinetics of Orai1 channels. J Biol Chem. 2009;284:19164–8. doi: 10.1074/jbc.C109.010900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–45. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–39. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berna-Erro A, Braun A, Kraft R, Kleinschnitz C, Schuhmann MK, Stegner D, et al. STIM2 regulates capacitive Ca2+ entry in neurons and plays a key role in hypoxic neuronal cell death. Sci Signal. 2009;2:ra67. doi: 10.1126/scisignal.2000522. [DOI] [PubMed] [Google Scholar]
- Steinbeck JA, Henke N, Opatz J, Gruszczynska-Biegala J, Schneider L, Theiss S, et al. Store-operated calcium entry modulates neuronal network activity in a model of chronic epilepsy. Exp Neurol. 2011;232:185–94. doi: 10.1016/j.expneurol.2011.08.022. [DOI] [PubMed] [Google Scholar]
- Klejman ME, Gruszczynska-Biegala J, Skibinska-Kijek A, Wisniewska MB, Misztal K, Blazejczyk M, et al. Expression of STIM1 in brain and puncta-like co-localization of STIM1 and ORAI1 upon depletion of Ca(2+) store in neurons. Neurochem Int. 2009;54:49–55. doi: 10.1016/j.neuint.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Skibinska-Kijek A, Wisniewska MB, Gruszczynska-Biegala J, Methner A, Kuznicki J. Immunolocalization of STIM1 in the mouse brain. Acta Neurobiol Exp (Wars) 2009;69:413–28. doi: 10.55782/ane-2009-1753. [DOI] [PubMed] [Google Scholar]
- Ghribi O. The role of the endoplasmic reticulum in the accumulation of beta-amyloid peptide in Alzheimer's disease. Curr Mol Med. 2006;6:119–33. doi: 10.2174/156652406775574514. [DOI] [PubMed] [Google Scholar]
- Mattson MP. ER calcium and Alzheimer's disease: in a state of flux. Sci Signal. 2010;3:e10. doi: 10.1126/scisignal.3114pe10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Kauppinen A, Suuronen T, Kaarniranta K, Ojala J. ER stress in Alzheimer's disease: a novel neuronal trigger for inflammation and Alzheimer's pathology. J Neuroinflammation. 2009;6:41. doi: 10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]